| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 7, Number 5-6, December 2016, pages 109-118
Incidence and Mortality of Nasopharynx Cancer and Its Relationship With Human Development Index in the World in 2012
Neda Mahdavifara, Farhad Towhidib, Behnam Reza Makhsosib, Reza Pakzadc, Ali Moinid, Abbas Ahmadib, Sarah Lotfib, Hamid Salehiniyae, f, g
aHealth Promotion Research Center, Department of Epidemiology and Biostatistics, School of Public Health, Zahedan University of Medical Sciences, Zahedan, Iran
bImam Reza Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran
cStudent Research Committee, Ilam University of Medical Sciences, Ilam, Iran
dDepartment of Internal Medicine, Imam Reza Hospital, Kermanshah University of Medical Sciences, Kermanshah, Iran
eZabol University of Medical Sciences, Zabol, Iran
fDepartment of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
gCorresponding Author: Hamid Salehiniya, Zabol University of Medical Sciences, Zabol, Iran; Department of Epidemiology and Biostatistics, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
Manuscript accepted for publication August 15, 2016
Short title: Nasopharynx Cancer and HDI in the World
doi: https://doi.org/10.14740/wjon980w
| Abstract | ▴Top |
Background: One of the most common cancers in head and neck is nasopharynx. Knowledge about the incidence and mortality of this disease and its distribution in terms of geographical areas is necessary for further study, better planning and prevention. Therefore, this study aimed to determine the incidence and mortality of nasopharynx cancer and its relationship with human development index (HDI) in the world in 2012.
Methods: This study was an ecological study conducted based on GLOBOCAN project of World Health Organization (WHO) for the countries in world. The correlation between standardized incidence rates (SIRs) and standardized mortality rates (SMRs) of nasopharynx cancer with HDI and its components was assessed with correlation coefficient by using SPSS 15.
Results: In 2012, 86,691 nasopharynx cancer cases occurred in the world, so that 60,896 new cases were seen in men and 25,795 new cases in women (sex ratio = 2.36). SIR of the cancer was 1.2 per 100,000 (1.7 in men and 0.7 in women per 100,000) in the world. In 2012, 50,831 nasopharynx death cases occurred in the world, so that 35,756 death cases were seen in men and 15,075 death cases in women (sex ratio = 2.37). SIR of mortality from the cancer was 0.7 per 100,000 (0.7 in women and 1 in men per 100,000) in the world. The results of correlation analysis showed a negative correlation between the SIR and HDI (r = -0.037, P = 0.629), and also the results of correlation analysis showed a negative correlation between the SMR and HDI (r = -0.237, P = 0.002).
Conclusion: Nasopharyngeal cancer is native to Southeast Asia and the highest incidence and mortality were seen in countries with moderate and low HDI. It is suggested that studies are conducted on determining the causes of the cancer incidence and mortality in the world and the differences between various regions.
Keywords: Incidence; Mortality; Nasopharynx cancer; Human development index; World
| Introduction | ▴Top |
Cancer is a major cause of morbidity and mortality in all communities [1]. Head and neck malignancies are relatively common cancers in human and involve the various anatomical parts of the head and neck [2]. One of the most common cancers in head and neck is nasopharyngeal cancer [3, 4], which is associated with a very unique geographical distribution pattern. It is reported that there are about 86,500 cases of nasopharynx cancer and 50,000 deaths from the disease worldwide [5]. The cancer is malignant and its incidence is affected by geographical and racial variations from the epidemiological perspective. In most parts of the world, the age-standardized incidence rate (ASIR) of nasopharynx cancer, regardless of gender, was reported about less than 1 per 100,000 person-years [6-8]. The southern part of China, parts of Southeast Asia, North and South Africa and Alaska have the highest incidence rate. In Europe, ASIR is generally less than two cases per 100,000 in 1 year among men and less than one case per 100,000 among women [9]. In contrast, in China, Southeast Asia and the Arctic region [7], as well as in immigrants from those regions [10], an incidence of 30 cases per 100,000 in men and 10 cases per 100,000 in female subjects were observed [9]. The cancer has an uneven geographical distribution: 81% of new cases in Asia and 9% in Africa. According to the burden of disease, Southeast Asian countries include 67% of the global burden of cancer [11]. Nasopharyngeal cancer is mainly related to the use of tobacco and alcohol [12] and other known risk factors, such as human papilloma virus (HPV) [13], infection with Epstein-Barr virus (EBV) [7] and low intake of fruits and vegetables [14]. There is an increased risk of nasopharyngeal cancer and some other cancers in first-degree relatives because of genetic and environmental risk factors [15]. Nasopharyngeal cancer is diagnosed in advanced stages, but associated with very poor prognosis. However, the cancer is potentially curable in early stages. Therefore, early detection screening may lead to improved outcomes [16]. A lack of knowledge of general practitioners working in health centers in Asian countries about the various aspects of nasopharyngeal cancer may lead to delay in diagnosis [17]. Due to the sensitive nature of the cancer, chemotherapy and radiation therapy are major treatments of this cancer [18-21]. However, about 10-15% of recurrences occur in patients with metastasis in distant organs when chemotherapy and radiation therapy are operated [22, 23].
One of the reasons for differences in cancer incidence and mortality in different regions is socio-economic status (SES), education level and life expectancy that are highlighted by the human development index (HDI). HDI classification is useful to compare the incidence and mortality of cancer on a global level [23] and is one of the indicators which evaluate the status of illnesses and deaths between countries [24]. In fact, this index is associated with the incidence and mortality of many diseases and is an appropriate indicator for awareness of the status of countries in terms of a specific disease [25-27]. The relationship between HDI and some types of cancer is studied and investigation on this relationship can lead to a more accurate understanding of cancer distribution and its risk factors [28]. Knowing nasopharynx cancer incidence and mortality can be useful for health programs and research activities and considering the possible role of the HDI, this study aimed to determine the incidence and mortality of nasopharyngeal carcinoma and its relationship with HDI and its components in the world in 2012.
| Materials and Methods | ▴Top |
This study was an ecologic study in the world for assessing the correlation between age-specific incidence and mortality rate (ASR) with HDI and its details, including life expectancy at birth, mean years of schooling, and gross national income (GNI) per capita. Data were about the ASR for all country in 2012 obtained from the global cancer project; available at http://globocan.iarc.fr/Default.aspx [29] and HDI from Human Development Report 2013 [30], that includes information about HDI and its details for every country in the world in 2012.
Method for estimating the ASRs in global cancer project by international agency for research on cancer
Age-specific incidence rate estimate
The methods of estimation are country specific, and the quality of the estimation depends upon the quality and on the amount of the information available for each country. In theory, there are as many methods as countries, and because of the variety and the complexity of these methods, an overall quality score for the incidence and mortality estimates combined is almost impossible to establish. However, an alphanumeric scoring system which independently describes the availability of incidence and mortality data has been established at the country level. The combined score is presented together with the estimates for each country with an aim of providing a broad indication of the robustness of the estimation.
