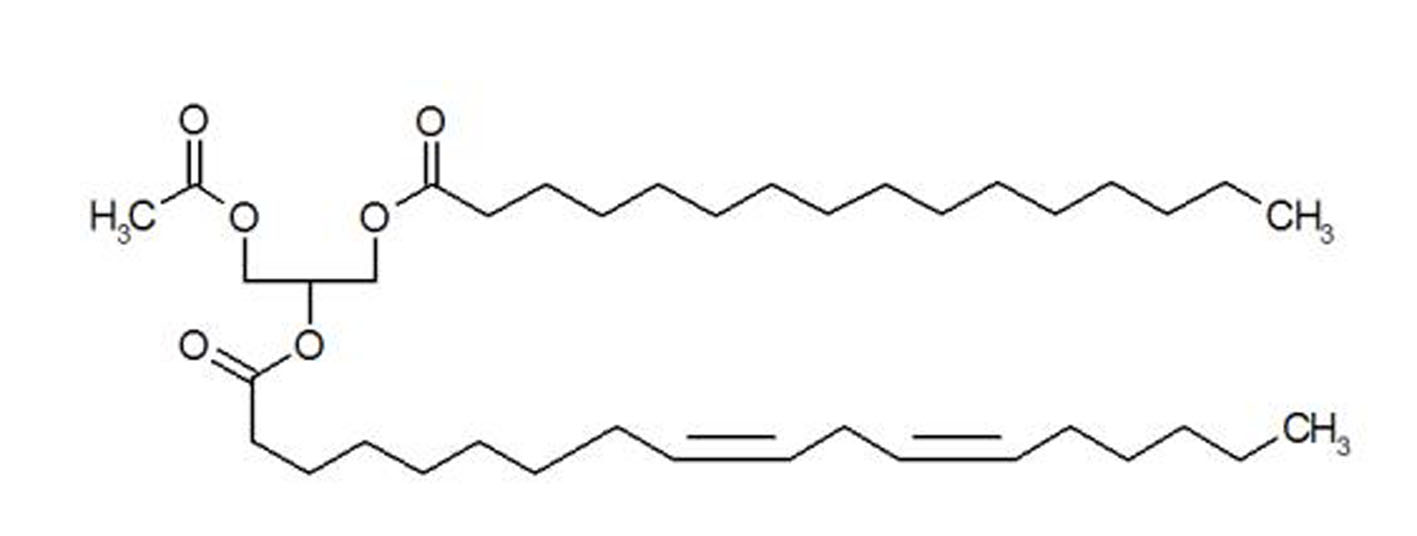
Figure 1. Structure of synthetic PLAG.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 6, Number 4, August 2015, pages 410-415
1-Pamitoyl-2-Linoleoyl-3-Acetyl-rac-Glycerol May Reduce Incidence of Gemcitabine-Induced Neutropenia: A Pilot Case-Controlled Study
Figures

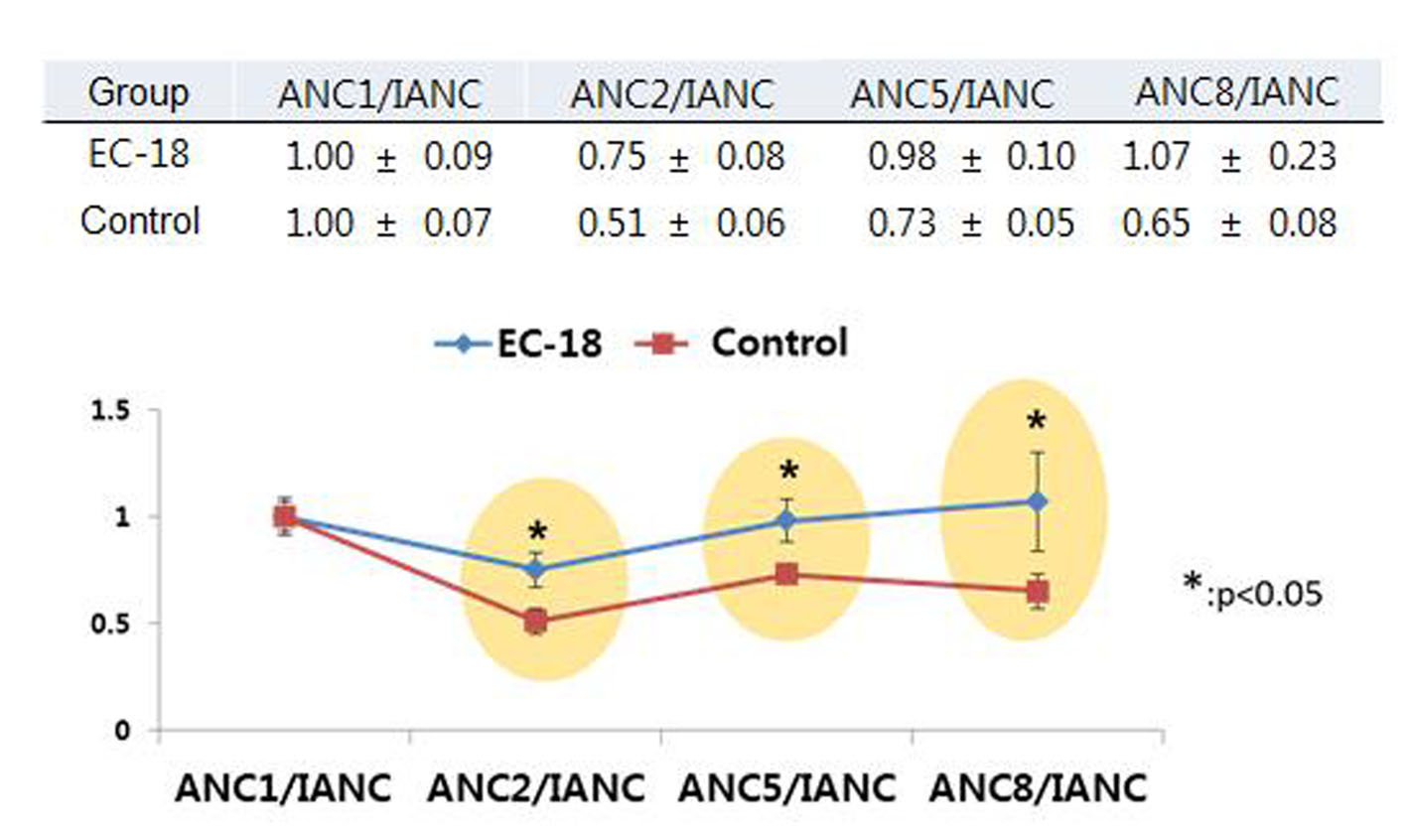
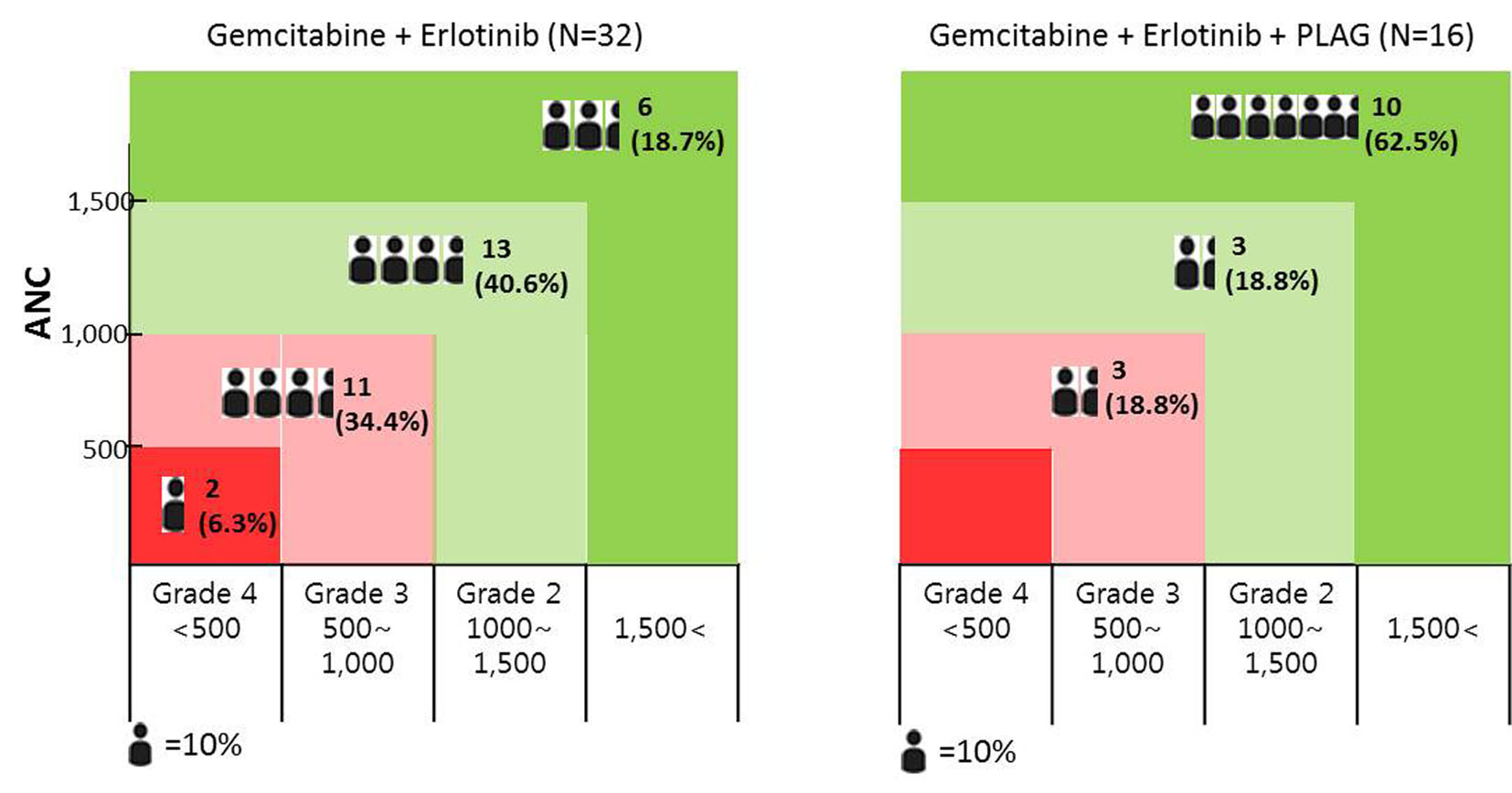
Tables
| PLAG group (N = 16) | Control group (N = 32) | |
|---|---|---|
| Male/female | 7:9 | 23:9 |
| Median age (years, range) | 56.5 (44 - 72) | 59 (44 - 69) |
