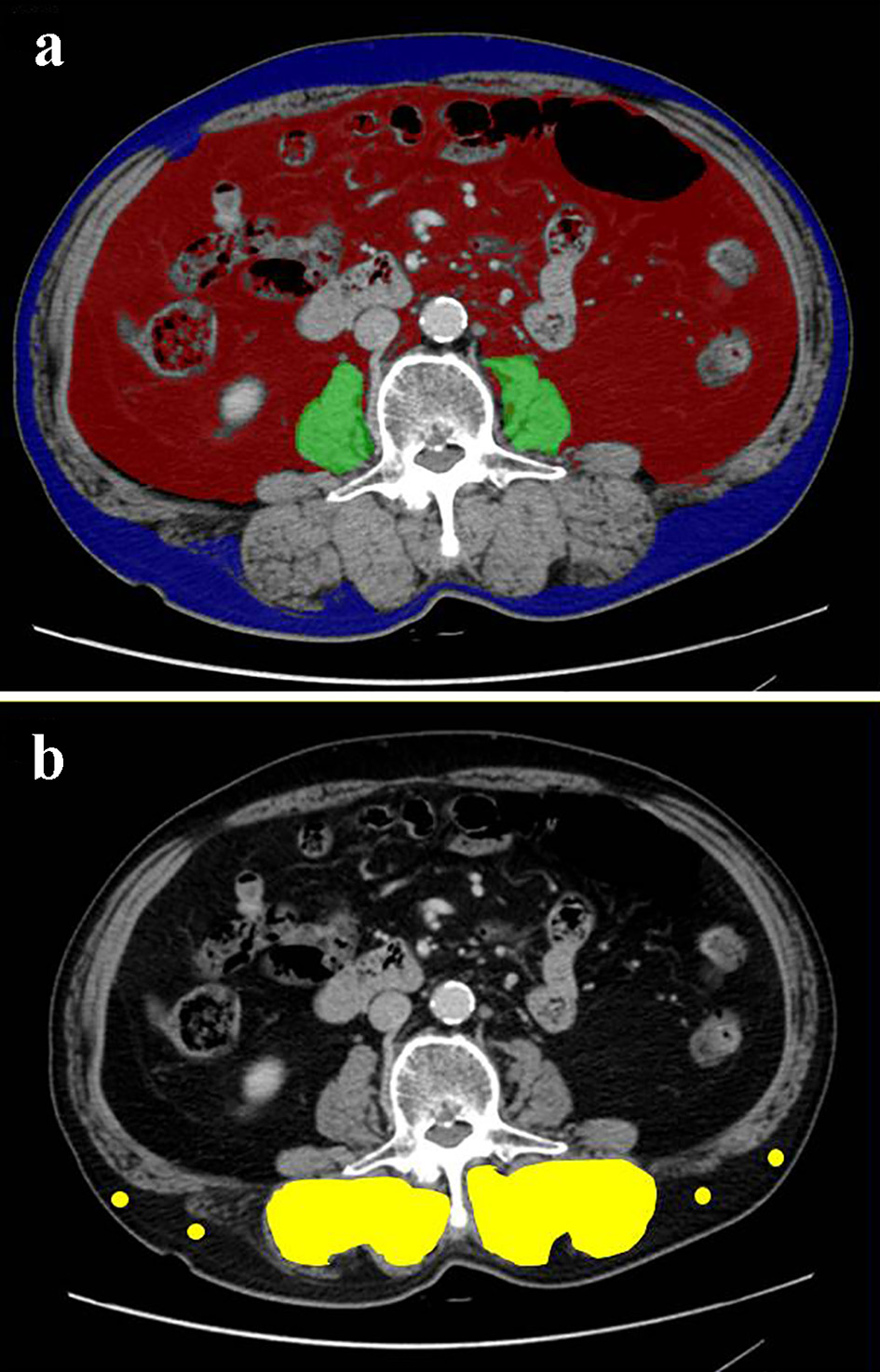
Figure 1. Cross-sectional CT images at the third lumber vertebra level of a 75-year-old male patient. (a) The areas of bilateral psoas muscles (green area), visceral (red) and subcutaneous (blue) adipose tissue area were identified. (b) The multifidus muscle (yellow area) was precisely traced. Four points (yellow dots) were placed on subcutaneous fat away from major vessels. CT: computed tomography.
