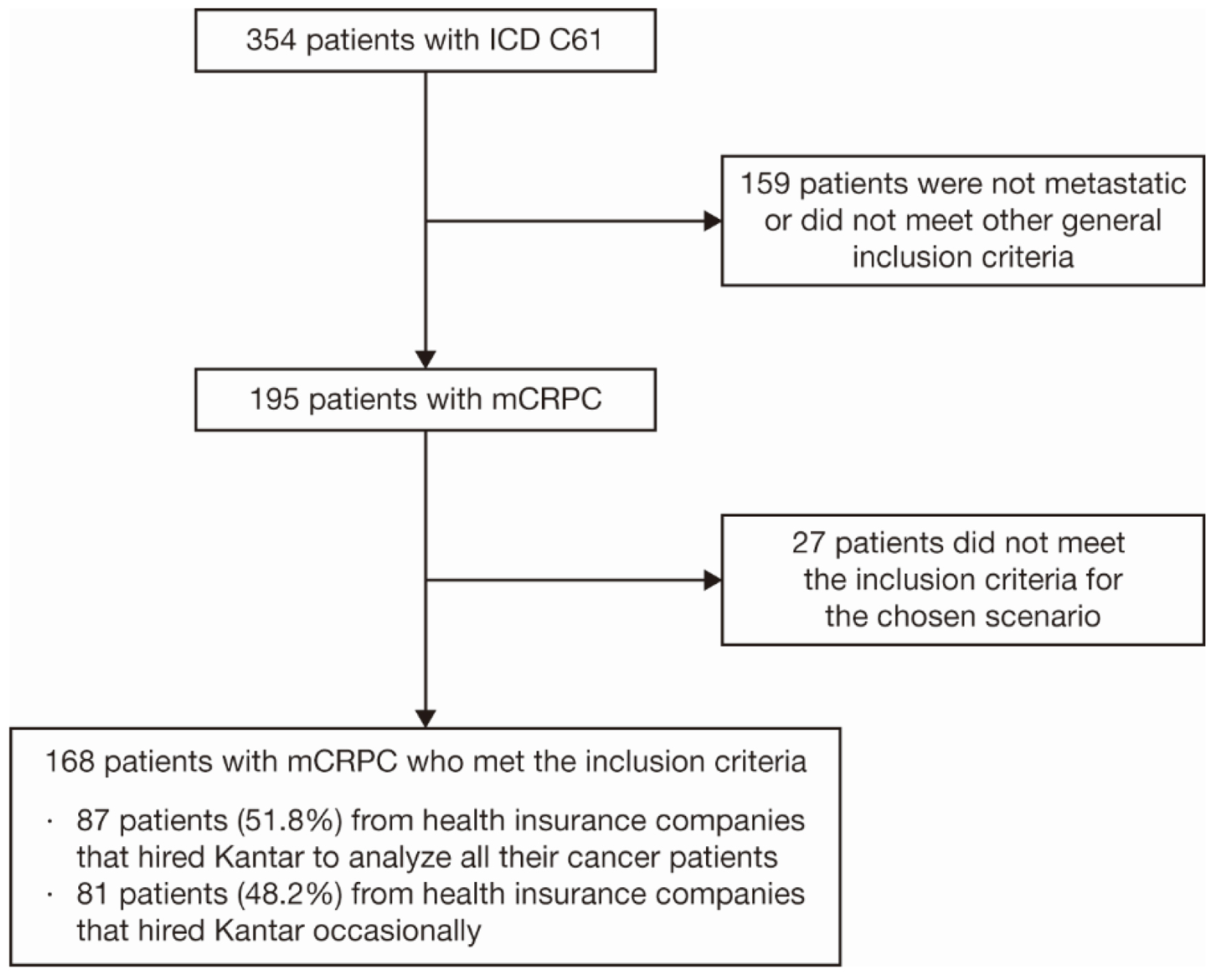
Figure 1. Patient selection criteria. Twenty-seven patients fell into the exclusion criteria: incomplete treatment, defined as having no information on first-line treatment for mCRPC within the database, or patients whose mCRPC diagnosis was made more than 90 days before the first information on treatment. ICD: International Classification of Diseases; mCRPC: metastatic castration-resistant prostate cancer.
