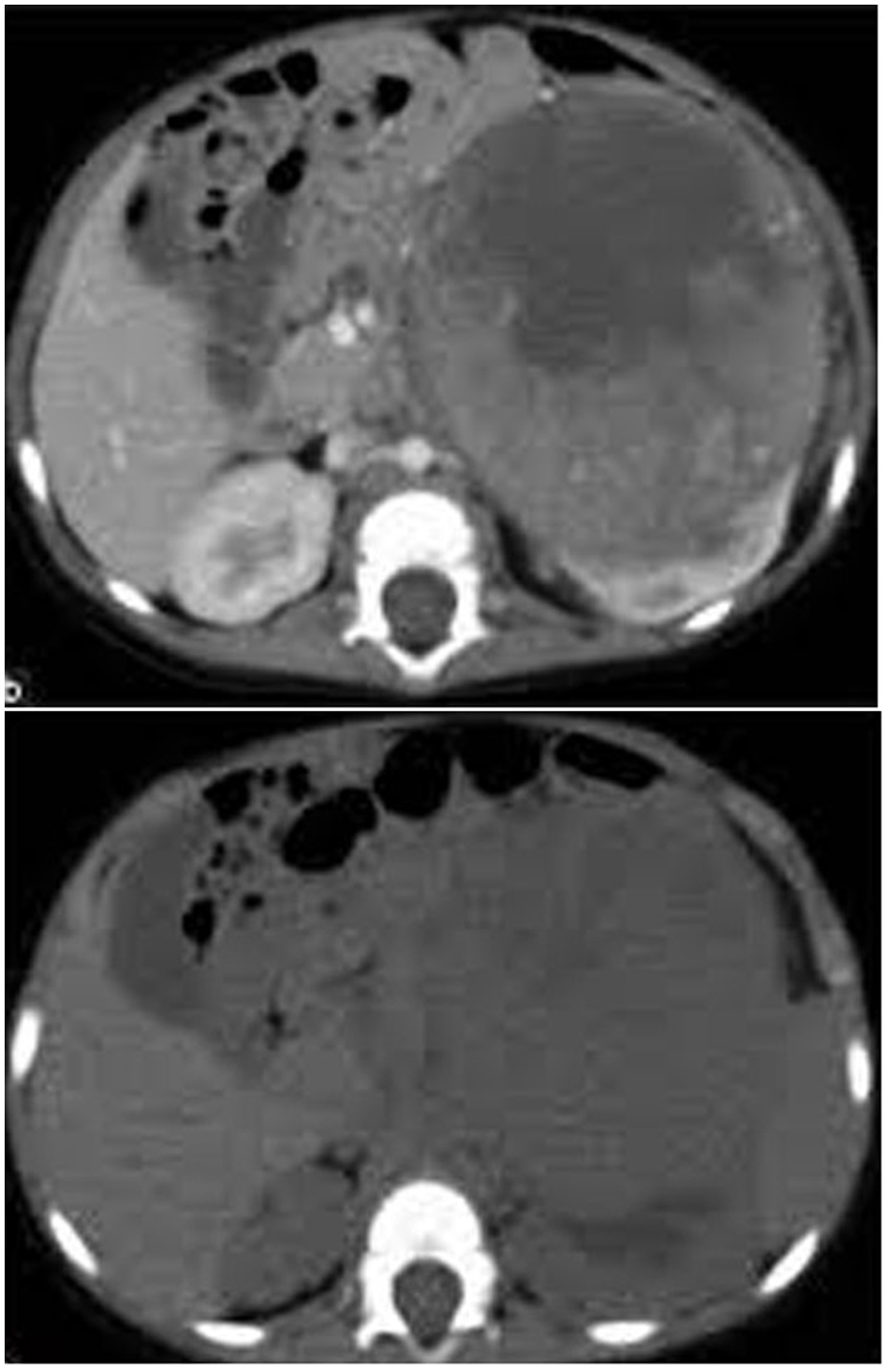
Figure 1. CT image showing a left renal mass with enhancing renal parenchyma posteriorly. Lobulated tumor thrombus is present in the left renal vein and IVC.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 6, Number 5, October 2015, pages 441-445
Initial Surgery in Tailoring Treatment for Children With Stage II and III Wilms’ Tumor: An Experience From Resource Challenged Settings
Figure

Tables
| Drug name | Pediatric dose |
|---|---|
| Dactinomycin | 0.015 mg/kg IV push qd for 5 days |
| Vincristine | 1.5 mg/m2 IV q1-3 weeks, not to exceed 2 mg/dose |
| Cyclophosphomide | 1.2 - 2.2 g/m2 IV qd for 1 - 3 days |
| Etoposide | 100 mg/m2 IV qd for 5 days |
| Doxorubicin (adriamycin) | 45 mg/m2 IV |
| Stage | Chemotherapeutic regimen |
|---|---|
| FH: favorable histology; AMD: dactinomycin; VCR: vincristine; DOX: doxorubicin; CPM: cyclophosphamide: E: etoposide. | |
| Stage II (FH), stage III (FH) | DD-4A (AMD, VCR, and DOX; 24 weeks |
| Stage II or stage III (focal or diffuse anaplasia) | I (VCR + CPM + E; 24 weeks) |
| Relapse | Group I | Group II | P value |
|---|---|---|---|
| Stage II | 3 (20%) | 0 | < 0.02 |
| Stage III | 2 (13.3%) | 1 | < 0.04 |
| Complication | Number of patients |
|---|---|
| Bone marrow depression | 9 (30%) |
| Bowel obstruction | 4 (13.3%) |
| Hepatic dysfunction | 6 (20%) |
| Interstitial pneumonitis | 2 (6.7%) |
| Cardiomyopathy | 1 (3.3%) |