| Chemotherapy cycle | ||
| Two cycles | 8 | 16 |
| Three cycles | 8 | 16 |
| Cancer stage | ||
| Locally advanced | 6 | 12 |
| Metastatic | 10 | 20 |
| ECOG performance | ||
| Grade 1 | 16 | 32 |
| Absolute neutrophil count (ANC) or % | PLAG group (N = 16) | Control group (N = 32) | P-value |
|---|---|---|---|
| ANC < 1,500 (grade 2 or higher) | 6 (37.5%) | 26 (81.3%) | P < 0.05 |
| Depth of the ANC nadir > 50% of baseline level | 6 (37.5%) | 29 (90.6%) | P < 0.05 |
| Depth of the ANC nadir > 75% of baseline level | 0 | 12 (37.5%) | P < 0.05 |
| Long-acting G-CSF (pegfilgrastim) | PLAG | |
|---|---|---|
| Route of administration | Parenteral (subcutaneous injection) | Per oral |
| Administration mode | Once per chemotherapy cycle | Daily administration continuing throughout the course of chemotherapy |
| Timing of starting administration | Recommended to be administered 24 h after chemotherapy completion | Can be safely administered prior to or simultaneously with the initiation of chemotherapy |
| Side effects | Bone pain, fatigue, nausea, headache, splenic rupture, etc. | Not observed |