| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 6, Number 4, August 2015, pages 410-415
1-Pamitoyl-2-Linoleoyl-3-Acetyl-rac-Glycerol May Reduce Incidence of Gemcitabine-Induced Neutropenia: A Pilot Case-Controlled Study
Dongwook Oha, Myung-Hwan Kima, d, Tae Jun Songa, Charles J. Choa, Kwangwoo Nama, Min Keun Choa, Joo Hyun Chuna, Kyoungwon Junga, Kyu-pyo Kimb, Jae Wha Kimc
aDivision of Gastroenterology, Department of Internal Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea
bDivision of Oncology, Department of Internal Medicine, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea
cBiomedical Translational Research Center, Korea Research Institute of Bioscience and Biotechnology, Deajeon, Korea
dCorresponding Author: Myung-Hwan Kim, Department of Internal Medicine, University of Ulsan College of Medicine, Asan Medical Center, 88-Olympic-Ro 43-Gil, Songpa-gu, Seoul 138-736, Korea
Manuscript accepted for publication July 22, 2015
Short title: Chemotherapy-Induced Neutropenia Prevention
doi: http://dx.doi.org/10.14740/wjon937e
| Abstract | ▴Top |
Background: Chemotherapy-induced neutropenia (CIN) may compromise planned chemotherapy, resulting in severe infection, dose reduction or delayed treatment. Orally administered 1-pamitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG) is a synthetic monoacetyldiglyceride, a product found in the antlers of sika deer. The aim of this study was to evaluate the effectiveness of PLAG for the prevention of CIN.
Methods: A total of 48 patients with unresectable pancreatic cancer received gemcitabine-based palliative chemotherapy. Among those patients, 16 patients received PLAG (500 mg) twice daily from the start of chemotherapy to the completion.
Results: The PLAG group showed a significantly lower incidence of neutropenia (absolute neutrophil count < 1,500 cells/mm3, grade 2-4), as compared to the control group (37.5% vs. 81.3%, P < 0.05). The absolute neutrophil counts (ANCs) of the PLAG group significantly less decreased from the baseline level compared to those of the control group (P < 0.05) and this significant difference in the reduction percentage of ANCs between the two groups was sustained throughout the courses of chemotherapy. No adverse events related to PLAG were observed.
Conclusions: PLAG was shown to be clinically effective and safe in reducing the incidence of CIN in pancreatic cancer patients receiving gemcitabine-based chemotherapy.
Keywords: Pancreatic cancer; Chemotherapy-induced neutropenia; Gemcitabine; G-CSF; PLAG
| Introduction | ▴Top |
Pancreatic cancer is one of the leading causes of death among gastrointestinal malignancies in the United States and Europe [1, 2]. Gemcitabine has been the standard first-line therapy for unresectable pancreatic cancer [3]. As the survival benefit of gemcitabine monotherapy is modest, however, various drugs have been investigated in combination with gemcitabine [4-9]. Gemcitabine with erlotinib combination chemotherapy showed significant survival benefits over gemcitabine monotherapy [7-9]. Myelosuppression, in particular neutropenia, is not uncommon during gemcitabine-based chemotherapy [9].
Severe neutropenia may be associated with life-threatening infections [10]. It also results in delays of the next cycle chemotherapy and/or dose reductions, leading to suboptimal chemotherapy delivery that may affect treatment outcomes [10, 11]. There is a need for hematopoietic stimulating agents for use in the prevention and/or recovery of neutropenia during cytotoxic chemotherapy. In current practice, long-acting G-CSFs have been used for the prevention of chemotherapy-induced neutropenia (CIN) [12]. However, these agents are administered parenterally and have considerable side effects.
1-Pamitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG) is an orally available synthetic monoacetyldiglyceride that has been isolated from the antlers of sika deer (Cervus nippon Temminck) (Fig. 1) [13]. Deer antler is a traditional Asian medicine, prepared by drying the uncornified antler of a deer. It has been known that chemically synthetic PLAG can stimulate the proliferation of hematopoietic stem cells, bone marrow stromal cells, immune system cells including T and B lymphocytes, dendritic cells and macrophages, both in vivo and in vitro [13, 14].