The methods to estimate the sex- and age-specific incidence rates of cancer for a specific country fall into one of the following broad categories, in priority order: 1) rates projected to 2012 (38 countries); 2) most recent rates applied to 2012 population (20 countries); 3) estimated from national mortality by modeling, using incidence mortality ratios derived from recorded data in country-specific cancer registries (13 countries); 4) estimated from national mortality estimates by modeling, using incidence mortality ratios derived from recorded data in local cancer registries in neighboring countries (nine European countries); 5) estimated from national mortality estimates using modeled survival (32 countries); 6) estimated as the weighted average of the local rates (16 countries); 7) one cancer registry covering a part of a country is used as representative of the country profile (11 countries); 8) age-/sex-specific rates for “all cancers” were partitioned using data on relative frequency of different cancers (by age and sex) (12 countries); 9) the rates are those of neighboring countries or registries in the same area (33 countries) [29].
Age-specific mortality rate estimate
Depending on the degree of detail and accuracy of the national mortality data, six methods have been utilized in the following order of priority: 1) rates projected to 2012 (69 countries); 2) most recent rates applied to 2012 population (26 countries); 3) estimated as the weighted average of regional rates (one country); 4) estimated from national incidence estimates by modeling, using country-specific survival (two countries); 5) estimated from national incidence estimates using modeled survival (83 countries); 6) the rates are those of neighboring countries or registries in the same area (three countries) [31].
HDI
HDI is a composite measure of indicators along three components, including life expectancy, educational attainment, and command over the resources needed for a decent living. All groups and regions have seen notable improvement in all HDI components, with faster progress in low and medium HDI countries. On this basis, the world is becoming less unequal. Nevertheless, national averages hide large variations in human experience. Wide disparities remain within countries of both the North and the South, and income inequality within and between many countries has been rising. According to HDI, countries in the world are divided into four categories as follows: countries with very high HDI (HDI ≥ 0.80), countries with a high HDI (0.80 > HDI > 0.710), medium HDI countries (0.710 ≥ HD ≥ 0.535), and countries with a low HDI (HDI < 0.535) [30].
Statistical analysis
In this study, we used correlation bivariate method for assessment of the correlation between ASR with HDI and its details, which include life expectancy at birth, mean years of schooling, and GNI per capita. Statistical significance was assumed if P < 0.05. All reported P-values are two-sided. Statistical analyses were performed using SPSS (version 15.0, SPSS Inc.).
| Results | ▴Top |
Nasopharynx cancer cases
In 2012, 86,691 nasopharynx cancer cases occurred in the world, so that 60,896 new cases were seen in men and 25,795 new cases in women (sex ratio = 2.36). Of all cases, 8,863 cases occurred in countries with very high HDI, 8,053 cases in countries with high HDI, 61,229 cases in countries with moderate HDI, and 8,535 cases in countries with low HDI. The highest number of cancer cases was seen in five countries such as China with about 33,198 cases, Indonesia 13,084, Vietnam about 4,931, India 3,947 and Malaysia with about 2,030 cases, respectively. Five countries that had the largest number of nasopharynx cancer in men were China about 23,581, Indonesia about 9,355, Vietnam 3,301, India 2,956 and Malaysia 1,487 cases, respectively. The highest number in women was seen in five countries including China 9,617, Indonesia 3,729 cases, Vietnam 1,630, India 991 cases and the United States about 599 cases, respectively.
ASIR
SIR of the cancer was 1.2 per 100,000 (1.7 in men and 0.7 in women per 100,000) in the world. The incidence rate in countries with very high HDI was 0.6 per 100,000, in countries with a high HDI 0.7 per 100,000, in countries with medium HDI 1.7 per 100,000, and in countries with a low HDI 1 per 100,000. Five countries that had the highest ASIR were Malaysia 7.2 per 100,000, Singapore 6.4 per 100,000, Indonesia 5.6 per 100,000, Vietnam 5.4 per 100,000 and Brunei 5 per 100,000, respectively. Five countries with the highest ASIR for men included Malaysia 10.6 per 100,000, Singapore 9.7 per 100,000, Indonesia 8.3 per 100,000, Vietnam 7.7 per 100,000 and Brunei 7.6 per 100,000, respectively. Five countries also had the highest ASIR for women were Malaysia 9.3 per 100,000, Vietnam 9.3 per 100,000, Singapore 3.2 per 100,000, Indonesia 3 per 100,000 and Kenya 2 per 100,000, respectively (Fig. 1).
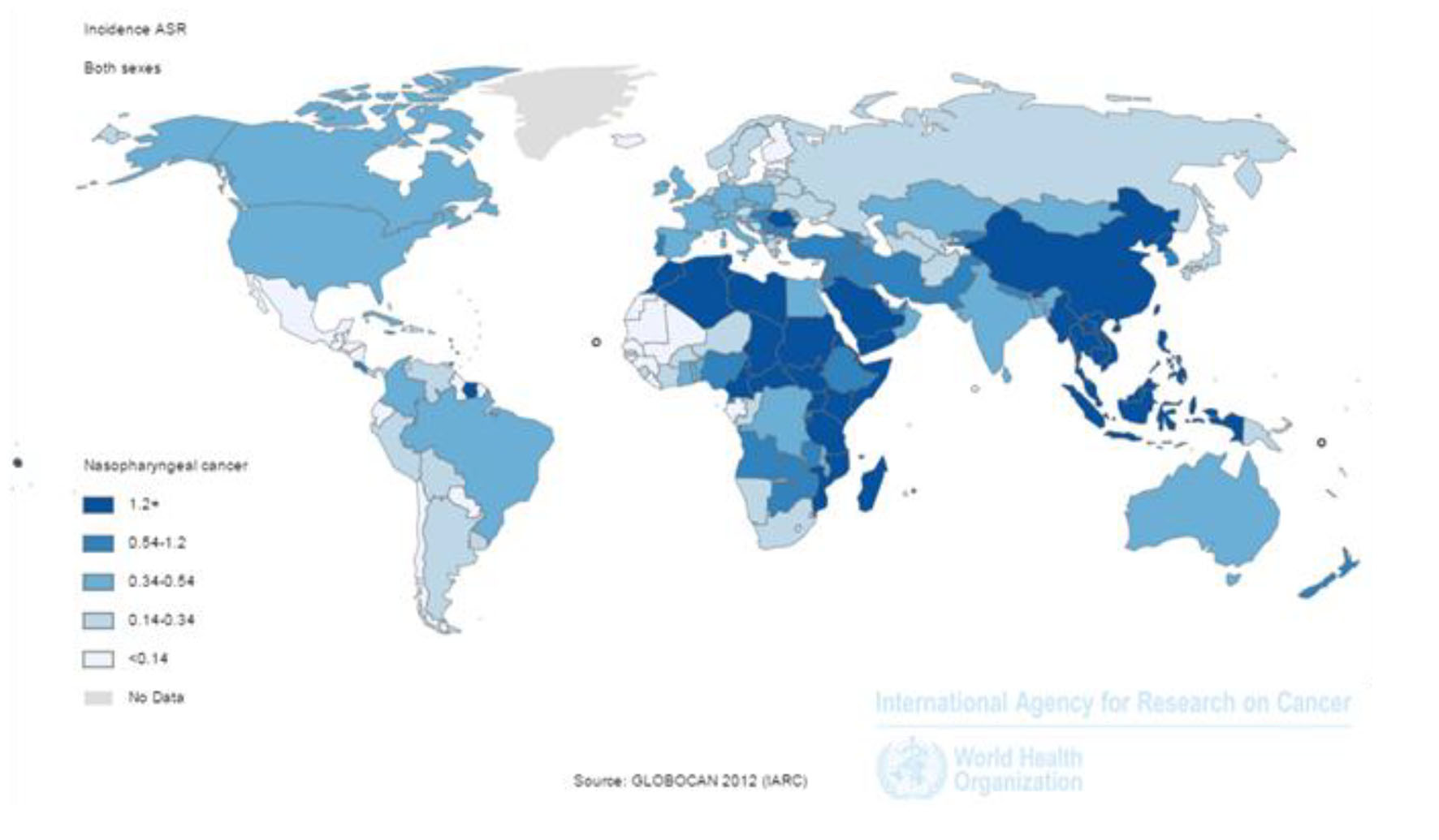 Click for large image | Figure 1. Distribution of standardized nasopharynx cancer incidence rate in the world (extracted from GLOBOCAN, 2012). |
The number of deaths from cancer
In 2012, 50,831 nasopharynx death cases occurred in the world, so that 35,756 death cases were seen in men and 15,075 death cases in women (sex ratio = 2.37). Of all cases, 3,948 death cases occurred in countries with very high HDI, 3,852 cases in countries with high HDI, 36,923 cases in countries with moderate HDI and 6,105 cases in countries with low HDI. The highest number of death cases was seen in countries such as China 20,404 cases, Indonesia 7,391, Vietnam 2,885, India 2,836 and Thailand 1,114 cases, respectively (Figs. 2 and 3).
