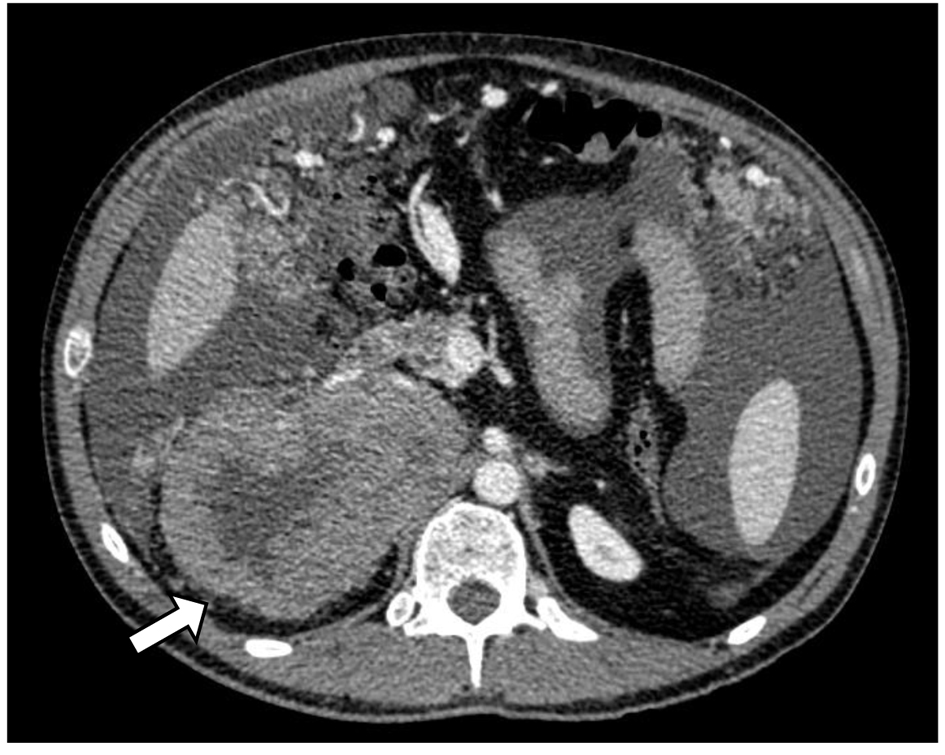
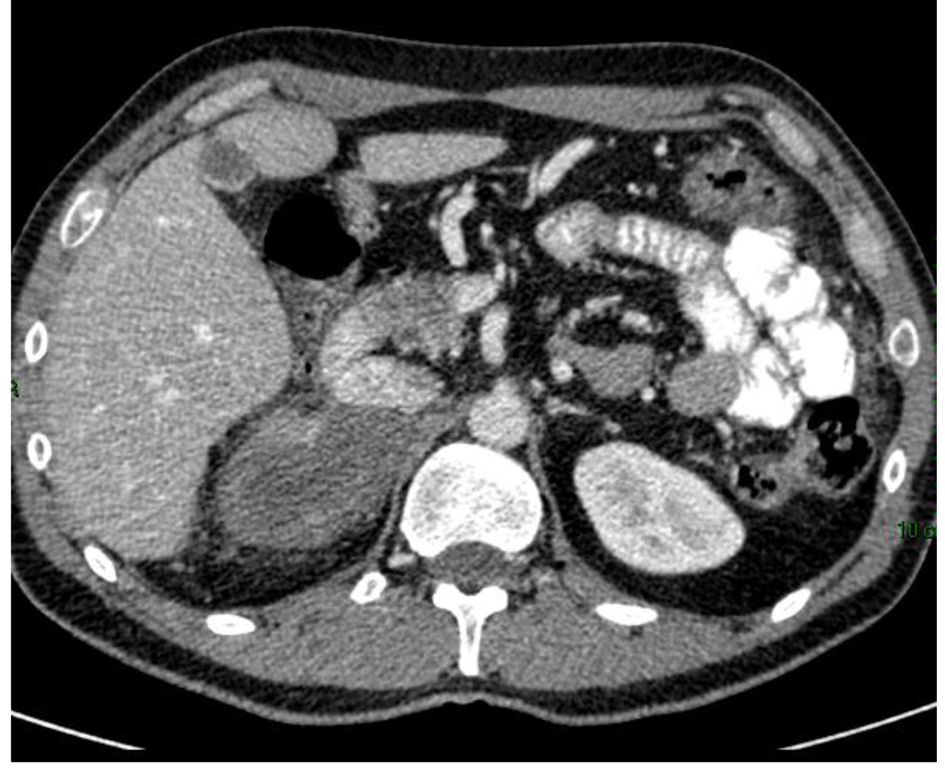
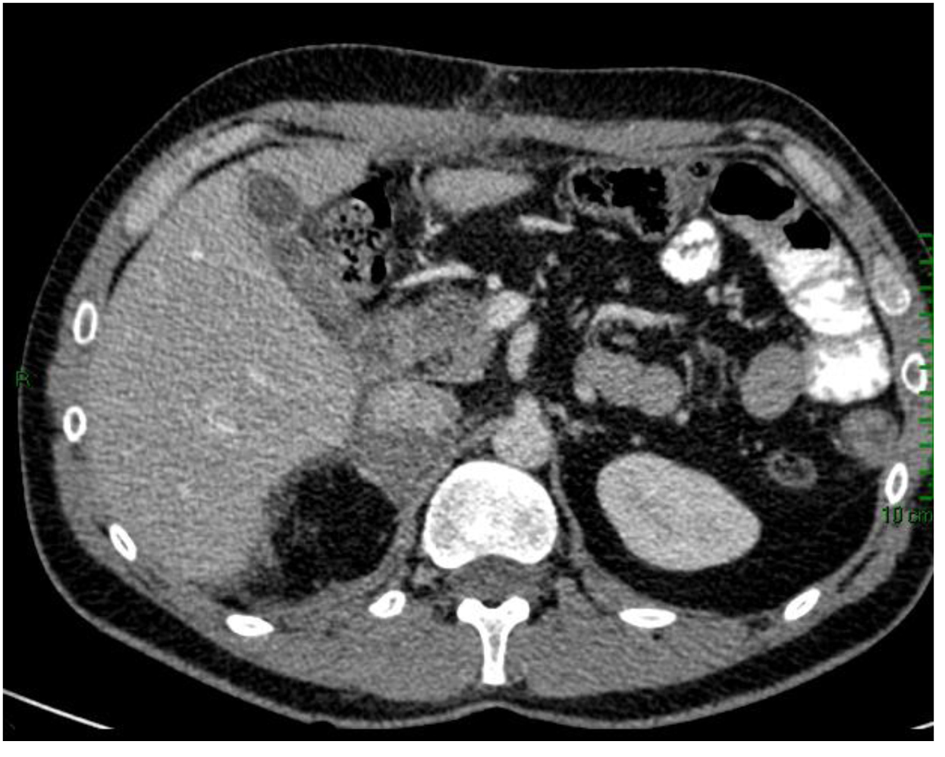
Figure 1. CT scan of adrenocortical carcinoma showing necrotic right adrenal mass in close proximity to the body/tail of pancreas and third portion of the duodenum (white arrow).
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Case Report
Volume 6, Number 6, December 2015, pages 485-490
Clinical Case of Metastatic Adrenocortical Carcinoma With Unusual Evolution: Review the Literature
Figures

Tables
| Drug | Study phase/n | Clinical benefit |
|---|---|---|
| M/EDP: mitotane + etoposide, doxorubicin and cisplatin; SM: streptozotocin + mitotane. | ||
| Cisplatin + etoposide followed by mitotane [24] | II/47 | ORR: 11% Median OS: 10 months |
| M/EDP [25] | II/28 | ORR: 53.5% CR: two patients PR: 13 patients |
| SM [26] | II/22 | ORR: 36.4% |
| M/EDP vs. SM [27] | III/304 | ORR: 23.2 vs. 9.2% PFS: 5.0 vs. 2.1 months OS: 14 vs. 12 months |
| Drug | Target | Study phase/n | Clinical benefit |
|---|---|---|---|