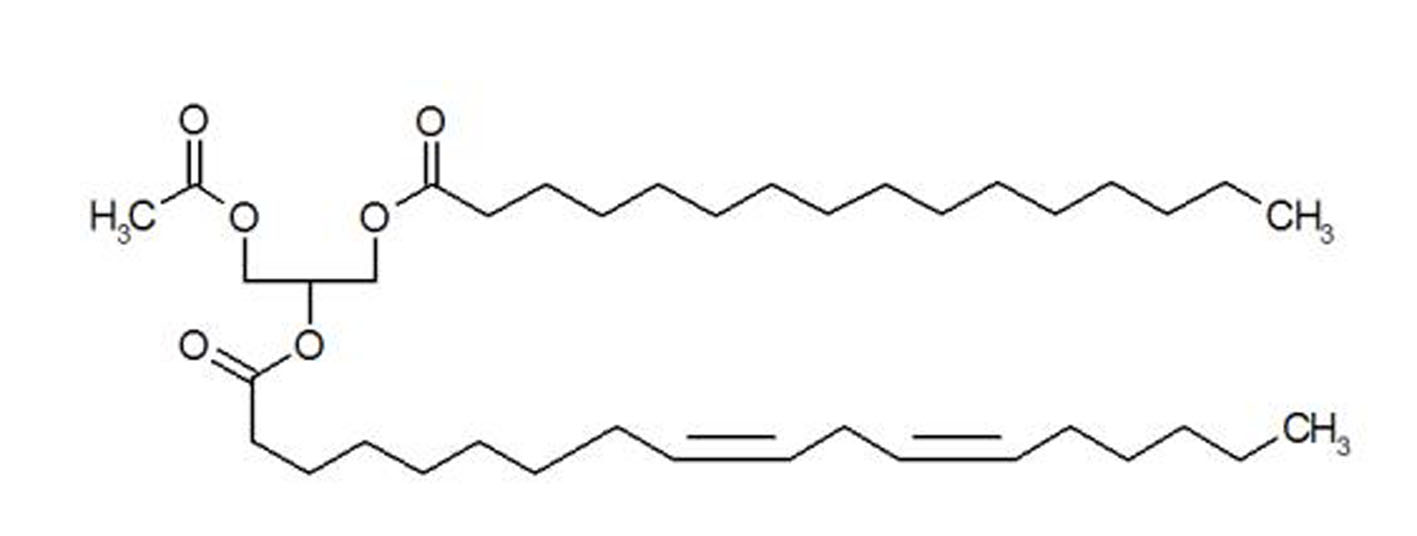 Click for large image | Figure 1. Structure of synthetic PLAG. |
Here we evaluate the effectiveness of synthetic PLAG for the prevention of CIN in patients with unresectable pancreatic cancer undergoing gemcitabine-based chemotherapy.
| Materials and Methods | ▴Top |
Patients
From January 2014 to September 2014, 16 patients with histologically or cytologically confirmed unresectable pancreatic cancer were enrolled in this study. Eligible patients had 1) locally advanced or metastatic cancer; 2) an age of ≥ 18 years; 3) an Eastern Cooperative Oncology Group (ECOG) performance status of ≤ 1; 4) adequate bone marrow function (absolute neutrophil count (ANC) ≥ 1,500/mm3, platelet count ≥ 105/mm3); 5) normal renal (creatinine clearance ≥ 50 mL/min) and hepatic function (alanine aminotransferase and total bilirubin ≥ 2 times the upper limit of normal).
Historical controls were also recruited from Asan Medical Center from March 2012 to December 2013. The eligibility criteria for the control group were the same as those for cases who intake PLAG during gemcitabine-based chemotherapy. The control group (n = 32) was matched to the PLAG group (n = 16) based on age, performance status, chemotherapy cycle, comorbidity and disease extent. This study was approved by our hospital institutional review board.