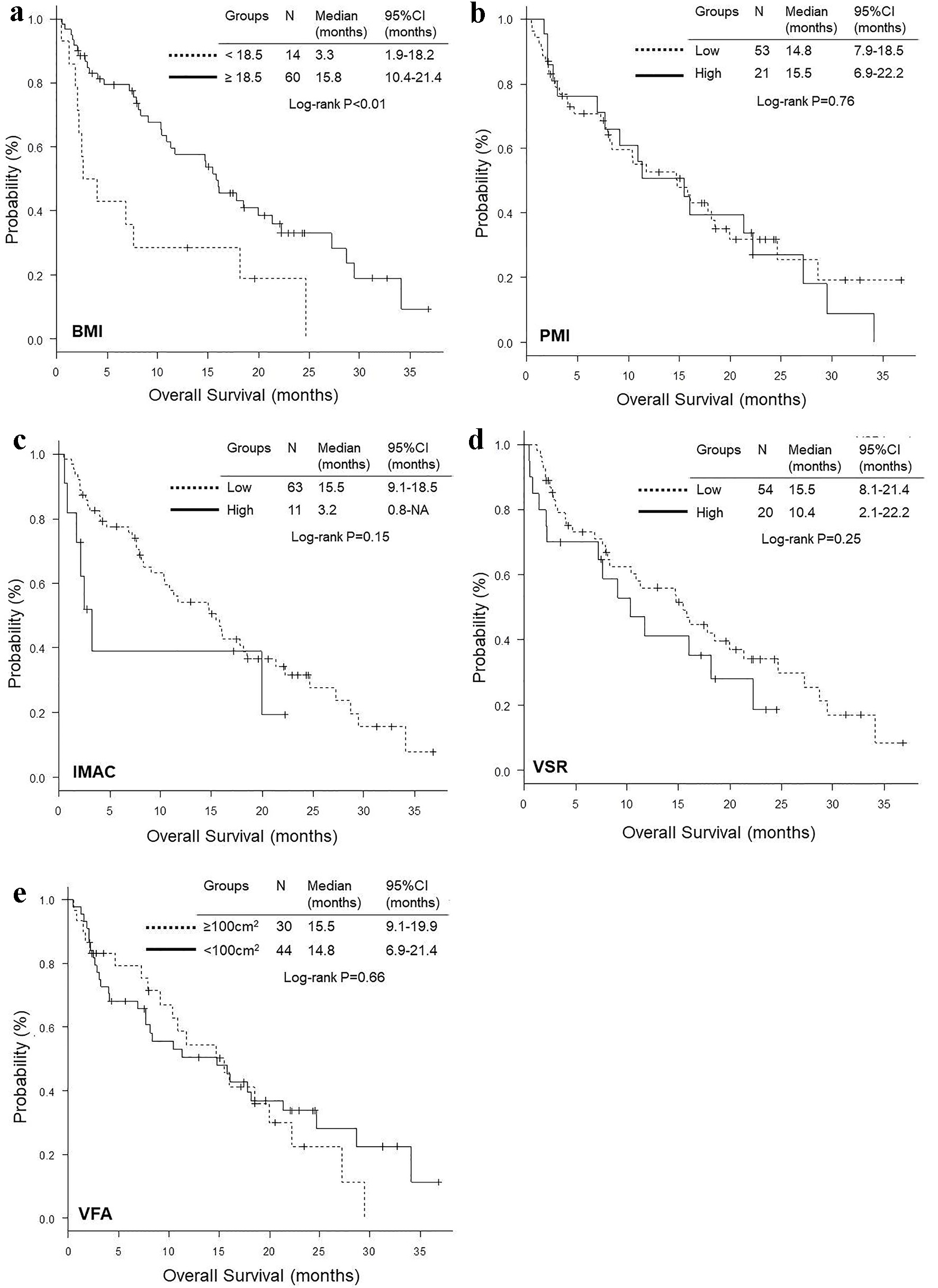
Figure 2. Kaplan-Meier curves of overall survival according to BMI (a), PMI (b), IMAC (c), VSR (d) and VFA (e). BMI: body mass index; PMI: psoas muscle index; IMAC: intramuscular adipose tissue content; VSR: visceral to subcutaneous adipose tissue area ratio; VFA: visceral fat area.
