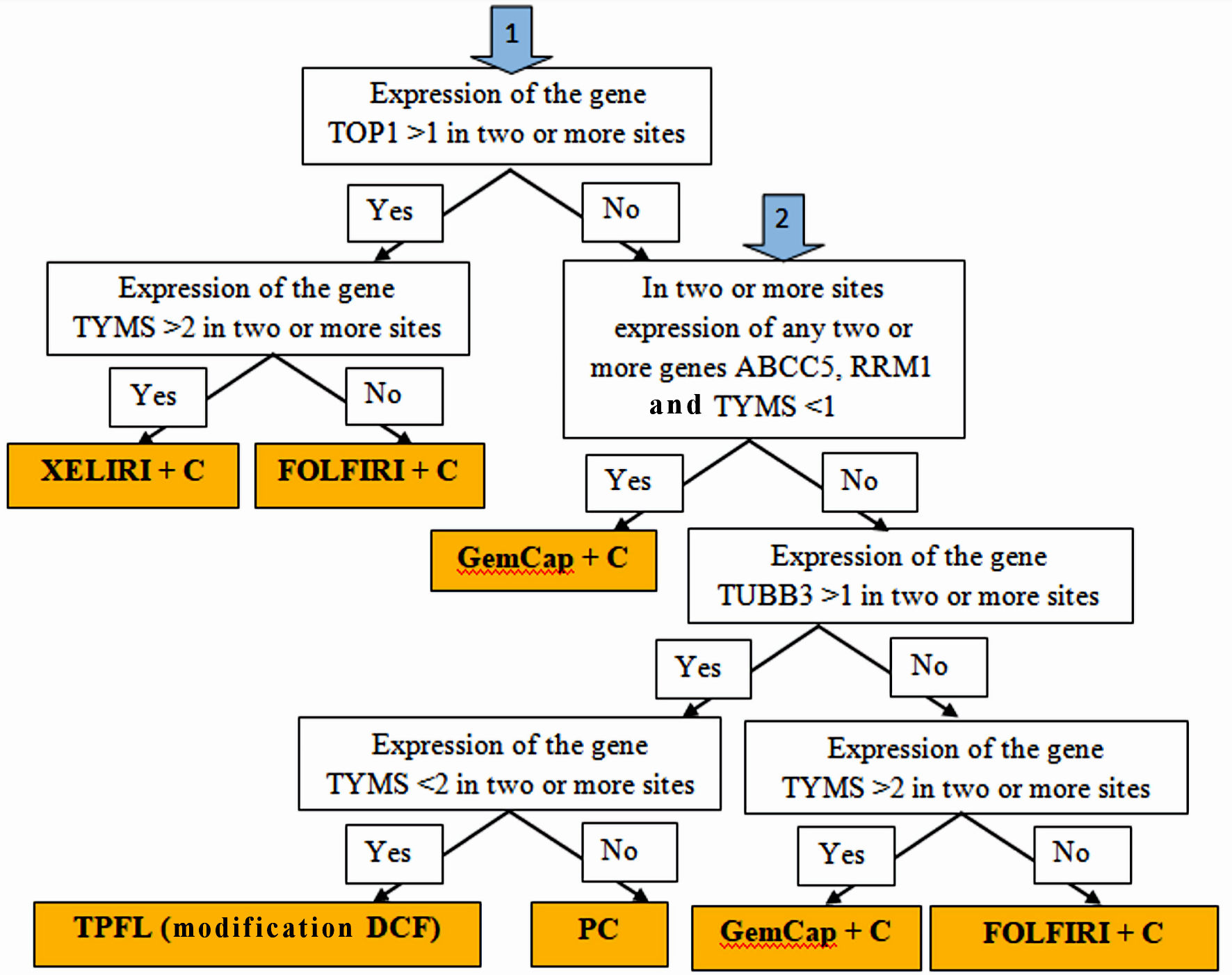
Figure 1. Algorithm for personalized chemotherapy depending on the chemotherapy genes expression at different sites: stomach tumors, peritoneal metastases, lymph node metastases and hematogenous metastases (if any).