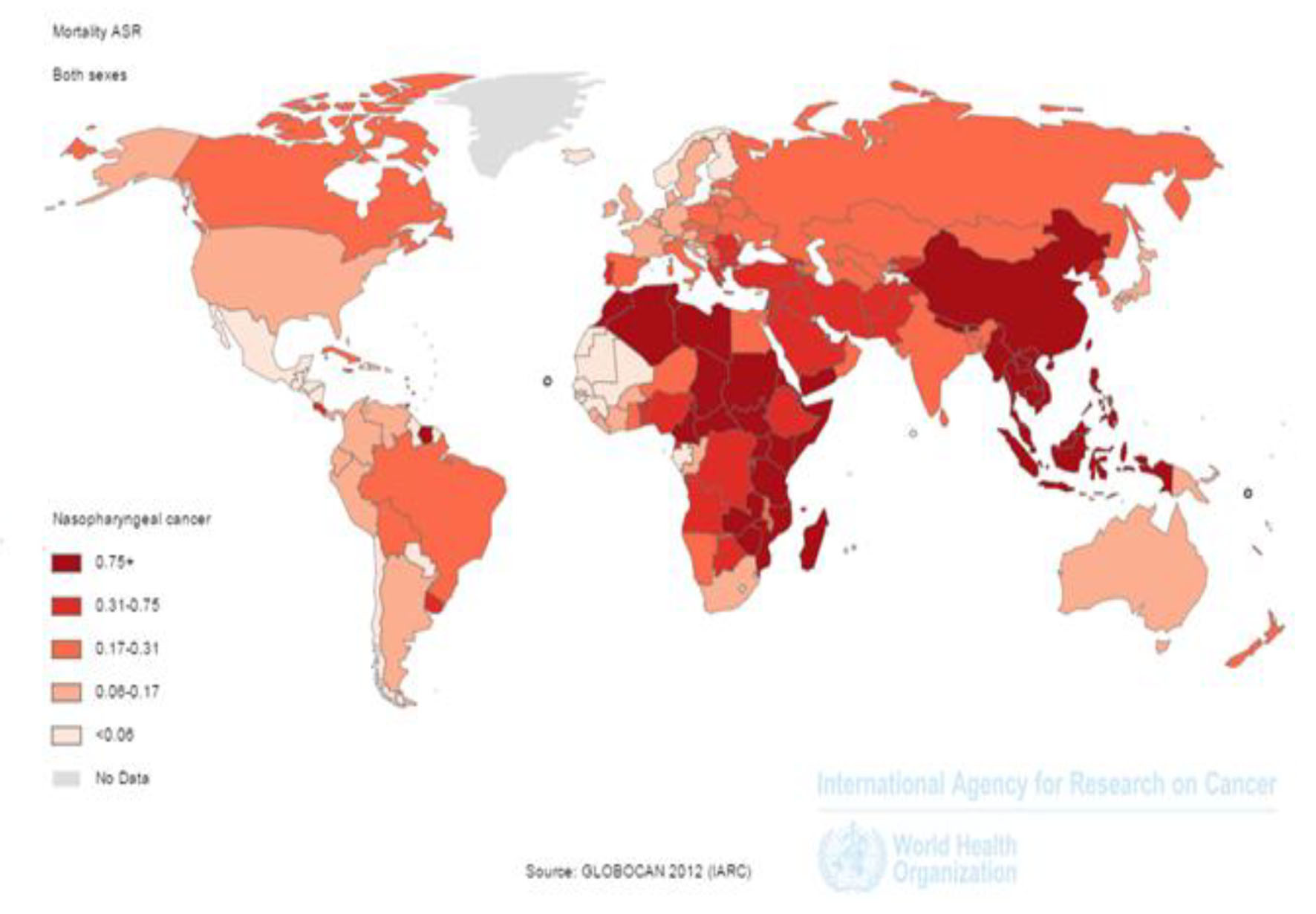 Click for large image | Figure 2. Distribution of standardized nasopharynx cancer mortality rate in the world (extracted from GLOBOCAN, 2012). |
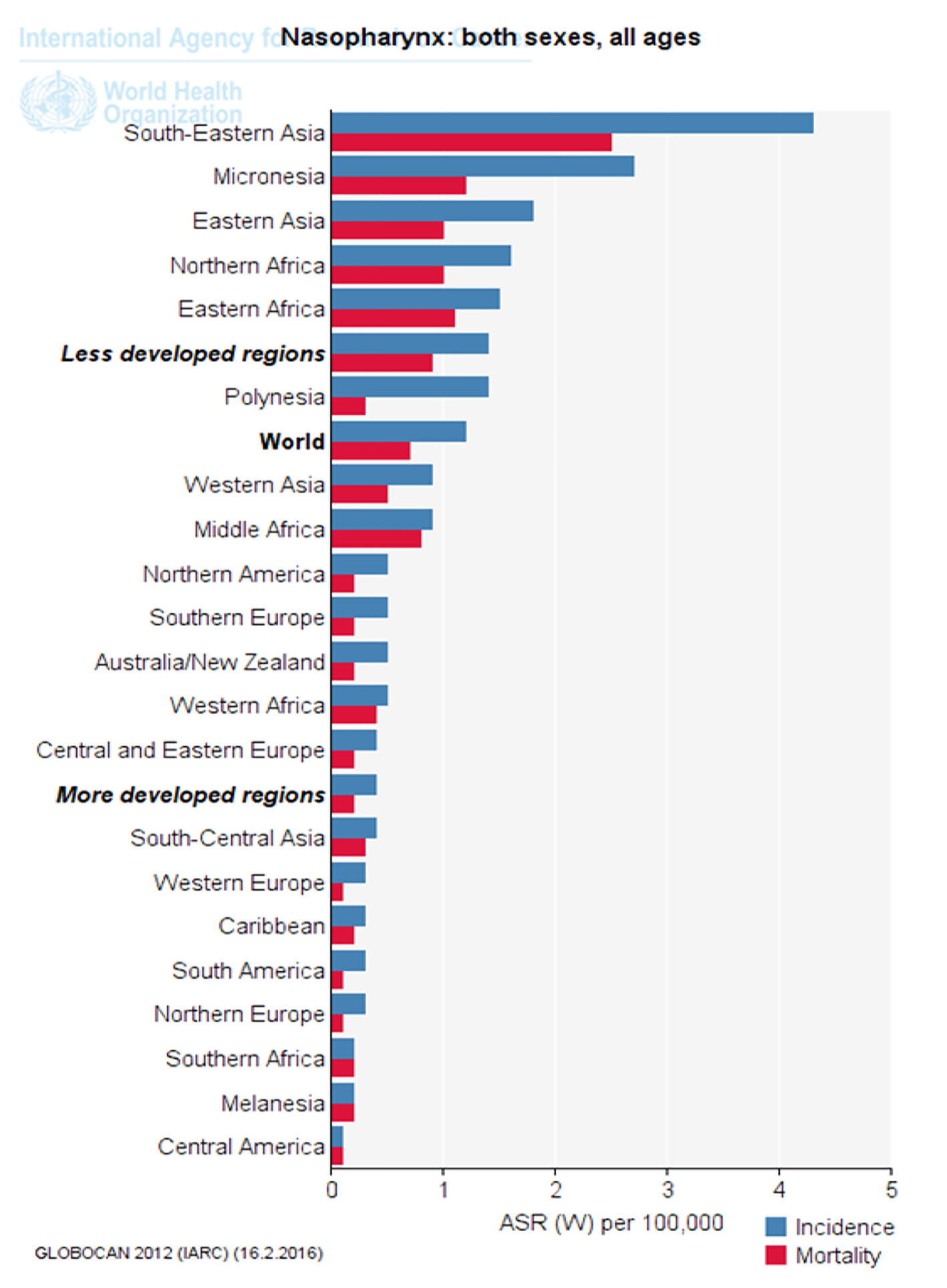 Click for large image | Figure 3. Standardized incidence and mortality of nasopharynx cancer in different parts of the UN (extracted from GLOBOCAN, 2012). |
The age-standardized mortality rate (ASMR)
SIR of mortality from the cancer was 0.7 per 100,000 (0.7 in women and 1in men per 100,000) in the world. The SMR in countries with very high HDI was 0.2 per 100,000, in countries with a high HDI 0.3 per 100,000, in countries with medium HDI 1 per 100,000, and in countries with a low HDI 0.7 per 100,000. Five countries that had the highest ASMR were Malaysia 3.3 per 100,000, Vietnam 3.3 per 100,000, Singapore 2.8 per 100,000, Malaysia 2.5 per 100,000 and Kenya 2.1 per 100,000, respectively. Five countries with the highest ASMR for men included Indonesia 5 per 100,000, Vietnam 4.8 per 100,000, Singapore 4.4 per 100,000, Malaysia 3.9 per 100,000 and Brunei 3.4 per 100,000, respectively. Five countries also had the highest ASMR for women were Vietnam 2 per 100,000, Indonesia 1.8 per 100,000, Bhutan 1.5 per 100,000, Uganda 1.4 per 100,000 and Burundi 1.4 per 100,000, respectively (Fig. 2).
Figure 3 shows nasopharynx cancer standardized incidence and mortality in different parts of the UN. As is clear, standardized cancer incidence and mortality of nasopharynx in underdeveloped countries, Southeast Asia and Africa was more than that in developed countries (Fig. 3).
Correlation analysis between the SIR, and HDI and its components
The results of correlation analysis showed a negative correlation between the SIR and HDI (r = -0.037, P = 0.629) (Fig. 4). A positive correlation was observed between HDI components and SIR, so that there was a positive correlation between the SIR and life expectancy at birth (r = 0.011, P = 0.891), a negative correlation between the SIR and average years of schooling (r = -0.121, P = 0.118), and a positive correlation between the SIR and the level of income per person of population (r = 0.054, P = 0.483) (Table 1).