Study design and treatment protocol
All patients received gemcitabine 1,000 mg/m2 on days 1, 8, and 15 of each 4-week schedule and daily erlotinib at 100 mg orally. In the PLAG group, PLAG 500 mg was orally administered twice daily from the start of the chemotherapy to the completion. Hematology and serum chemistry analyses were performed at screening baseline, then weekly until the end of the study. Febrile neutropenia (FN) was defined as an ANC of less than 1,000/mm3 and an oral temperature of more than 38 °C on the same day or the following day after chemotherapy. If, on the day of chemotherapy administration, a patient’s ANC was reduced to 500 - 1,000/mm3 or if the absolute platelet count was reduced to 50,000 - 100,000/mm3, the gemcitabine dose was reduced by 75%. Gemcitabine was omitted for 1 week if the neutrophil count was lower than 500/mm3 or the absolute platelet count was lower than 50,000/mm3. Chemotherapy was discontinued if disease progression was observed in a follow-up CT scan, which was performed within 2 or 3 months after the initiation of chemotherapy. Erlotinib dose was interrupted in patients within tolerable rash and was reduced or discontinued if symptoms persisted for 10 - 14 days. Erlotinib dose was reduced for grade 2 diarrhea persisting for 48 - 72 h and for grade 3 diarrhea following resolution to grade 1; erlotinib was permanently discontinued for grade 4 diarrhea. Treatment continued until disease progression, unacceptable toxicity, withdrawal of patient’s consent or physician’s decision. Safety was evaluated throughout the entire study. Toxicity was graded based on the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 3.0.
Statistical analysis
The primary endpoint was neutropenia and the secondary endpoint was a safety profile. All analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were used to evaluate demographics, and safety data continuous variables were compared using the Mann-Whitney U test, paired t-test, and independent T test. A P value of < 0.05 was considered statistically significant.