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 2, April 2024, pages 298-308
Gene Expression Profile-Guided Personalized Intraperitoneal Chemotherapy for Gastric Cancer Peritoneal Carcinomatosis
Figures

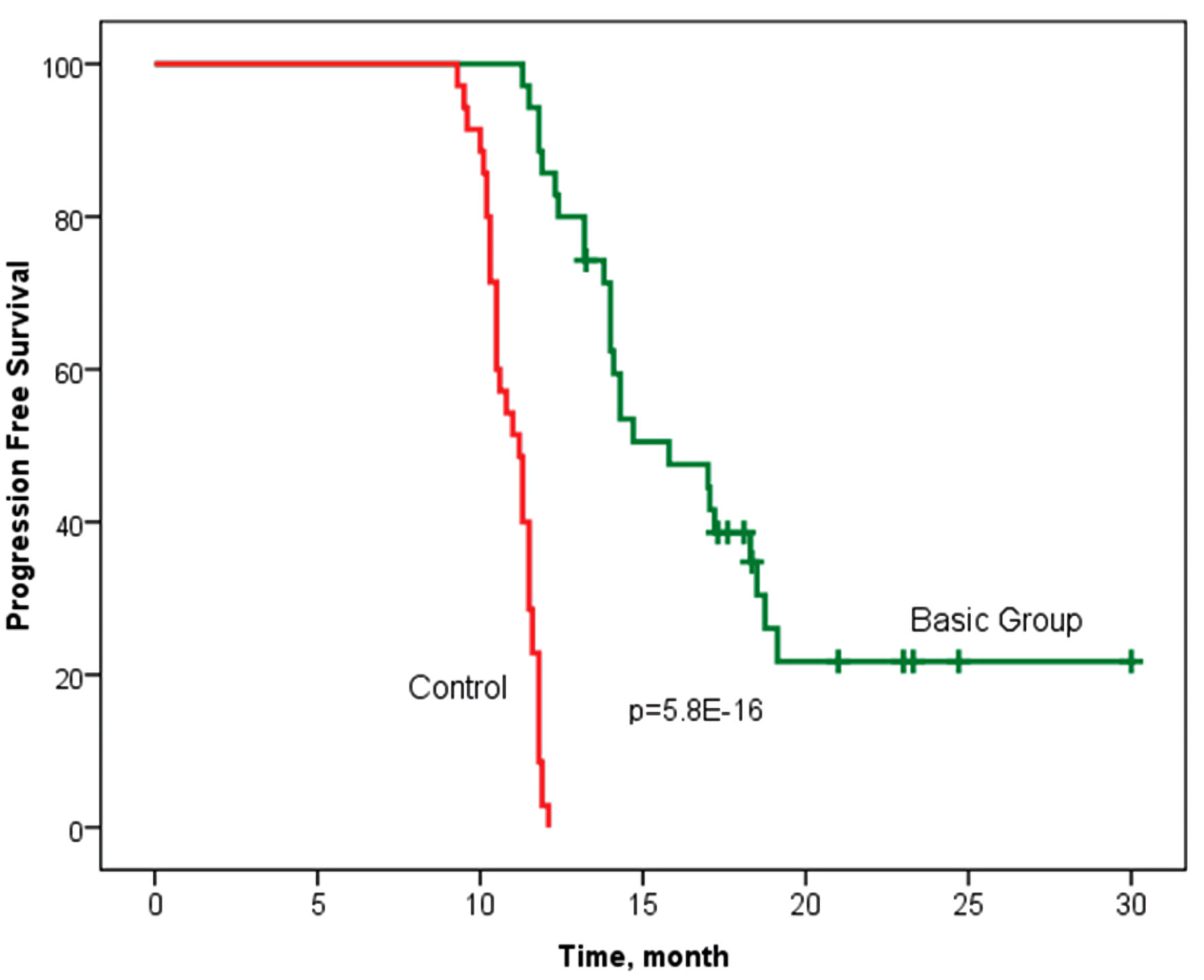
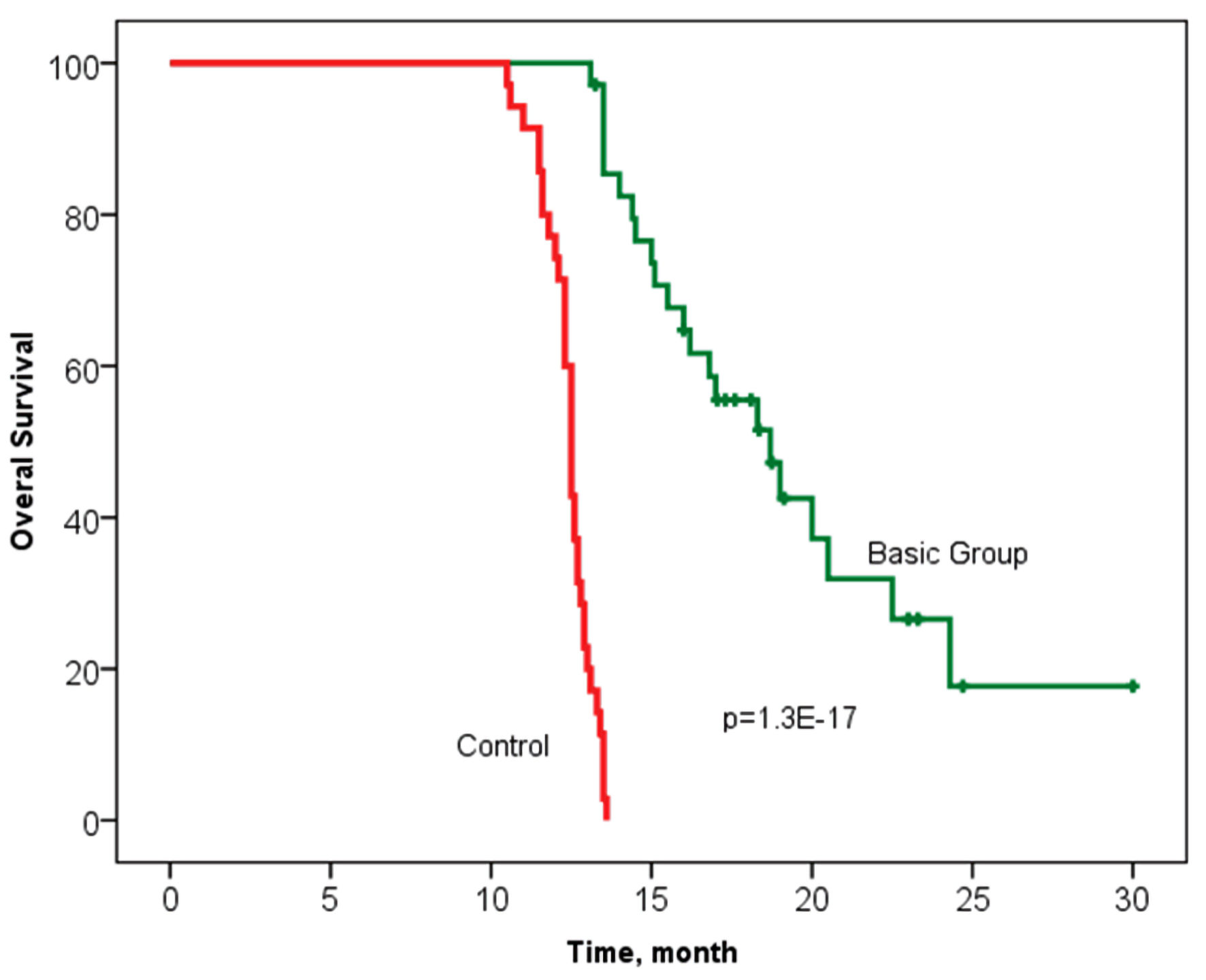
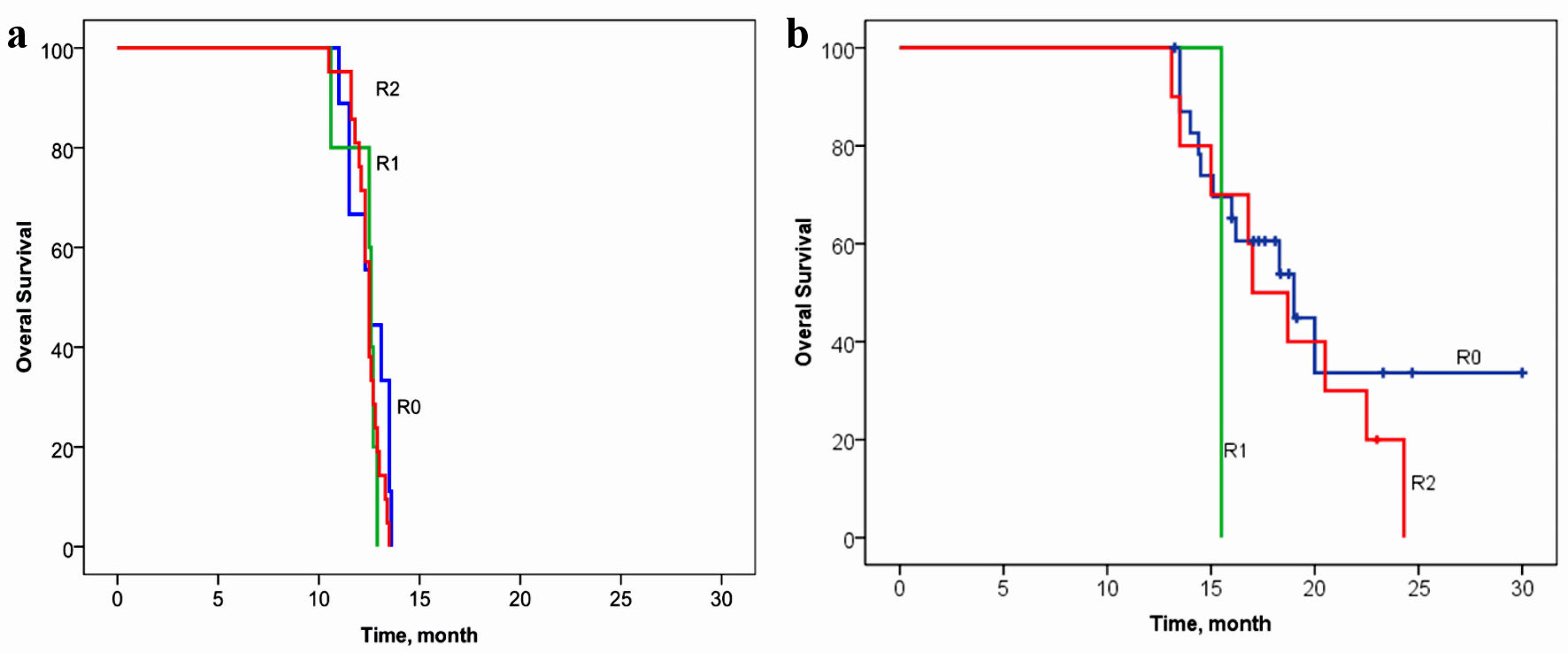
Tables
| Inclusion criteria | Exclusion criteria |
|---|---|
| HER2: human epidermal growth factor receptor 2; ECOG: Eastern Cooperative Oncology Group; WHO: World Health Organization; PCI: Peritoneal Carcinomatosis Index; CEC: cardioesophageal cancer. | |
| Age from 18 to 68 years | Age over 68 years |
| Histologically verified gastric and cardioesophageal cancer (Sievert III) with signs of dissemination (carcinomatosis, ascites, metastatic ovarian involvement) subject to cytoreductive surgery. | Patients with cardioesophageal cancer with lesions of the esophagus above 4 cm, requiring esophageal extirpation (CEC, Sievert I - II) |