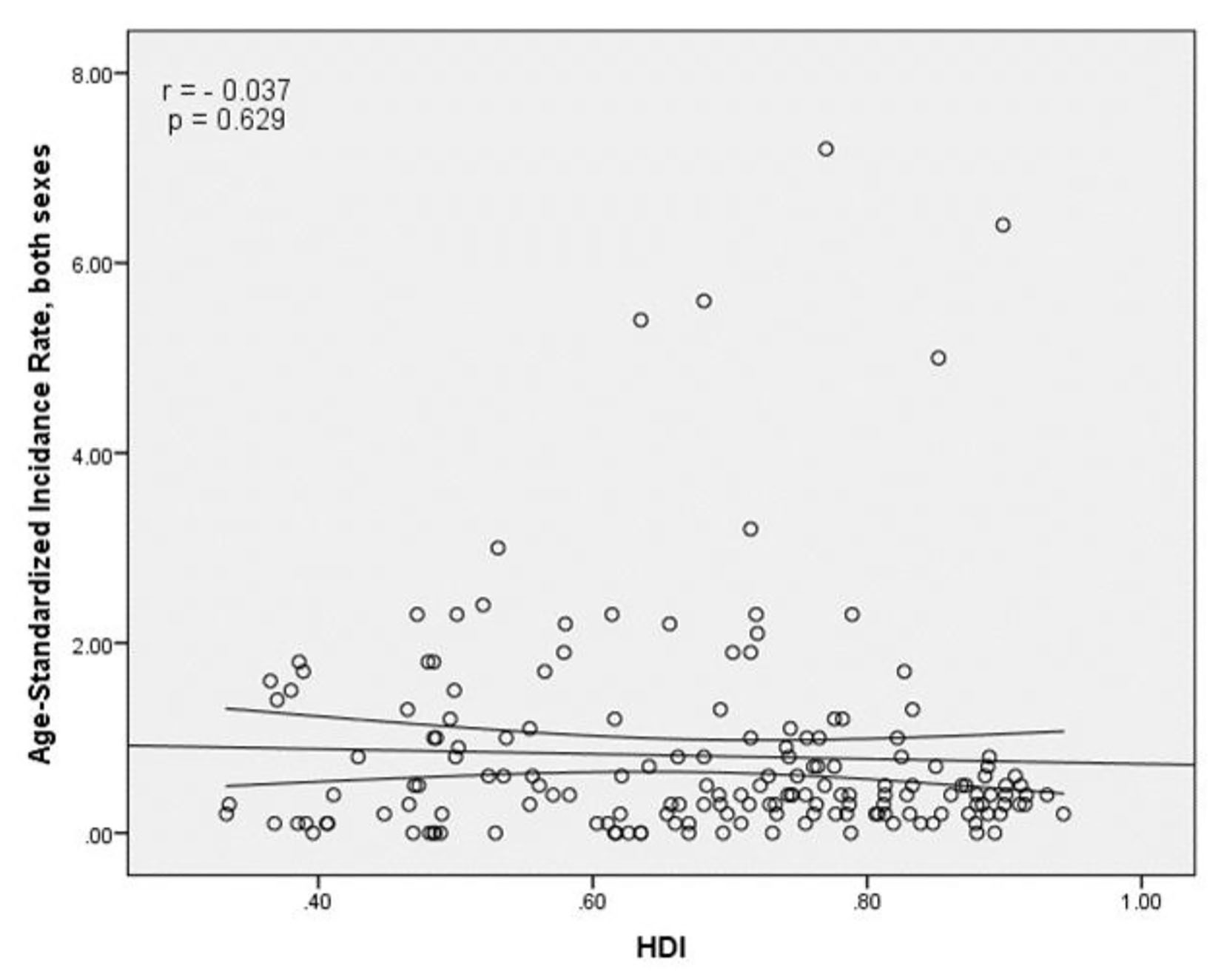 Click for large image | Figure 4. The relationship between the standardized incidence rate and the human development index. |
 Click to view | Table 1. Correlation Between ASIR, ASMR With HDI and Its Components |
Correlation analysis between the SMR, and HDI and its components
The results of correlation analysis showed a negative correlation between the SMR and HDI (r = -0.237, P = 0.002) (Fig. 5). A negative correlation was observed between HDI components and SMR, so that there was a negative correlation between the SMR and life expectancy at birth (r = -0.171, P < 0.027), the SMR and average years of schooling (r = -0.298, P < 0.001) and the SMR and the level of income per person of population (r = -0.11, P = 0.156) (Table 1).
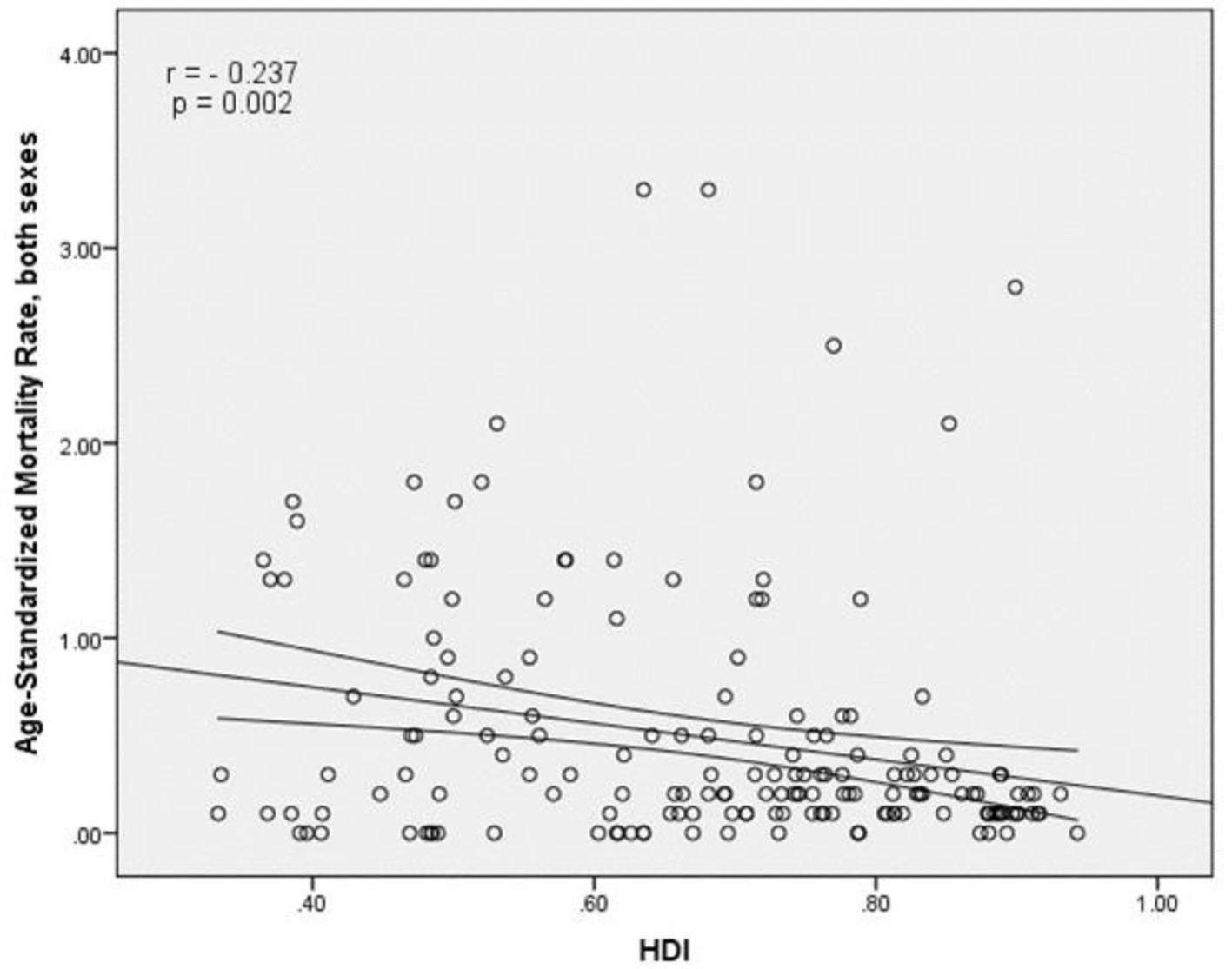 Click for large image | Figure 5. Relationship between standardized mortality rate and the human development index. |
| Discussion | ▴Top |
Overall, in 2012, 86,691 new cases and 50,831 deaths of nasopharyngeal cancer have been documented in the world with sex ratio (male to female) of nasopharyngeal cancer risk about 2.36 and sex ratio (male to female) of nasopharyngeal cancer deaths about 2.37. The highest incidence and mortality were seen in countries with medium and low HDI. Nasopharyngeal cancer prevalence in countries with medium and low HDI is greater due to higher exposure to a variety of risk factors related to the cancer and less budget allocations for health. Therefore, because of the diagnosis at an advanced stage and lack of access to treatment, metastasis of nasopharynx cancer can be observed more in these areas [32, 33].