| Results | ▴Top |
The baseline characteristics of patients are summarized in Table 1 and clinical outcomes are presented in Table 2. Six patients (37.5%) had locally advanced pancreatic cancer and the rest of the 16 patients (62.5%) had metastatic pancreatic cancer. The median number of treatment cycles administered was 2.5 (range 2 - 3). There were no significant differences between the PLAG group and the control group with respect to age, gender, disease extent, chemotherapy cycle, and ECOG performance.
 Click to view | Table 1. Baseline Characteristics of Patients |
 Click to view | Table 2. Clinical Outcomes of Patients |
The incidence of neutropenia (ANC < 1,500/mm3, grade 2-4) was significantly lower among patients who received PLAG, compared to the control group (37.5% vs. 81.3%, P < 0.05). By cycle, the reduction percentage of ANC was evaluated in both groups. At the baseline evaluation, the ANC did not differ between the two groups. However, the ANCs of the PLAG group significantly less decreased from the baseline level compared to those of the control group (P < 0.05) and this significant difference in the reduction percentage of ANCs between the two groups was sustained throughout the courses of chemotherapy (Fig. 2). Severe neutropenia (ANC < 500/mm3, grade 4) developed only in the control group (Fig. 3). The ANC nadir of the control group was significantly deeper than that of the PLAG group; a depth of ANC nadir of > 50% or 75% of the baseline level, respectively, was more frequently observed in the control group (Table 2, P < 0.05). FN did not occur in both groups.