| HER2/neu status: negative | Patients with gastric cancer stage I - IIIC (T1-4 N0-3 M0) |
| PCI is from 1 to 12 | HER2/neu status: positive |
| ECOG (WHO) is not more than two points, on the Karnofsky scale more than 70% | PCI is from 13 to 39 |
| Consent of the patient to be included in the study | ECOG (WHO) is more than two points, on the Karnofsky scale less than 70% |
| Multiple distant metastases (liver, lungs, bones, etc.) | |
| Obstructive jaundice (a consequence of the bile duct block, at the level of the junction of the liver/choledochus, metastasis - affected lymph nodes). True germination into the head and body of the pancreas. Impaired liver function and kidney function | |
| PC: peritoneal carcinomatosis; 5-FU: 5-fluorouracil. | ||
| 1 | PC | Paclitaxel 135 mg/m2 on the first day, intravenously, drip for 3 h, cisplatin 50 mg on the second day, intraperitoneally with 200 mL of saline, paclitaxel 65 mg/m2 on the eighth day, intraperitoneally with 200 mL of saline. Interval: 21 days. |
| 2 | GemCap + С (intraperitoneally) | Gemzar 800 mg/m2, intravenously on the first and eighth days; cisplatin 50 mg intraperitoneally per 200 mL of saline on the fourth day; capecitabine 1,500 mg/m2 for 14 days. Interval: 21 days. |
| 3 | TPFL (modification DCF) with intraperitoneal insertion of cisplatin | Docetaxel 75 mg/m2 intravenously over 1 h on the second day; cisplatin 50 mg intravenously, 1 h on the second day; cisplatin 50 mg intraperitoneally per 200 mL saline on the third day; 5-FU 500 mg/m2 3-h infusion on the 1 - 3 days; leucovorin 50 mg intravenously, on the 1 - 3 days before 5-FU insertion. Interval: 21 days. |
| 4 | FOLFIRI + С (intraperitoneally) | Irinotecan 170 mg/m2 intravenously, infusion 90 min; leucovorin 400 mg/m2 intravenously, infusion 2 h on the first day; 5-FU 400 mg/m2 intravenously, then 2,400 mg/m2 intravenously, infusion 46 h. Cisplatin 50 mg intraperitoneally per 200 mL of saline on the second day. Interval: 21 days. |