The countries with the highest incidence of nasopharyngeal cancer had higher HDI that includes very high HDI countries of Singapore and Brunei, high HDI countries of Malaysia and countries with the moderate HDI of Indonesia and Vietnam. According to previous studies in Singapore, nasopharyngeal cancer is the eighth most common cancer in men, with ASIR of 9.5 per 100,000 per year [34]. The relatively high incidence was reported from Indonesia, at least 5.7 per 100,000 among men and 1.9 per 100,000 among women, compared with the incidence of 1.9 per 100,000 among men and 0.8 per 100,000 among women worldwide [35], while significant lowest incidence of nasopharyngeal carcinoma has been reported from parts of the United States and Europe. Nasopharyngeal cancer in Europe and North America included less than 1% of all cancer cases [9]. This peculiar geographical distribution reflects differences between pathology of nasopharyngeal carcinoma and its epidemiology in these areas [9, 36]. Regardless of ethnicity, genetics plays an important part in the pathogenesis of nasopharyngeal carcinoma. The incidence of the cancer is higher about 20 - 50 times in southern China compared with Western countries. In spite of immigration of the second and third generation Chinese to the United States (low prevalence area), in past years, they are at risk for nasopharyngeal cancer [37]. According to the causes of cancer (tobacco and alcohol), despite the declining prevalence of smoking in Europe [38], the incidence of epithelial cancers of the nasopharynx, hypopharynx and larynx cancer remains quite stable, while the oropharynx and oral cavity cancer is on the rise [39]. The results showed that wood dust severely causes to paranasal sinuses and nasal passages cancer, but its association with nasopharyngeal cancer has been weak [40]. Evidence is scattered for a link between diet and the risk of nasopharyngeal cancer, though in general a protective effect has been accessed from non-starchy vegetables, fresh fruit [41-44], vegetables and yellow/red fruits [45-48]. Although examination of the role of dietary habits in the cause of nasopharyngeal cancer, especially about features of some foods and cooking methods and food preservation, are widely surveyed in areas with higher incidence, the results also were generalized to areas with lower incidence [42, 49]. In the United States (population with low incidence), only two studies were conducted [47, 50] that mainly had focus on fruits and vegetables [51]. In our study, the countries with the highest mortality rate of nasopharyngeal cancer were medium and low HDI countries. As well as other studies have shown that one of the main causes of death in these countries is nasopharyngeal cancer which in early detection, 80% of patients are at an advanced stage [17]. Older patients diagnosed with stage 2 or stages 3 are more at higher risk of recurrence and lower overall survival [52]. However, nasopharyngeal cancer treated with radiation therapy, but the outcome varies widely around the world. Almost 80% of new cases occur in countries with poor treatment outcome. Global inequality in access and need to a plan for optimal services shows a significant relationship between survival and access to radiation therapy [11]. In the past two decades, the treatment of nasopharyngeal cancer remarkably improved through introducing chemotherapy and radiotherapy at the same time. However, the overall incidence of patients with metastatic status remains 25-34% and survival of these patients is low [53, 54]. In low- and middle-income countries, the expertise required to delivering the appropriate therapy is a major health challenge. Only there are a third of global radiation therapy centers to treat nearly 60% of cancer patients in the world, in countries with low and middle income [55].
In this study, no significant relationship was seen between the incidence of nasopharyngeal cancer and HDI, while the SMRs of nasopharyngeal cancer were correlated with the index. Also, between the SIR of nasopharyngeal cancer and one of the components of the HDI (life expectancy), there was no significant relationship, but between SMR and life expectancy, a significant relationship has been observed. In other studies, increased life expectancy leads to an increase in the global cancer burden and future changes in population growth and aging shows that new cases of nasopharyngeal cancer and mortality from it will increase in elderly people and the increase in developing countries with a low HDI is more than in developed countries with high HDI (76% vs. 25%) [56-59]. Also in this study between the SIR of the cancer and another component of the HDI (level of education or average years of schooling), there was no significant relationship, while a significant relationship was seen between the SMR and level of education or average years of schooling. According to other studies, in countries with highly educated individuals typically they have healthier habits and more appropriate behaviors when exposed to risk factors such as smoking, inappropriate diet, compared with those in countries with a poor education [60, 61]. In the United States, the risk factors with a higher prevalence of cancers associated with head and neck were as follows: single in terms of marital status, lower educational (pre high school) as well as lower income (household income less than $20,000) [62]. In some developed countries, such as Ontario and Canada, the incidence of cancers associated with head and neck similarly with lower levels of education (less than grade 8 of education) and average family income is less relevant [63]. The incidence and mortality of nasopharyngeal cancer had no significant relationship with another component of the HDI, income levels (GDP). In studies on nasopharyngeal cancer, the relationship between nasopharyngeal cancer, and socioeconomic status, lifestyle and geographical position is known. In Asian countries, an increase in the cancer can be seen more among men and women with lower economic status [64]. Also, no difference has been seen in the death of people living in rural areas with lower socio-economic compared with urban areas [65, 66]. Studies in the United States, Canada and Europe have shown that nasopharyngeal cancer is more common in deprived socio-economic population [62, 63, 67-70]. The evidence is mainly related to the risk factors associated with lower SES, such as tobacco and alcohol use and poor health so that after controlling for the risk factors, higher prevalence of nasopharyngeal cancer is associated with lower SES [62, 63, 69]. MacKillop et al concluded that there is a strong inverse relationship between income level and areas involved with head and neck cancer in the United States and Ontario [71].
Conclusion
Nasopharyngeal cancer is native to Southeast Asia and the highest incidence and mortality were seen in countries with moderate and low HDI. It is suggested that studies are conducted on determining the causes of the cancer incidence and mortality in the world and the differences between various regions.
Limitations
This study was an ecological study and the limitations include ecological misleading and lack of relation of group results with individuals. Also, in this study, the exact survey about the connection between exposure and outcome is not clearly obvious and all other seen relations were in group and regional level.
Conflicts of Interest
None.
| References | ▴Top |
- Esmail Nasab N, Moradi G, Zareie M, Ghaderi E, Gheytasi B. Survey of epidemilogic status and incidence rates of cancers in the patients above 15 years old in Kurdistan province. Scientific Journal of Kurdistan University of Medical Sciences. 2007;11(4):18-25.
- Devita, Principles and practice of oncology. 2001;1880-904.
- Tsao SW LK, Huang DP. Nasopharyngeal carcinoma. In: Tselis AC, Jenson H, editors. Epsteion-Bar virus. New York: Taylor & Francis. 2006:p. 273-295.
doi - N R-T. Epstein-Bar virus in the pathogenesis of NPC. In: Robertson ES, editor. Epstein-Barr virus Wymondham. Norfolk: Caister Academic Press. 2005: p. 71-92.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74-108.
doi pubmed - Busson P, Keryer C, Ooka T, Corbex M. EBV-associated nasopharyngeal carcinomas: from epidemiology to virus-targeting strategies. Trends Microbiol. 2004;12(8):356-360.
doi pubmed - Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1765-1777.
doi pubmed - Ferlay J, Shin H, Bray F, Forman D, Mathers C, Parkin D. Incidence/mortality data. GLOBOCAN 2008 v2.0. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer. 2010.