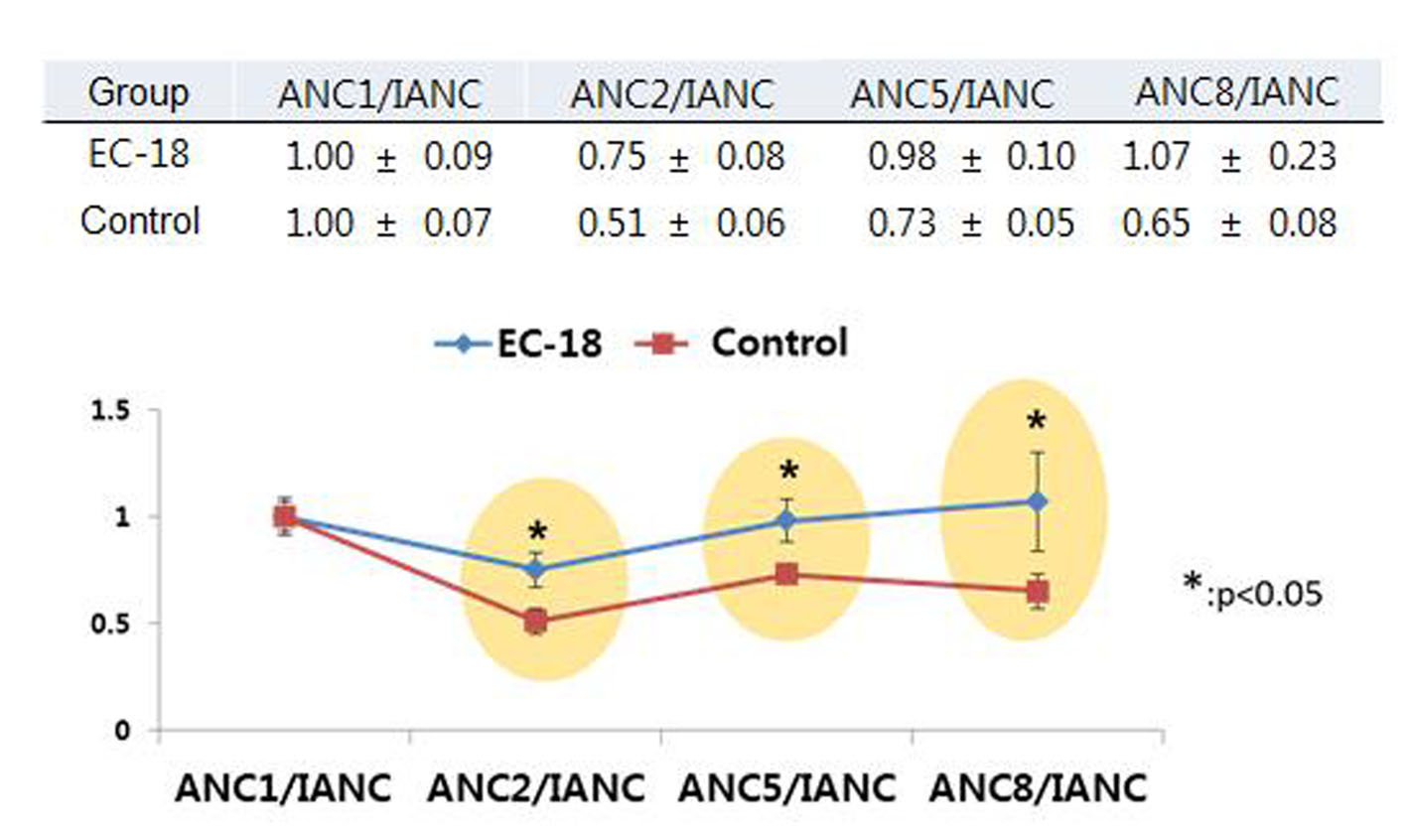 Click for large image | Figure 2. Trends in ANCs over the courses of chemotherapy between PLAG and control groups. The ANCs of PLAG group significantly less decreased from the baseline level compared to those of control group and this significant difference in reduction percentage of ANCs between the two groups was sustained throughout the courses of chemotherapy. Mean ± SE: mean ± standard error of mean. ANC: absolute neutrophil count; IANC: baseline neutrophil count prior to chemotherapy; ANC1: ANC obtained just prior to second time gemcitabine injection; ANC2: ANC obtained just prior to third time gemcitabine injection; ANC5: ANC obtained just prior to sixth time gemcitabine injection; ANC 8: ANC obtained just prior to ninth time gemcitabine injection. |
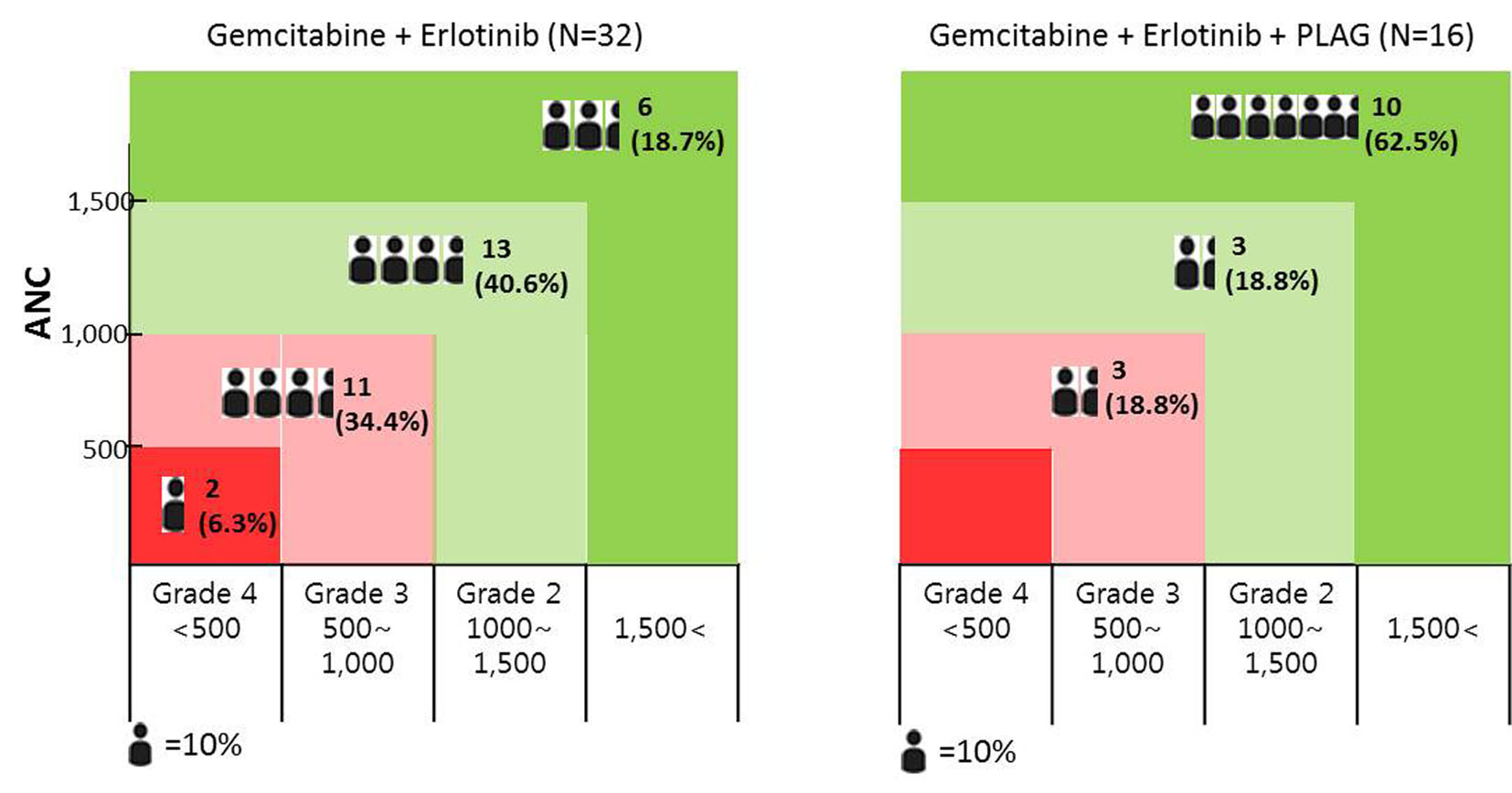 Click for large image | Figure 3. The distribution of chemotherapy-induced neutropenic patients according to the degree of neutropenia; the incidence of grade 2-4 neutropenia was significantly lower among patients who received PLAG compared to control group. Grade 4 neutropenia developed only in the control group. |
PLAG was well tolerated in PLAG group. All patients completed intake of PLAG during the study period. There were no adverse events related to PLAG during chemotherapy including nausea/vomiting, bone pain, fatigue, and liver dysfunction.
| Discussion | ▴Top |
This study was focused on the incidence of CIN for assessing the preventive effect of orally administered PLAG in patients who receive gemcitabine-based chemotherapy. PLAG administered orally during the courses of chemotherapy significantly reduced grade 2-4 neutropenia which may need chemotherapy dose modifications (dose delay/reduction) as compared with the control group (Table 2). In addition, severe neutropenia (ANC < 500/mm3) developed only in the control group (Fig. 3). In our study, FN did not develop in both groups, probably because gemcitabine is a chemotherapy regimen associated with a low risk for FN [12].