| 5 | XELIRI + C (intraperitoneally) | Irinotecan 230 mg/m2 intravenously, infusion 60 - 90 min, on the first day; cisplatin 50 mg intraperitoneally per 200 mL saline, on the second day; capecitabine 1,800 mg/m2 daily for 14 days. Interval: 21 days. |
| Characteristics | Basic group (n = 35) | Control group (n = 35) | P value (Mann-Whitney U-test or χ2 Pearson test) |
|---|---|---|---|
| Age, mean (Q25, Q75) years | 57 (45, 63) | 57.5 (43, 62) | U = 612.5/P = 0.99 |
| Men, n (%) | 18 (51%) | 18 (51%) | χ2 = 0,000000/P = 1 |
| Women, n (%) | 17 (49%) | 17 (49%) | |
| Histological subtype | |||
| Low-grade adenocarcinoma | 20 (57%) | 25 (71%) | P = 0.32 |
| Cricoid cell carcinoma | 15 (43%) | 10 (29%) | |
| The volume of the operational aid | |||
| Extended combined palliative gastrectomy according to Roux | 16 (46%) | 12 (34%) | P = 0.47 |
| Palliative gastrectomy according to Roux | 19 (54%) | 23 (66%) | |
| Postoperative complications | |||
| No complications | 18 (51%) | 14 (40%) | χ2 = 9.960361/P = 0.13 |
