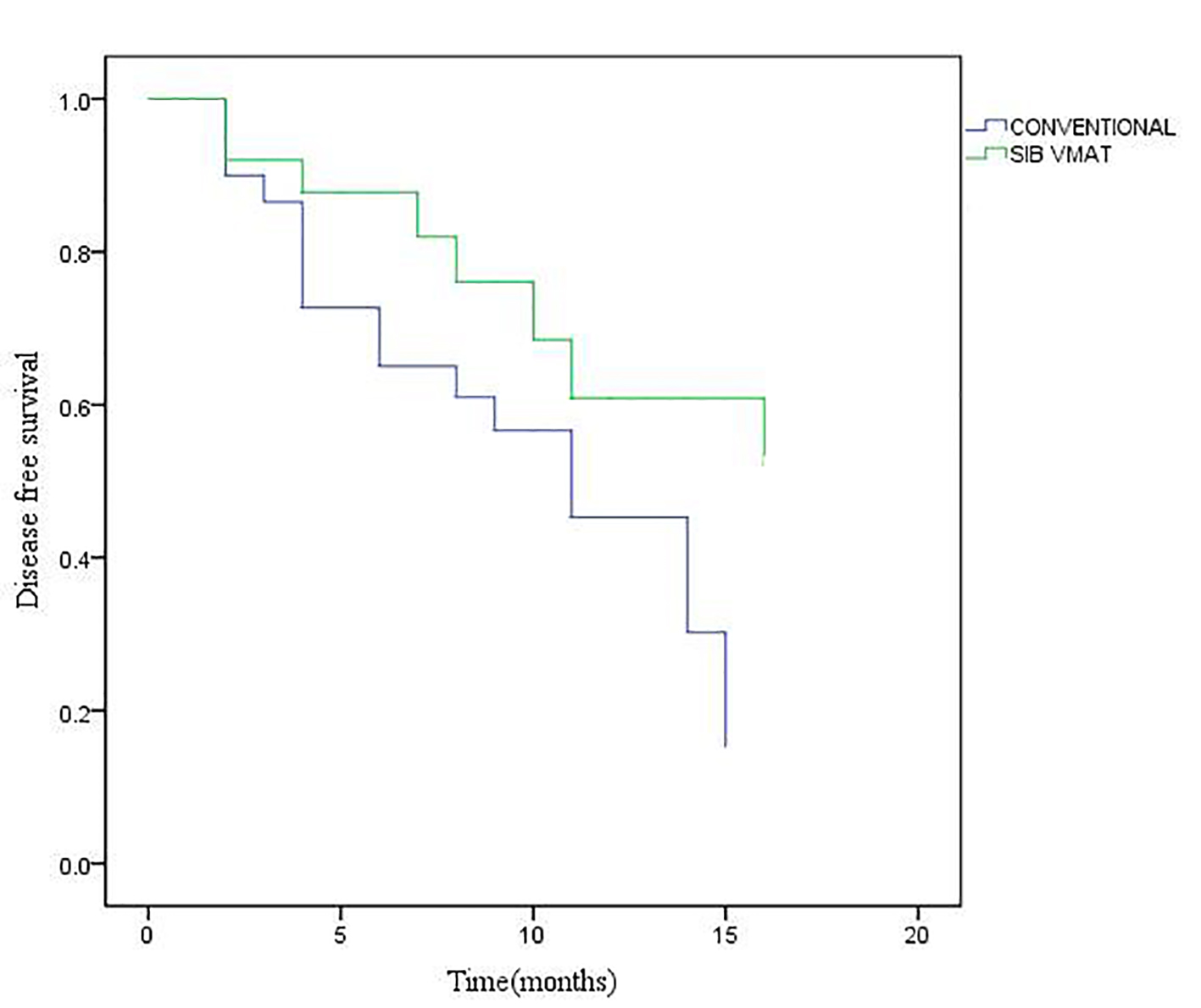
Figure 1. Disease-free survival (DFS) for patients under study.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Short Communication
Volume 8, Number 4, August 2017, pages 117-121
Evaluation of Acute Toxicity and Early Clinical Outcome in Head and Neck Cancers Treated With Conventional Radiotherapy and Simultaneous Integrated Boost Arc Radiotherapy
Figure

Tables
| Conventional radiotherapy, n (%) | SIB-VMAT, n (%) | P | |
|---|---|---|---|
| SIB-VMAT: simultaneous integrated boost-volumetric modulated arc therapy. | |||
| N | 30 (54.55%) | 25 (45.45%) | |
| Age, median (range), years | 58 (35 - 70) | 52 (31 - 65) | 0.200 |
| Sex | |||
| Female | 2 (6.66%) | 6 (24%) | 0.069 |
| Male | 28 (93.33%) | 19 (76%) | |
| Disease subsite | |||
| Oral cavity | 8 (26.7%) | 6 (24%) | 0.027 |
| Oropharynx | 15 (50%) | 12 (48%) | |
| Hypopharynx | 5 (16.7%) | 4 (16%) | |
| Unknown primary | 1 (3.3%) | 0 (0%) | |
| Paranasal sinus | 1 (3.30%) | 3 (12%) | |
| Overall stage | |||
| I | 1(3.3%) | 1 (4%) | 0.333 |
| II | 5 (16.7%) | 0 (0%) | |
| III | 9 (30%) | 9 (36%) | |
| IV | 15 (50%) | 15 (60%) | |
| Chemotherapy | |||
| Weekly | 11 (36.7%) | 9 (36%) | 0.497 |
| Thrice weekly | 7 (23.3%) | 3 (12%) | |
| Conventional radiotherapy (mean ± SD) | SIB-VMAT (mean ± SD) | P | |
|---|---|---|---|
| OTT: overall treatment time; SD: standard deviation. | |||
| OTT (days) | 52.93 ± 22.81 | 48.24 ± 5.0 | 0.31 |
| Treatment breaks (days) | 4.67 ± 8.19 | 1.0 ± 2.08 | 0.001 |
| Variable | Grade of toxicity | Conventional radiotherapy | SIB-VMAT | P value |
|---|---|---|---|---|
| Radiation dermatitis | 3-4 | 26.7% | 16% | 0.340 |
| Mucositis | 3-4 | 83.3% | 56.0% | 0.026 |
| Xerostomia | 2-3 | 80.0% | 44.0% | 0.006 |
| Trismus | 3-4 | 6.7% | 4% | 0.440 |
| Dysphagia | 2-3 | 80.0% | 40.0% | 0.008 |
| Constipation | 2 | 6.7% | 0% | 0.330 |
| Fatigue | 2-3 | 6.7% | 16.0% | 0.227 |
| Insomnia | 2 | 3% | 0% | 0.214 |
| Pain | 2 | 36.7% | 20.0% | 0.175 |
| Dysgeusia | 2 | 66.7% | 76.0% | 0.448 |
| Response | Conventional radiotherapy, n (%) | SIB-VMAT, n (%) | P value |
|---|---|---|---|
| CR: complete response; non-CR: partial response, stable disease and progressive disease. | |||
| CR | 14 (46.7%) | 17 (68%) | |
| Non-CR | 16 (53.3%) | 8 (32%) | 0.040 |