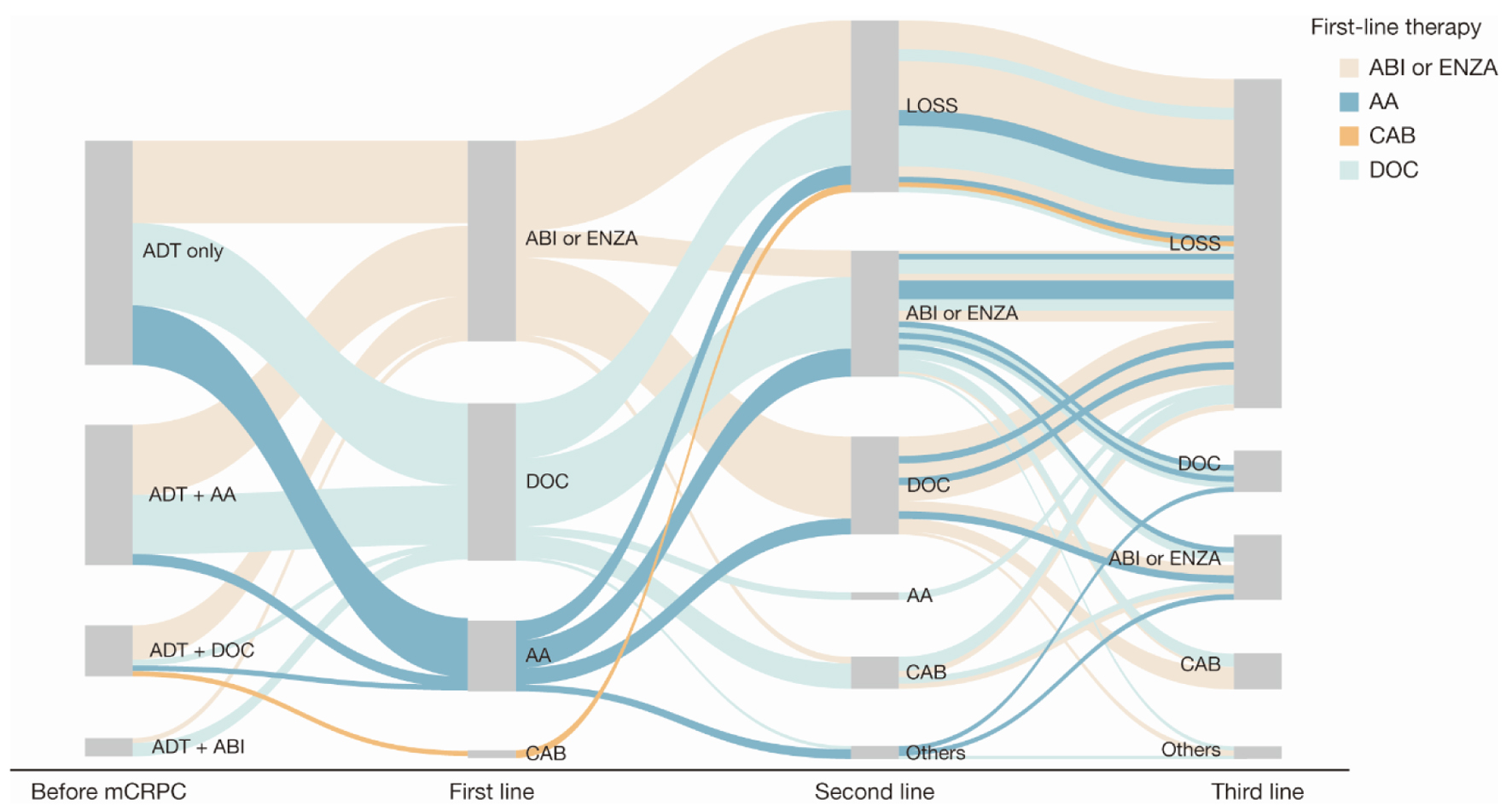
Figure 2. Sankey diagram of treatment pattern of patients with mCRPC (n = 168). AA: first-generation anti-androgen (bicalutamide or flutamide); ABI: abiraterone; ADT: androgen deprivation therapy; CAB: cabazitaxel; DOC: docetaxel; ENZA: enzalutamide; LOSS: information lost in tracking; mCRPC: metastatic castration-resistant prostate cancer.