- Curado MP EB, Shin HR, Storm H, Ferlay J, Heanue M, (eds). Cancer incidence in five continents, Vol. IX. IARC Sci Publ No. 160. IARC, Lyon. 2007.
- Mousavi SM, Sundquist J, Hemminki K. Nasopharyngeal and hypopharyngeal carcinoma risk among immigrants in Sweden. International Journal of Cancer. 2010;127(12):2888-2892.
doi pubmed - Lam KO, Lee AW, Choi CW, Sze HC, Zietman AL, Hopkins KI, Rosenblatt E. Global Pattern of Nasopharyngeal Cancer: Correlation of Outcome With Access to Radiation Therapy. Int J Radiat Oncol Biol Phys. 2016;94(5):1106-1112.
doi pubmed - Lubin JH, Purdue M, Kelsey K, Zhang ZF, Winn D, Wei Q, Talamini R, et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2009;170(8):937-947.
doi pubmed - Mork J, Lie AK, Glattre E, Clark S, Hallmans G, Jellum E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. New England Journal of Medicine. 2001;344(15):1125-1131.
doi pubmed - Keane MG, Horsfall LJ, Rait G, Pereira SP. Sociodemographic trends in the incidence of pancreatic and biliary tract cancer in UK primary care. PLoS One. 2014;9(9):e108498.
doi pubmed - Liu Z, Fang F, Chang ET, Ye W. Cancer risk in the relatives of patients with nasopharyngeal carcinoma-a register-based cohort study in Sweden. Br J Cancer. 2015;112(11):1827-1831.
doi pubmed - Yang S, Wu S, Zhou J, Chen XY. Screening for nasopharyngeal cancer. 2015(11).
- Fles R, et al. Knowledge of general practitioners about nasopharyngeal cancer at the Puskesmas in Yogyakarta, Indonesia. BMC medical education. 2010;10(1):1.
doi pubmed - Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, Forastiere AA, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16(4):1310-1317.
pubmed - Huncharek M, Kupelnick B. Combined chemoradiation versus radiation therapy alone in locally advanced nasopharyngeal carcinoma: results of a meta-analysis of 1,528 patients from six randomized trials. Am J Clin Oncol. 2002;25(3):219-223.
doi pubmed - Langendijk JA, Leemans CR, Buter J, Berkhof J, Slotman BJ. The additional value of chemotherapy to radiotherapy in locally advanced nasopharyngeal carcinoma: a meta-analysis of the published literature. J Clin Oncol. 2004;22(22):4604-4612.
doi pubmed - Zhang L, Zhao C, Ghimire B, Hong MH, Liu Q, Zhang Y, Guo Y, et al. The role of concurrent chemoradiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma among endemic population: a meta-analysis of the phase III randomized trials. BMC Cancer. 2010;10:558.
doi pubmed - Wang J, Shi M, Hsia Y, Luo S, Zhao L, Xu M, Xiao F, et al. Failure patterns and survival in patients with nasopharyngeal carcinoma treated with intensity modulated radiation in Northwest China: a pilot study. Radiat Oncol. 2012;7:2.
doi pubmed - Liu MT, Hsieh CY, Chang TH, Lin JP, Huang CC, Wang AY. Prognostic factors affecting the outcome of nasopharyngeal carcinoma. Jpn J Clin Oncol. 2003;33(10):501-508.
doi pubmed - Rafiemanesh H, Mehtarpour M, Khani F, Hesami SM, Shamlou R, Towhidi F, Salehiniya H, et al. Epidemiology, incidence and mortality of lung cancer and their relationship with the development index in the world. J Thorac Dis. 2016;8(6):1094-1102.
doi pubmed - Pakzad R, Mohammadian-Hafshejani A, Ghoncheh M, Pakzad I, Salehiniya H. The incidence and mortality of prostate cancer and its relationship with development in Asia. Prostate Int. 2015;3(4):135-140.
doi pubmed - Pakzad R, Mohammadian-Hafshejani A, Ghoncheh M, Pakzad I, Salehiniya H. The incidence and mortality of lung cancer and their relationship to development in Asia. Transl Lung Cancer Res. 2015;4(6):763-774.
pubmed - Mahdavifar N, Ghoncheh M, Pakzad R, Momenimovahed Z, Salehiniya H. Epidemiology, Incidence and Mortality of Bladder Cancer and their Relationship with the Development Index in the World. Asian Pac J Cancer Prev. 2016;17(1):381-386.
doi pubmed - Franceschi S, Wild CP. Meeting the global demands of epidemiologic transition - the indispensable role of cancer prevention. Mol Oncol. 2013;7(1):1-13.
doi pubmed - Ferlay Jea. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr, accessed on 2/2/2016. 2016.
- Malik K. Human Development Report 2013. The rise of the South: Human progress in a diverse world. The Rise of the South: Human Progress in a Diverse World (March 15, 2013) UNDP-HDRO Human Development Reports. 2013.
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-386.
doi pubmed - Adham M, Stoker SD, Wildeman MA, Rachmadi L, Gondhowiardjo S, Atmakusumah D, Gatot D, et al. Current status of cancer care for young patients with nasopharyngeal carcinoma in Jakarta, Indonesia. PLoS One. 2014;9(7):e102353.
doi pubmed - Wildeman MA, Fles R, Herdini C, Indrasari RS, Vincent AD, Tjokronagoro M, Stoker S, et al. Primary treatment results of Nasopharyngeal Carcinoma (NPC) in Yogyakarta, Indonesia. PLoS One. 2013;8(5):e63706.
doi pubmed - Teo MC, Soo KC. Cancer trends and incidences in Singapore. Jpn J Clin Oncol. 2013;43(3):219-224.
doi pubmed - Valean S, Acalovschi M, Diculescu M, Manuc M, Goldis A, Sfarti C, Trifan A. Mortality in Digestive Cancers, 2012: International Data and Data from Romania. J Gastrointestin Liver Dis. 2015;24(4):507-514.
pubmed - Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90.
doi pubmed - Buell P. The effect of migration on the risk of nasopharyngeal cancer among Chinese. Cancer Res. 1974;34(5):1189-1191.
pubmed - Eriksen M MJ, Ross H. The tobacco atlas. 4th ed. Atlanta: American Cancer Society; 2012.
