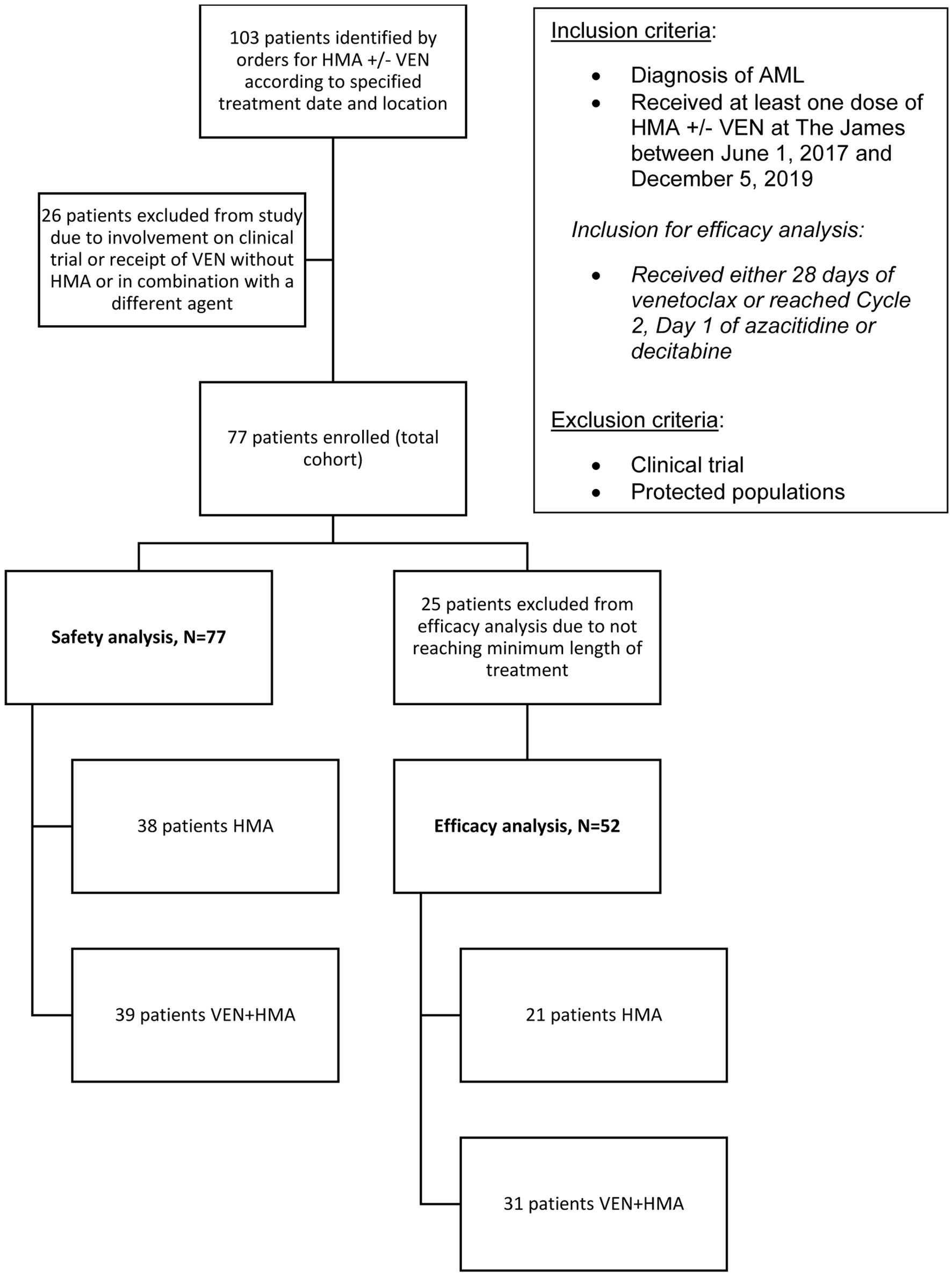
Figure 1. The selection process for study enrollment. Records were identified by electronic orders according to treatment date and location criteria. Diagnosis and treatment were verified creating the total cohort, all of which were included in the safety analysis. The efficacy analysis was comprised of patients that received the minimum length of treatment as specified.