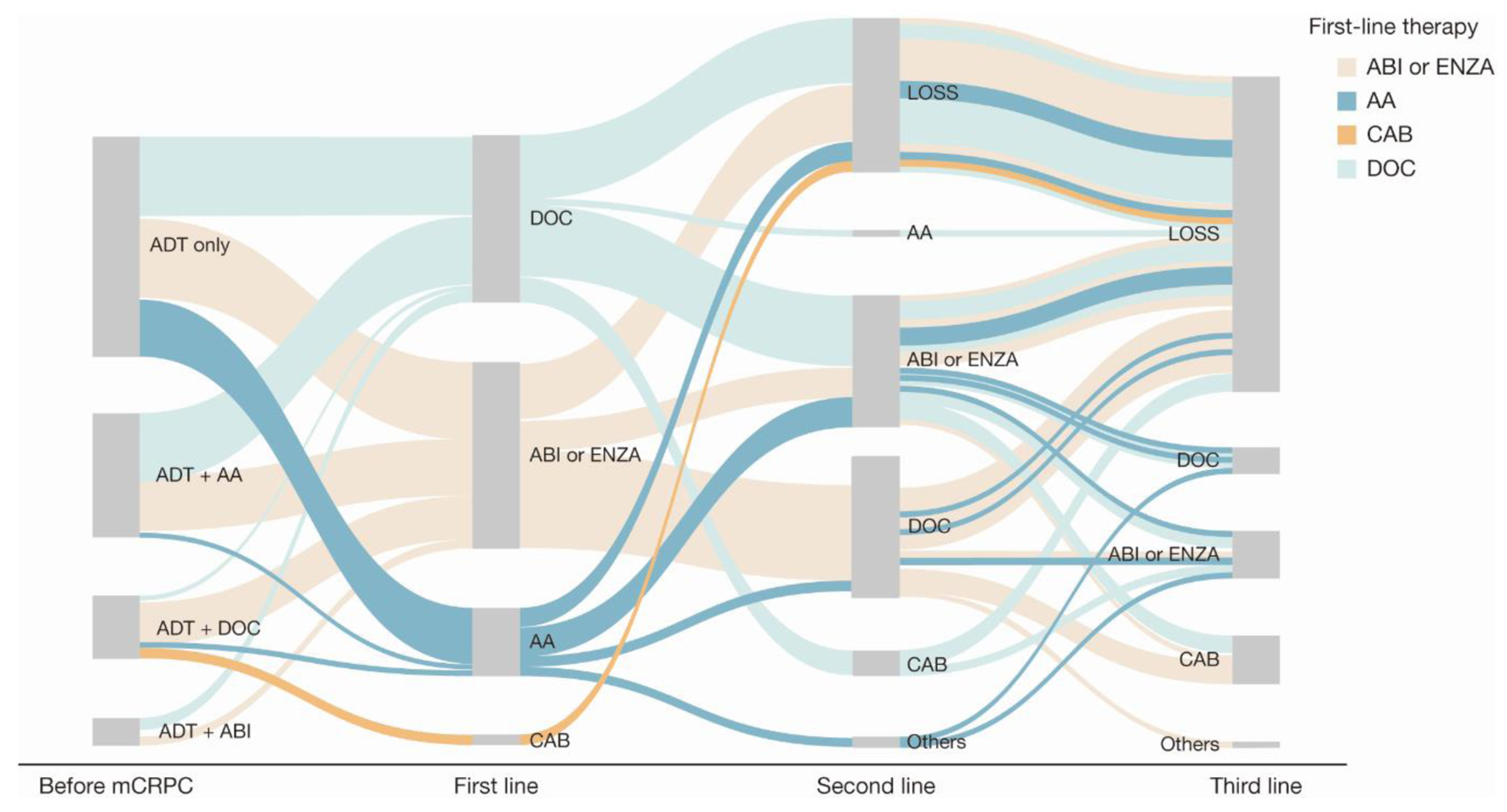
Figure 3. Sankey diagram of treatment pattern of patients who were metastatic before mCRPC diagnosis (n = 122). AA: first-generation anti-androgen (bicalutamide or flutamide); ABI: abiraterone; ADT: androgen deprivation therapy; CAB: cabazitaxel; DOC: docetaxel; ENZA: enzalutamide; LOSS: information lost in tracking; mCRPC: metastatic castration-resistant prostate cancer.