| VEGF: vascular endothelial growth factor; EGFR: epidermal growth factor receptor; IGF-1R: insulin growth factor 1 receptor; mTOR: mammalian target of rapamycin; PDGFR: platelet-derived growth factor receptor. | |||
| Sunitinib [32] | VEGF pathway | II/35 | Five patients with SD |
| Sorafenib + paclitaxel [33] | VEGF pathway + chemotherapy | II/9 | No benefit |
| Bevacizumab + capecitabina [34] | VEGF pathway + chemotherapy | II/10 | No benefit |
| Erlotinib + gemcitabina [35] | EGFR pathway + chemotherapy | II/10 | One patient with SD |
| Gefitinib [36] | EGFR pathway | II/19 | No benefit |
| Figitumumab [37] | IGF-1R pathway | II/14 | Eight patients with SD |
| Cixutumumab [38] | IGF-1R pathway | II/10 | One patient with SD |
| Cixutumumab + temsirolimus [39] | IGF-1R pathway + mTOR pathway | I/10 | Four patients with SD |
| Everolimus [40] | mTOR pathway | II/4 | No benefit |
| Imatinib [41] | c-ABL, PDGFR and c-Kit pathway | II/4 | No benefit |
| Linsitinib [31] | Dual inhibitor of IGFR and IR tyrosine kinase | III/139 | No benefit |