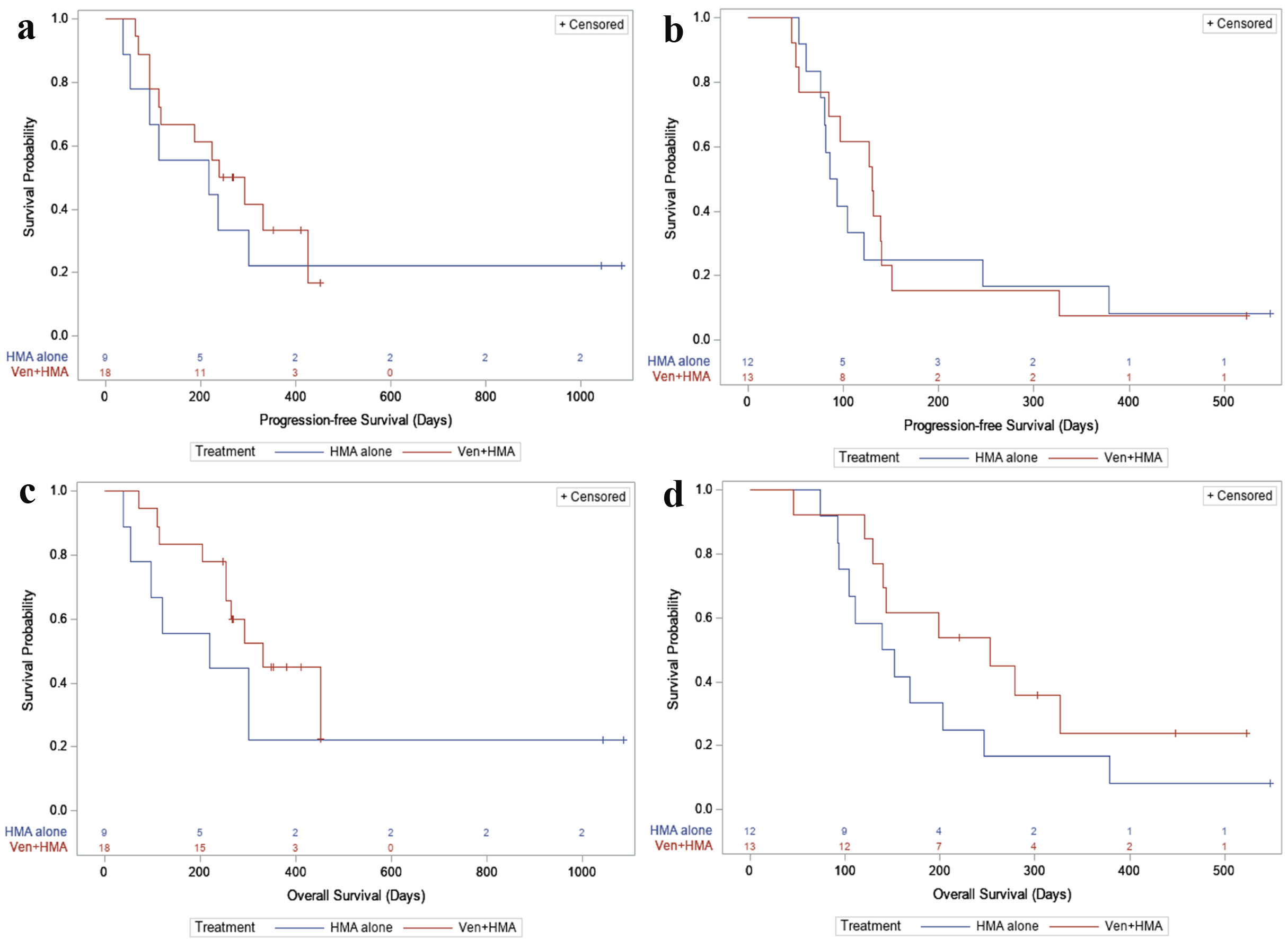
Figure 2. (a) PFS, first-line. For newly diagnosed patients: in the HMA alone group, 7/9 (77.8%) had a PFS event during follow-up, with a median PFS time of 218 days (95% CI: 36 - not reached). In the VEN + HMA group, 12/18 (66.7%) had a PFS event, with a median PFS time of 266 days (95% CI: 112 - 425). (b) PFS, relapsed or refractory. For relapsed/refractory patients: in the HMA alone group, 11/12 (91.7%) had a PFS event during follow-up, with a median PFS time of 89.5 days (95% CI: 61 - 246). In the VEN + HMA group, 12/13 (92.3%) had a PFS event, with a median PFS time of 130 days (95% CI: 53 - 140). (c) OS, first-line. In newly diagnosed patients: in the HMA alone group, 7/9 (77.8%) patients died during follow-up, with a median survival of 219 days (95% CI: 39 - not reached). In the VEN + HMA group, 10/18 (55.6%) patients died, with a median survival of 331 days (95% CI: 253 - not reached). (d) OS, relapsed or refractory. In relapsed/refractory patients: in the HMA alone group, 11/12 (91.7%) patients died during follow-up, with a median survival of 145.5 days (95% CI: 92 - 246). In the VEN + HMA group, 9/13 (69.2%) patients died, with a median survival of 253 days (95% CI: 129 - not reached). VEN: venetoclax; HMA: hypomethylating agent; PFS: progression-free survival; OS: overall survival; CI: confidence interval.