- Fuchs CS, Colditz GA, Stampfer MJ, Giovannucci EL, Hunter DJ, Rimm EB, Willett WC, et al. A prospective study of cigarette smoking and the risk of pancreatic cancer. Arch Intern Med. 1996;156(19):2255-2260.
doi pubmed - Muscat JE, Stellman SD, Hoffmann D, Wynder EL. Smoking and pancreatic cancer in men and women. Cancer Epidemiol Biomarkers Prev. 1997;6(1):15-19.
pubmed - Gallicchio L, Matanoski G, Tao XG, Chen L, Lam TK, Boyd K, Robinson KA, et al. Adulthood consumption of preserved and nonpreserved vegetables and the risk of nasopharyngeal carcinoma: a systematic review. Int J Cancer. 2006;119(5):1125-1135.
doi pubmed - Hassan MM, Bondy ML, Wolff RA, Abbruzzese JL, Vauthey JN, Pisters PW, Evans DB, et al. Risk factors for pancreatic cancer: case-control study. Am J Gastroenterol. 2007;102(12):2696-2707.
doi pubmed - Feng BJ, Jalbout M, Ayoub WB, Khyatti M, Dahmoul S, Ayad M, Maachi F, et al. Dietary risk factors for nasopharyngeal carcinoma in Maghrebian countries. Int J Cancer. 2007;121(7):1550-1555.
doi pubmed - Jia WH, Luo XY, Feng BJ, Ruan HL, Bei JX, Liu WS, Qin HD, et al. Traditional Cantonese diet and nasopharyngeal carcinoma risk: a large-scale case-control study in Guangdong, China. BMC Cancer. 2010;10:446.
doi pubmed - Armstrong RW, Imrey PB, Lye MS, Armstrong MJ, Yu MC, Sani S. Nasopharyngeal carcinoma in Malaysian Chinese: salted fish and other dietary exposures. Int J Cancer. 1998;77(2):228-235.
doi - Yuan JM, Wang XL, Xiang YB, Gao YT, Ross RK, Yu MC. Preserved foods in relation to risk of nasopharyngeal carcinoma in Shanghai, China. Int J Cancer. 2000;85(3):358-363.
doi - Kasum CM, Jacobs DR, Jr., Nicodemus K, Folsom AR. Dietary risk factors for upper aerodigestive tract cancers. Int J Cancer. 2002;99(2):267-272.
doi pubmed - Liu YT, Dai JJ, Xu CH, Lu YK, Fan YY, Zhang XL, Zhang CX, et al. Greater intake of fruit and vegetables is associated with lower risk of nasopharyngeal carcinoma in Chinese adults: a case-control study. Cancer Causes Control. 2012;23(4):589-599.
doi pubmed - Jia WH, Qin HD. Non-viral environmental risk factors for nasopharyngeal carcinoma: a systematic review. Semin Cancer Biol. 2012;22(2):117-126.
doi pubmed - Farrow DC, Vaughan TL, Berwick M, Lynch CF, Swanson GM, Lyon JL. Diet and nasopharyngeal cancer in a low-risk population. Int J Cancer. 1998;78(6):675-679.
doi - Polesel J, Serraino D, Negri E, Barzan L, Vaccher E, Montella M, Zucchetto A, et al. Consumption of fruit, vegetables, and other food groups and the risk of nasopharyngeal carcinoma. Cancer Causes Control. 2013;24(6):1157-1165.
doi pubmed - Mak HW, Lee SH, Chee J, Tham I, Goh BC, Chao SS, Ong YK, et al. Clinical Outcome among Nasopharyngeal Cancer Patients in a Multi-Ethnic Society in Singapore. PLoS One. 2015;10(5):e0126108.
doi pubmed - Chen MY, Jiang R, Guo L, Zou X, Liu Q, Sun R, Qiu F, et al. Locoregional radiotherapy in patients with distant metastases of nasopharyngeal carcinoma at diagnosis. Chin J Cancer. 2013;32(11):604-613.
doi pubmed - Lee AW, Lin JC, Ng WT, editors. Current management of nasopharyngeal cancer. Seminars in radiation oncology; 2012: Elsevier.
- Datta NR, Samiei M, Bodis S. Radiation therapy infrastructure and human resources in low- and middle-income countries: present status and projections for 2020. Int J Radiat Oncol Biol Phys. 2014;89(3):448-457.
doi pubmed - Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol. 2002;13(7):1007-1015.
doi pubmed - Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol. 2012;13(8):790-801.
doi - Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, Peto R, et al. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross-sectional household surveys. Lancet. 2012;380(9842):668-679.
doi - Bosetti C, Lucenteforte E, Silverman DT, Petersen G, Bracci PM, Ji BT, Negri E, et al. Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4). Ann Oncol. 2012;23(7):1880-1888.
doi pubmed - Stelmach W, Kaczmarczyk-Chalas K, Bielecki W, Drygas W. The impact of income, education and health on lifestyle in a large urban population of Poland (Cindi programme). Int J Occup Med Environ Health. 2004;17(3):393-401.
pubmed - Shi L. Sociodemographic characteristics and individual health behaviors. South Med J. 1998;91(10):933-941.
doi pubmed - Johnson S, McDonald JT, Corsten MJ. Socioeconomic factors in head and neck cancer. J Otolaryngol Head Neck Surg. 2008;37(4):597-601.
pubmed - Johnson S, McDonald JT, Corsten M, Rourke R. Socio-economic status and head and neck cancer incidence in Canada: a case-control study. Oral Oncol. 2010;46(3):200-203.
doi pubmed - Mimi CY, Yuan J-M, editors. Epidemiology of nasopharyngeal carcinoma. Seminars in cancer biology; 2002: Elsevier.
- Yu MC, Ho JH, Ross RK, Henderson BE. Nasopharyngeal carcinoma in Chinese - salted fish or inhaled smoke? Prev Med. 1981;10(1):15-24.
doi - Armstrong RW, Kannan Kutty M, Dharmalingam SK, Ponnudurai JR. Incidence of nasopharyngeal carcinoma in Malaysia, 1968 - 1977. Br J Cancer. 1979;40(4):557-567.
doi pubmed - Conway DI, Brewster DH, McKinney PA, Stark J, McMahon AD, Macpherson LM. Widening socio-economic inequalities in oral cancer incidence in Scotland, 1976-2002. Br J Cancer. 2007;96(5):818-820.
doi pubmed - Greenwood M, Thomson PJ, Lowry RJ, Steen IN. Oral cancer: material deprivation, unemployment and risk factor behaviour - an initial study. Int J Oral Maxillofac Surg. 2003;32(1):74-77.
doi pubmed - Greenberg RS, Haber MJ, Clark WS, Brockman JE, Liff JM, Schoenberg JB, Austin DF, et al. The relation of socioeconomic status to oral and pharyngeal cancer. Epidemiology. 1991;2(3):194-200.
doi pubmed - Arsenijevic S, Pantovic V, Gledovic Z, Stojanovic J, Belic B. Demographic characteristics of patients with laryngeal cancer and their socioeconomic status. J BUON. 2010;15(1):131-135.
pubmed - Mackillop WJ, Zhang-Salomons J, Boyd CJ, Groome PA. Associations between community income and cancer incidence in Canada and the United States. Cancer. 2000;89(4):901-912.
doi
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.