G-CSF is a recombinant growth factor that decreases the incidence and duration of severe neutropenia and minimizes infections as manifested by FN by stimulating the proliferation, differentiation, and activation of the neutrophil lineage, thereby reducing the neutrophil maturation time [12, 15-17]. Recently, pegylated G-CSF (pegfilgrastim) has been used for the prevention of CIN. Compared with original G-CSF (filgrastim), pegfilgrastim has a longer-acting effect equivalent to 10 - 11 days of filgrastim [18]. The administration of G-CSF within 24 h before or after chemotherapy is not recommended because of the theoretical potential for increasing chemotherapy toxicity to myeloid progenitor cells after growth factor stimulation (Table 3) [19]. Current guidelines recommend the use of prophylactic G-CSFs when the chemotherapy regimen is associated with a high risk (> 20%) for FN [12, 15-17]. In patients who receive a chemotherapy regimen associated with an intermediate risk (10-20%) for FN, however, long-acting G-CSFs can be used when patients have additional patient-related risk factors for FN such as old age or comorbidity [15-17].
 Click to view | Table 3. Comparison of PLAG and Long-Acting G-CSF for the Prevention of Chemotherapy-Induced Neutropenia |
The administration of G-CSF can cause various adverse events in patients who receive chemotherapy. Injection-site discomfort is common with G-CSF because it is administered by subcutaneous injection. Constitutional symptoms, such as fever, malaise, and influenza-like symptoms, are commonly developed after G-CSF administration. Bone pain, the most common side effect, develops in 10-30% of patients [15]. Serious adverse events such as splenic rupture, acute respiratory distress syndrome, though rare, can occur in patients receiving G-CSF [20-23]. In our study, side effects such as bone pain, fatigue, nausea, headache, or splenic rupture, which has been reported in the use of G-CSF, were not observed in any patients receiving PLAG.
In a cost-analysis of G-CSF, previous studies seem to heavily concentrate on medical costs related to FN and its consequences such as the incidence of FN, rate of hospitalization, IV antibiotic use and early mortality. However, “afebrile” neutropenia (ANC < 1,500/mm3, grade 2-4) may lead to dose delays or dose reductions as well as interference with the delivery of the full doses of the chemotherapy on time [24]. The resulting reduced dose intensity may worsen outcomes, especially in the setting of curative/adjuvant chemotherapy [25]. It is unlikely that much consideration was given to the impact of afebrile neutropenia on the relative dose intensity of the treatment received. This is because FN has an immediate impact on both mortality and the cost of treatment, whereas survival outcome is such a distant end point which needs long-term analysis [26]. Cost issues are likely to play a major role in limiting the use of long-acting G-CSF. If orally available PLAG with proven safety and effectiveness is much cheaper than long-acting G-CSF, PLAG may replace pegfilgrastim for the prevention of CIN in real world. This is because long-acting G-CSF (including biosimilar) is expensive, parenterally administered, and has considerable adverse events.
Due to the inherent limitation of a retrospective design and small-population of the current study, the effect of PLAG in the present study may become more evident in the large-scale prospective randomized placebo-controlled studies. Further studies are warranted to verify the effectiveness of PLAG in terms of FN prevention in patients receiving chemotherapeutic agent with higher myelosuppressive potency.
In conclusion, PLAG was shown clinically to be effective and safe in reducing the incidence of CIN in pancreatic cancer patients receiving gemcitabine-based chemotherapy.
Disclosure
This study did not receive grants from any funding agency in the public, commercial, or not-for-profit sector. The authors declare no conflict of interest.
| References | ▴Top |
- Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2013. Ann Oncol. 2013;24(3):792-800.
doi pubmed - Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9-29.
doi pubmed - Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403-2413.
pubmed - Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB, 3rd. Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20(15):3270-3275.
doi pubmed - Colucci G, Labianca R, Di Costanzo F, Gebbia V, Carteni G, Massidda B, Dapretto E, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with single-agent gemcitabine as first-line treatment of patients with advanced pancreatic cancer: the GIP-1 study. J Clin Oncol. 2010;28(10):1645-1651.
doi pubmed - Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, Harper PG, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2009;27(33):5513-5518.
doi pubmed - Gresham GK, Wells GA, Gill S, Cameron C, Jonker DJ. Chemotherapy regimens for advanced pancreatic cancer: a systematic review and network meta-analysis. BMC Cancer. 2014;14:471.
doi pubmed - Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960-1966.
