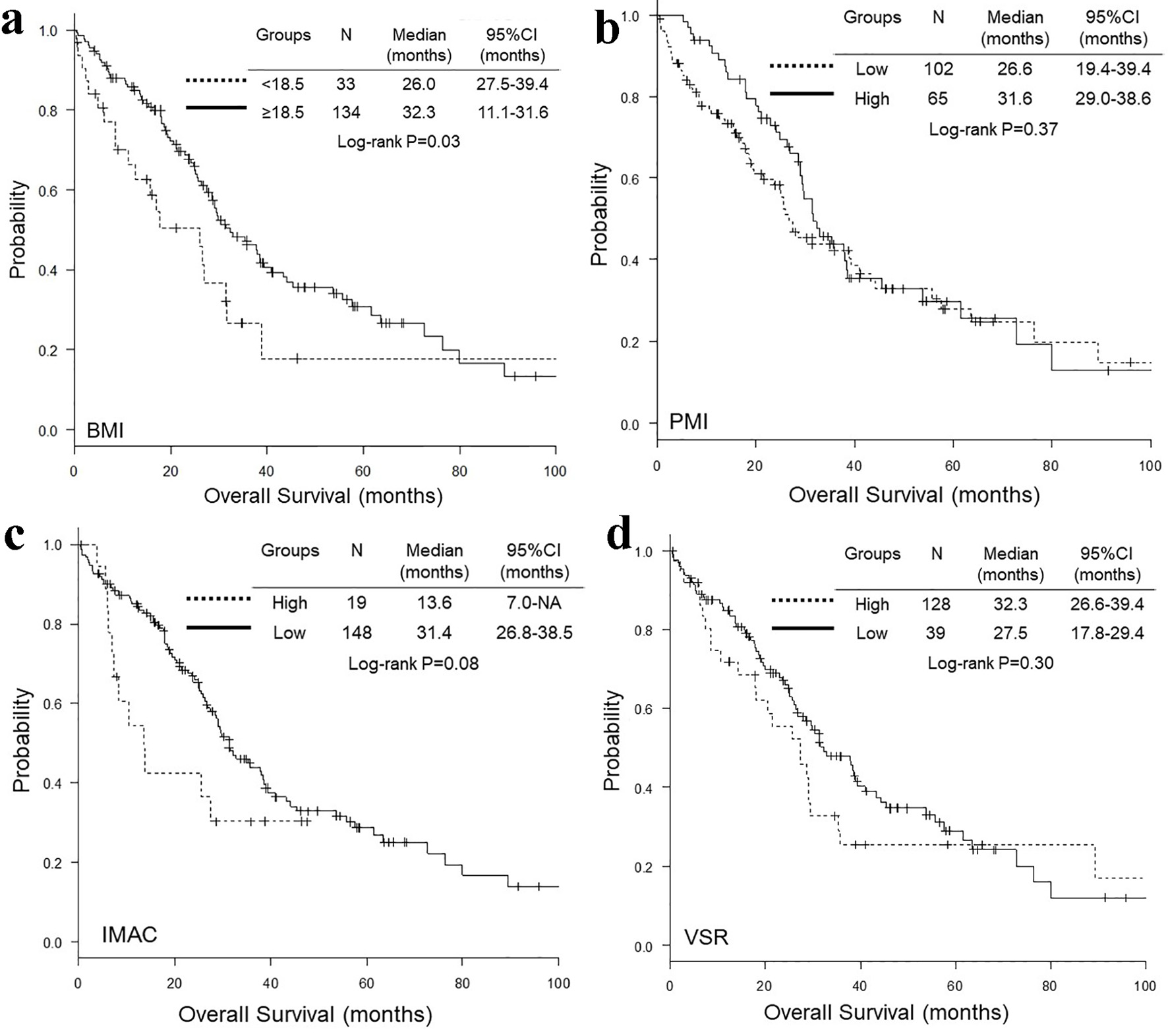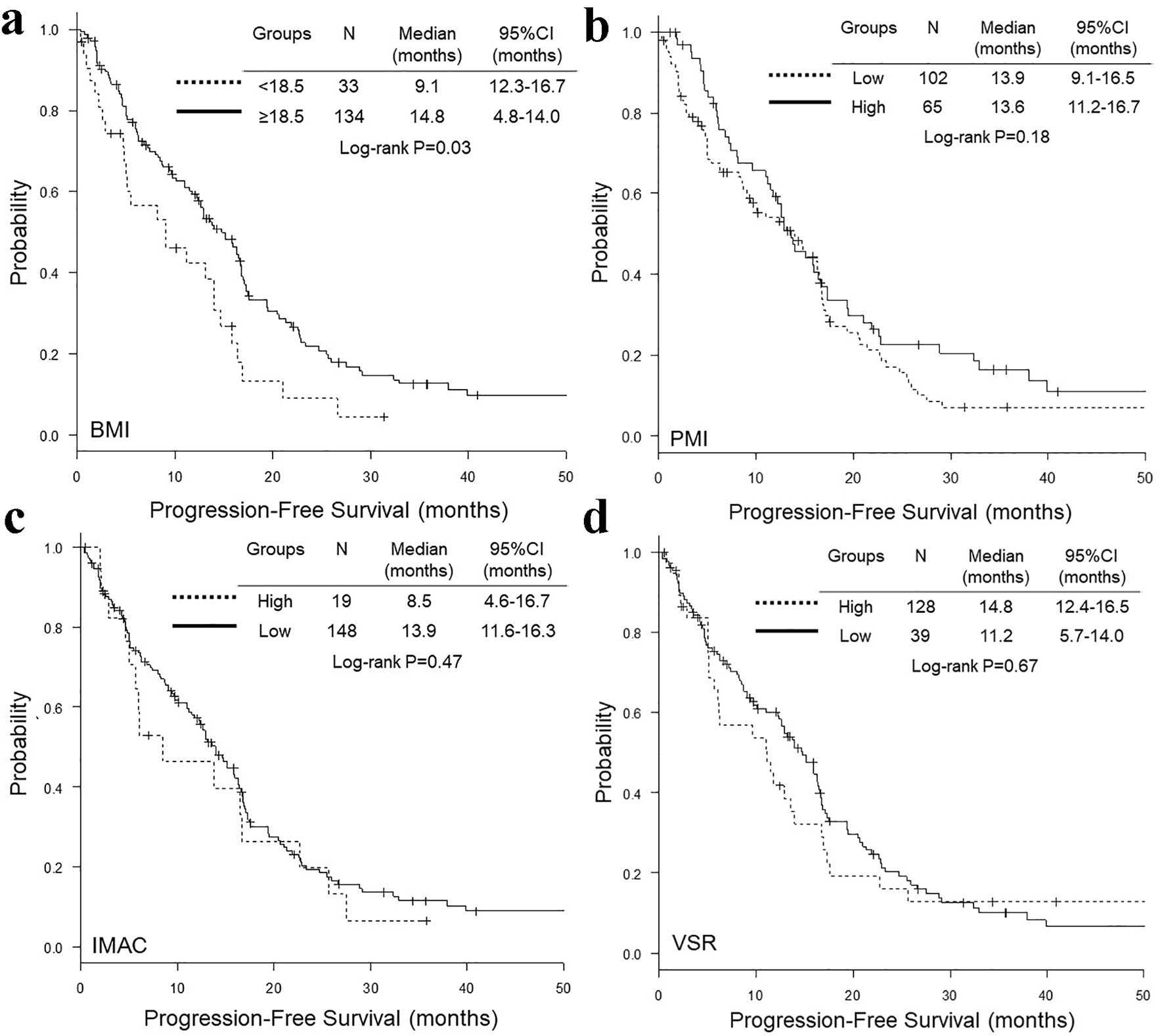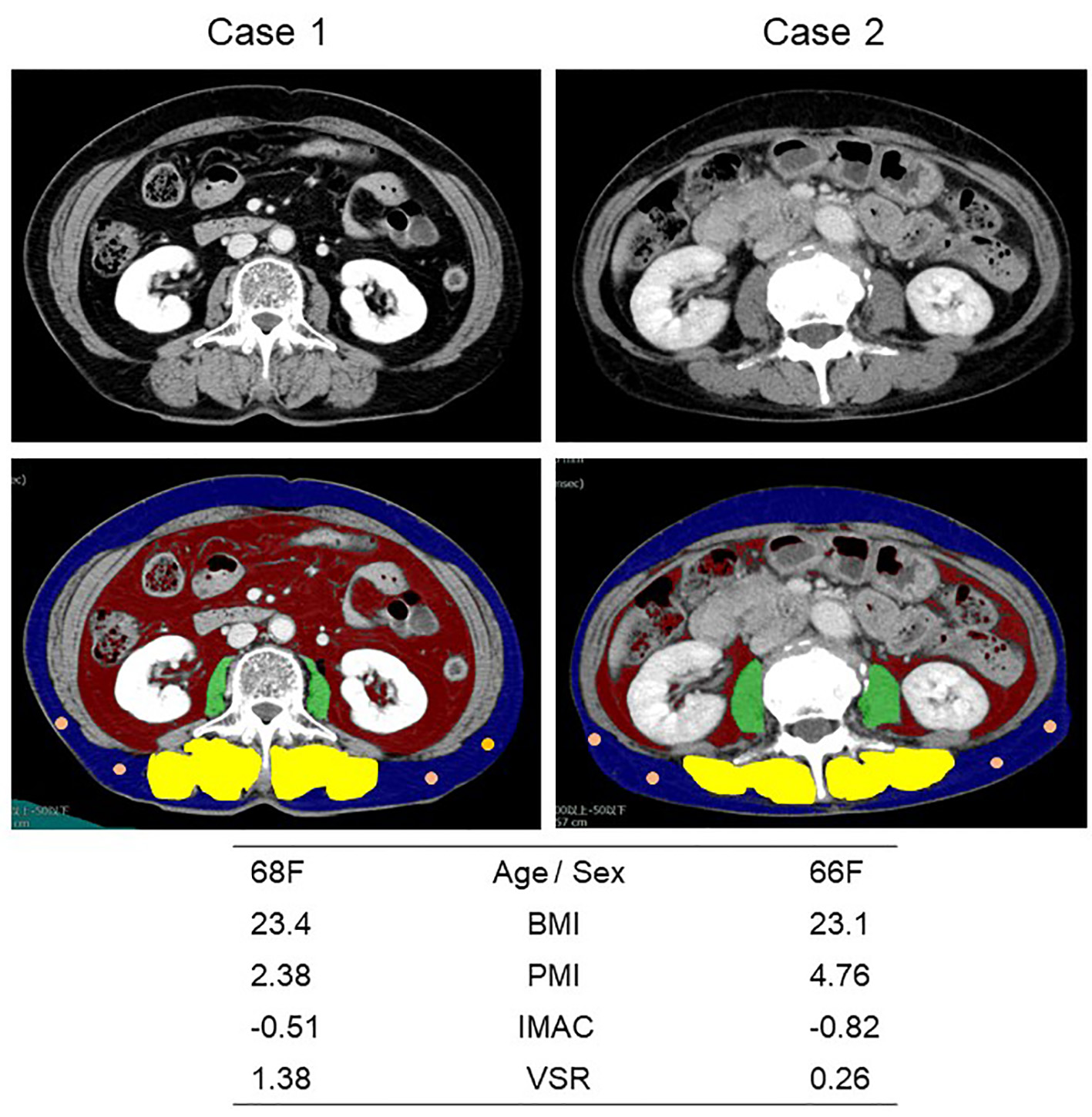
Figure 1. Representative cross-sectional CT images obtained at the third lumber vertebra. Two female patients had similar BMI and age, but different PMI, IMAC and VSR. The areas of bilateral psoas muscles, visceral fat, subcutaneous fat, multifidus muscle and subcutaneous four small circles were identified by green, red, blue, yellow and orange. CT: computed tomography; BMI: body mass index; PMI: psoas muscle index; IMAC: intramuscular adipose tissue content; VSR: visceral-to-subcutaneous adipose tissue area ratio.