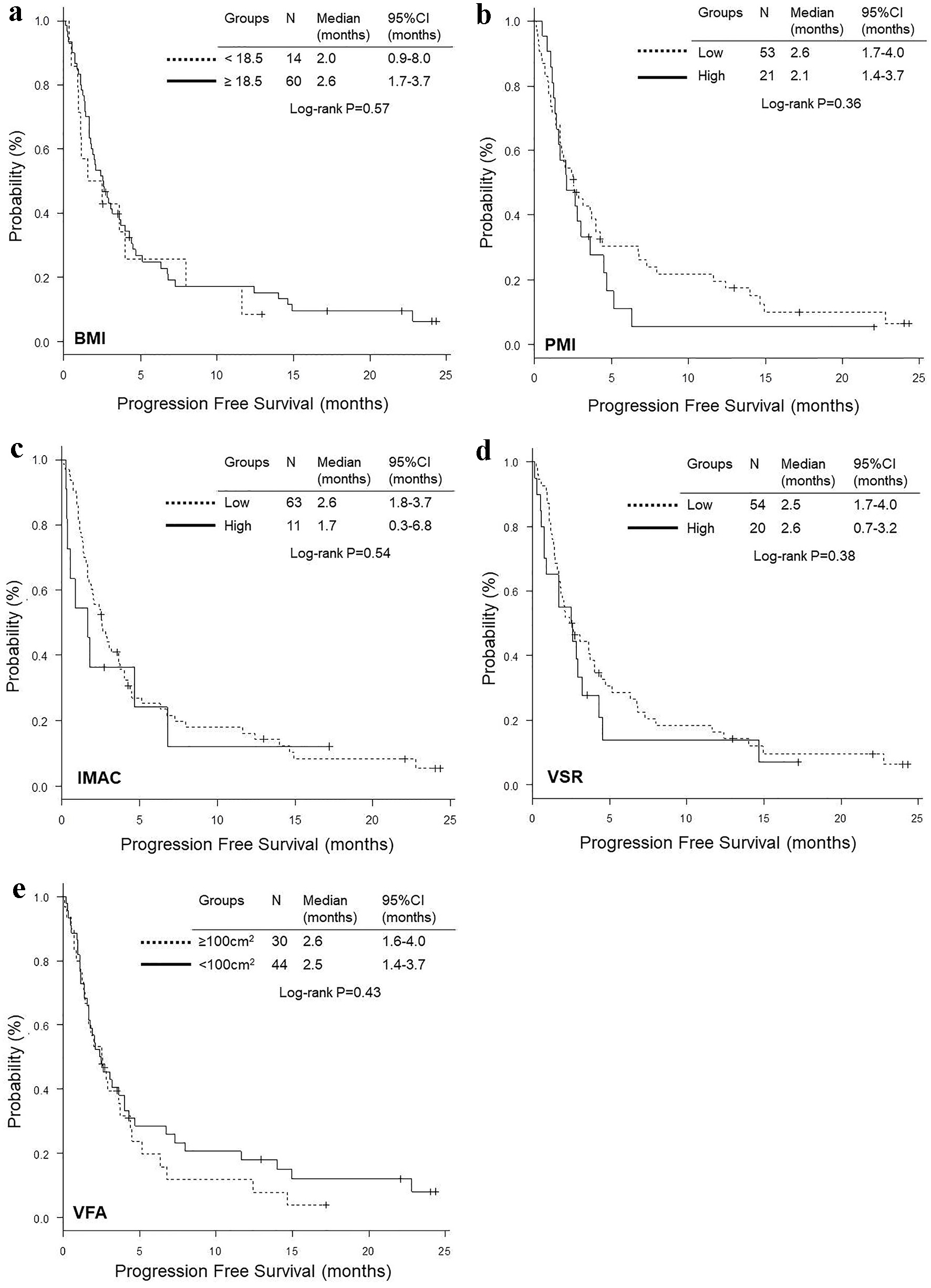
Figure 3. Kaplan-Meier curves of progression-free survival according to BMI (a), PMI (b), IMAC (c), VSR (d) and VFA (e). BMI: body mass index; PMI: psoas muscle index; IMAC: intramuscular adipose tissue content; VSR: visceral to subcutaneous adipose tissue area ratio; VFA: visceral fat area.