| Variables | Basic group (n = 35) | Control group (n = 35) | P value (Mann-Whitney U-test or χ2 Pearson test) |
|---|---|---|---|
| PCI: Peritoneal Carcinomatosis Index; CC: completeness of cytoreduction. | |||
| R: resection boundary | |||
| R0 | 24 (69%) | 9 (26%) | χ2 = 13.39/P = 0.0012 |
| R1 | 1 (3%) | 5 (14%) | |
| R2 | 10 (29%) | 21 (60%) | |
| D: volume of lymph node dissection | |||
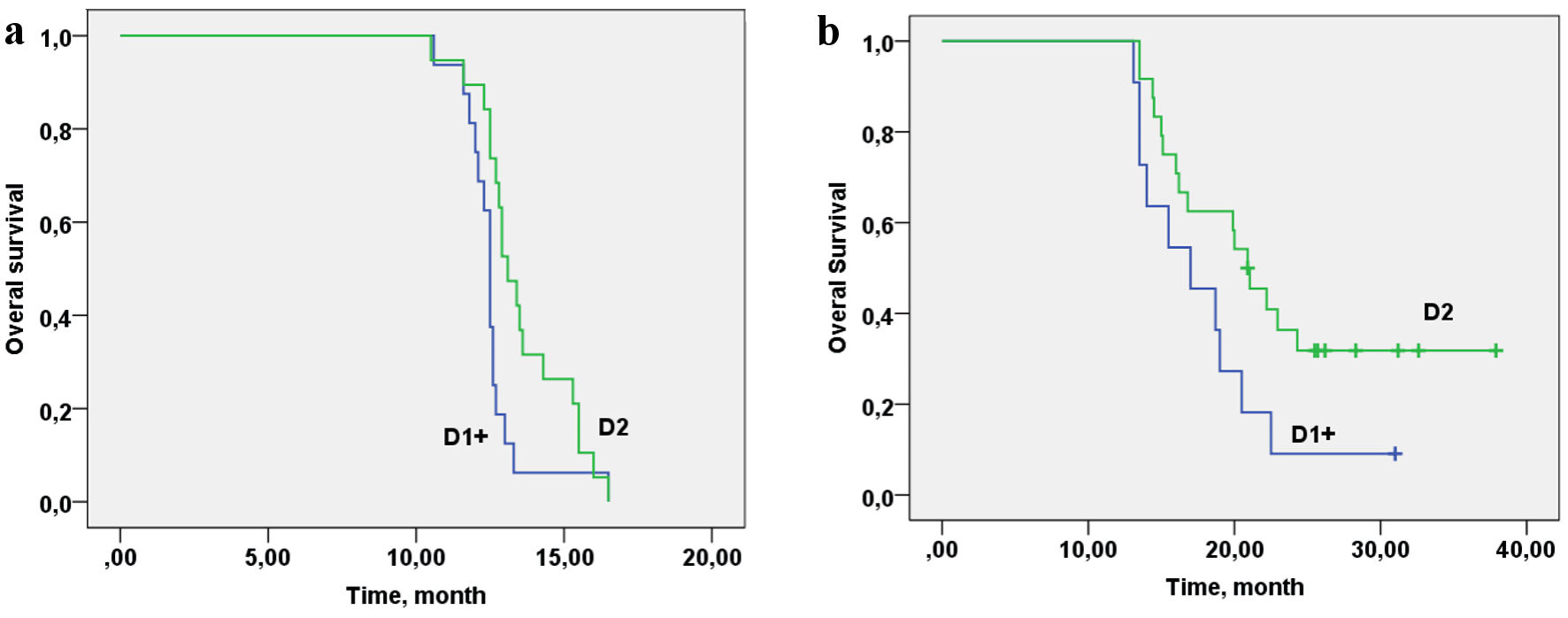
| D1+ | 11 (31%) | 25 (71%) | χ2 = 11.94/P = 0.003 |
| D2 | 24 (69%) | 10 (29%) | |
| PCI: (PCI from English: Peritoneal Cancer Index) | |||
| PCI (0 - 4) | 10 (29%) | 5 (14%) | χ2 = 2.12/P = 0.3465 |
| PCI (5 - 8) | 11 (31%) | 13 (37%) | |
| PCI (9 - 12) | 14 (40%) | 17 (49%) | |
| CC score: scale for assessing the completeness of cytoreduction | |||
| CC-0 score | 24 (69%) | 13 (37%) | χ2 = 7.97/P = 0.0466 |
| CC-1 score | 6 (17%) | 9 (26%) | |
| CC-2 score | 2 (6%) | 8 (23%) | |
| CC-3 score | 3 (8%) | 5 (14%) | |