doi pubmed - Yang ZY, Yuan JQ, Di MY, Zheng DY, Chen JZ, Ding H, Wu XY, et al. Gemcitabine plus erlotinib for advanced pancreatic cancer: a systematic review with meta-analysis. PLoS One. 2013;8(3):e57528.
doi pubmed - Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228-237.
doi pubmed - Schwenkglenks M, Pettengell R, Szucs TD, Culakova E, Lyman GH. Hodgkin lymphoma treatment with ABVD in the US and the EU: neutropenia occurrence and impaired chemotherapy delivery. J Hematol Oncol. 2010;3:27.
doi pubmed - Crawford J, Armitage J, Balducci L, Becker PS, Blayney DW, Cataland SR, Heaney ML, et al. Myeloid growth factors. J Natl Compr Canc Netw. 2013;11(10):1266-1290.
pubmed - Yang HO, Kim SH, Cho SH, Kim MG, Seo JY, Park JS, Jhon GJ, et al. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler). Chem Pharm Bull (Tokyo). 2004;52(7):874-878.
doi - Yang HO, Park JS, Cho SH, Yoon JY, Kim MG, Jhon GJ, Han SY, et al. Stimulatory effects of monoacetyldiglycerides on hematopoiesis. Biol Pharm Bull. 2004;27(7):1121-1125.
doi pubmed - Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colony-stimulating factors for febrile neutropenia during cancer therapy. N Engl J Med. 2013;368(12):1131-1139.
doi pubmed - Smith TJ, Khatcheressian J, Lyman GH, Ozer H, Armitage JO, Balducci L, Bennett CL, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187-3205.
doi pubmed - Aapro MS, Cameron DA, Pettengell R, Bohlius J, Crawford J, Ellis M, Kearney N, et al. EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphomas and solid tumours. Eur J Cancer. 2006;42(15):2433-2453.
doi pubmed - Zamboni WC. Pharmacokinetics of pegfilgrastim. Pharmacotherapy. 2003;23(8 Pt 2):9S-14S.
doi pubmed - Burris HA, Belani CP, Kaufman PA, Gordon AN, Schwartzberg LS, Paroly WS, Shahin S, et al. Pegfilgrastim on the Same Day Versus Next Day of Chemotherapy in Patients With Breast Cancer, Non-Small-Cell Lung Cancer, Ovarian Cancer, and Non-Hodgkin's Lymphoma: Results of Four Multicenter, Double-Blind, Randomized Phase II Studies. J Oncol Pract. 2010;6(3):133-140.
doi pubmed - Scott WR, Silberstein L, Flatley R, Ardeshna KM, Korostoff N, Dawe S. Cutaneous reaction to pegfilgrastim presenting as severe generalized skin eruption. Br J Dermatol. 2009;161(3):717-719.
doi pubmed - Veerappan R, Morrison M, Williams S, Variakojis D. Splenic rupture in a patient with plasma cell myeloma following G-CSF/GM-CSF administration for stem cell transplantation and review of the literature. Bone Marrow Transplant. 2007;40(4):361-364.
doi pubmed - Karlin L, Darmon M, Thiery G, Ciroldi M, de Miranda S, Lefebvre A, Schlemmer B, et al. Respiratory status deterioration during G-CSF-induced neutropenia recovery. Bone Marrow Transplant. 2005;36(3):245-250.
doi pubmed - Alvarez-Ruiz S, Penas PF, Fernandez-Herrera J, Sanchez-Perez J, Fraga J, Garcia-Diez A. Maculopapular eruption with enlarged macrophages in eight patients receiving G-CSF or GM-CSF. J Eur Acad Dermatol Venereol. 2004;18(3):310-313.
doi pubmed - Weycker D, Li X, Edelsberg J, Barron R, Kartashov A, Xu H, Lyman GH. Risk and Consequences of Chemotherapy-Induced Febrile Neutropenia in Patients With Metastatic Solid Tumors. J Oncol Pract. 2014.
doi - Crawford J, Dale DC, Kuderer NM, Culakova E, Poniewierski MS, Wolff D, Lyman GH. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6(2):109-118.
pubmed - Leonard RC, Miles D, Thomas R, Nussey F. Impact of neutropenia on delivering planned adjuvant chemotherapy: UK audit of primary breast cancer patients. Br J Cancer. 2003;89(11):2062-2068.
doi pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.