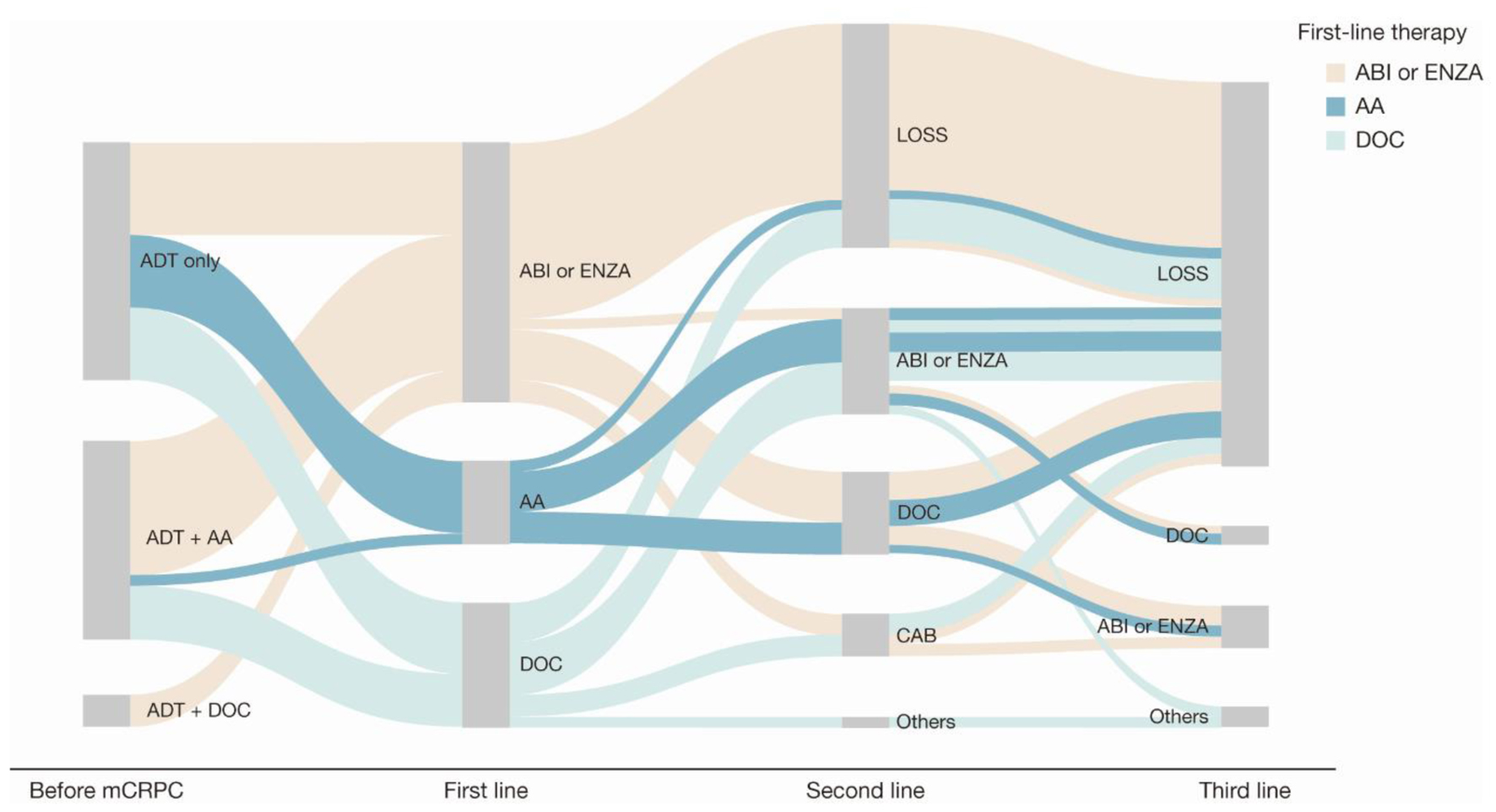
Figure 4. Sankey diagram of treatment pattern of patients who were non-metastatic before mCRPC diagnosis (n = 45). AA: first-generation anti-androgen (bicalutamide or flutamide); ABI: abiraterone; ADT: androgen deprivation therapy; CAB: cabazitaxel; DOC: docetaxel; ENZA: enzalutamide; LOSS: information lost in tracking; mCRPC: metastatic castration-resistant prostate cancer.



