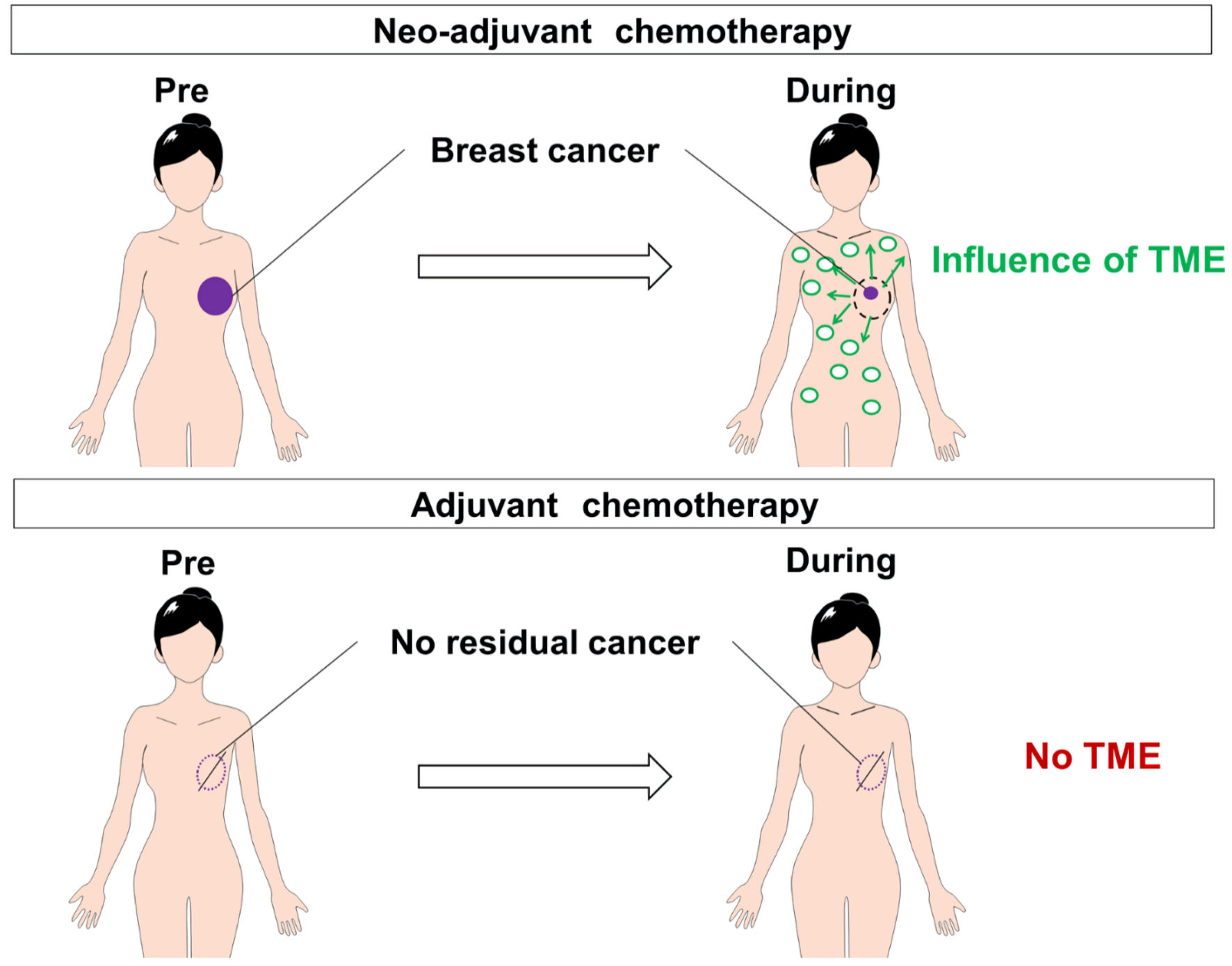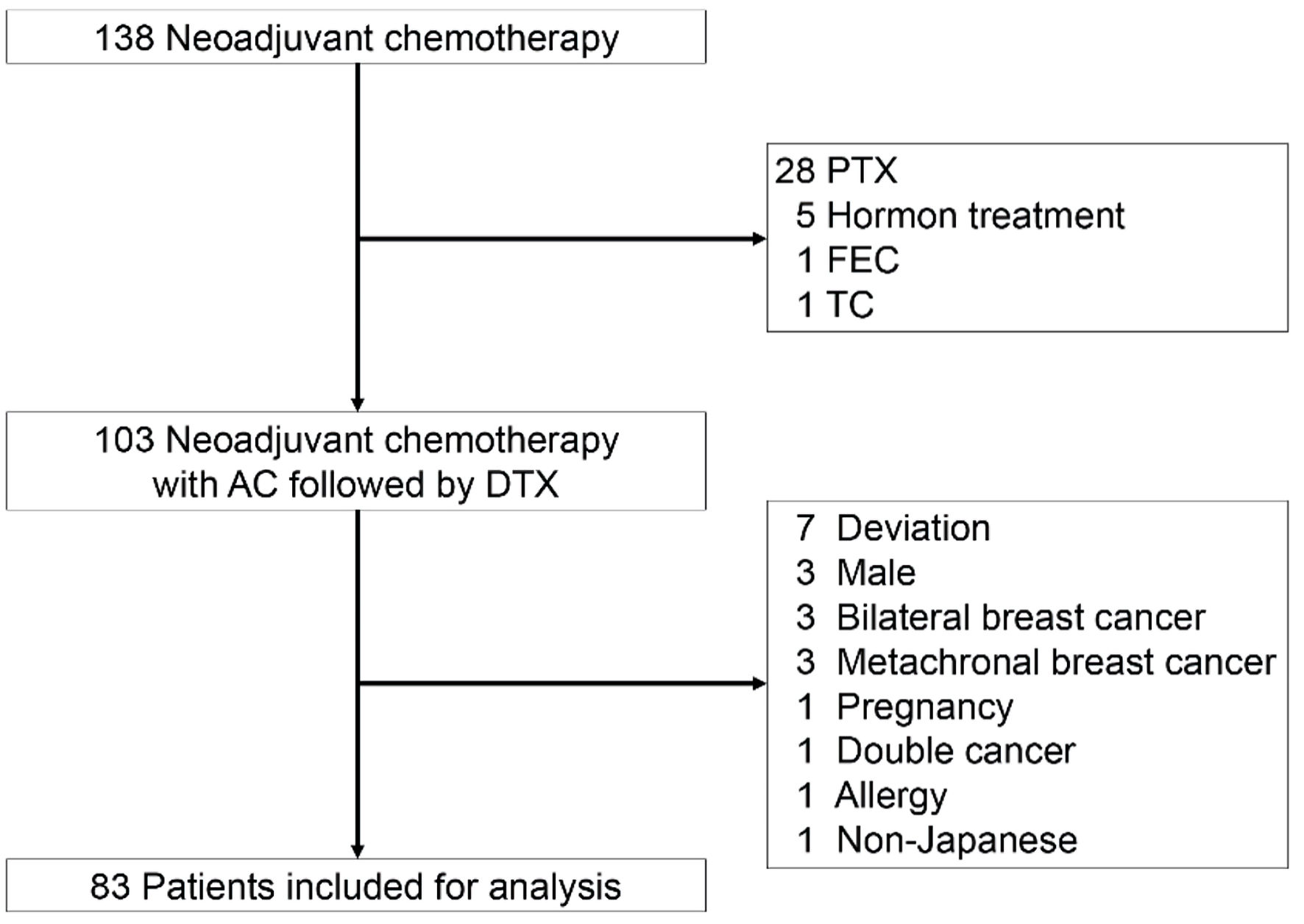
Figure 1. The CONSORT diagram with inclusion and exclusion criteria. Eighty-three patients were included for analysis. AC: adriamycin/cyclophosphamide; DTX: docetaxel; FEC: fluorouracil/epirubicin hydrochloride/cyclophosphamide; PTX: paclitaxel; TC: docetaxel/cyclophosphamide.