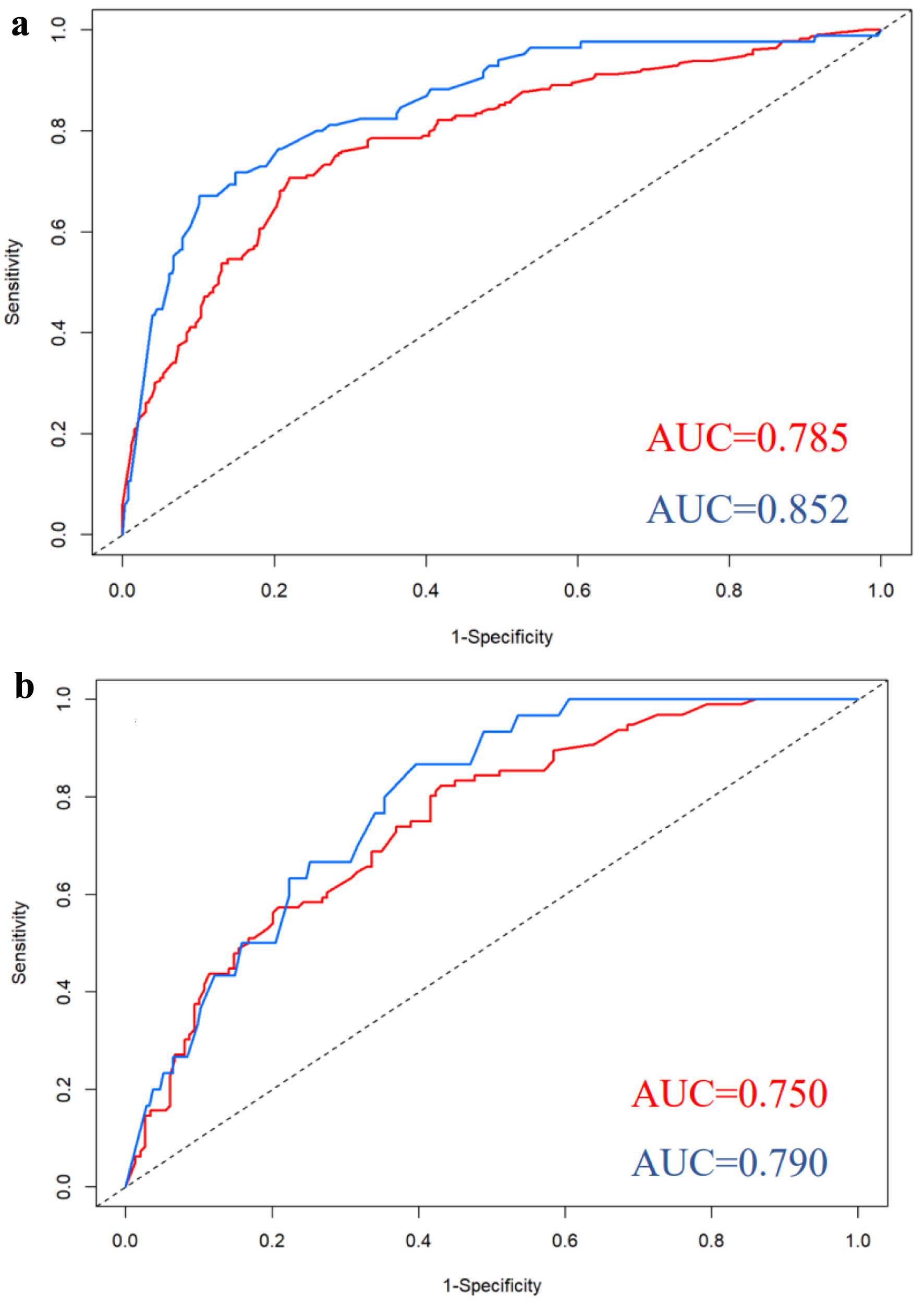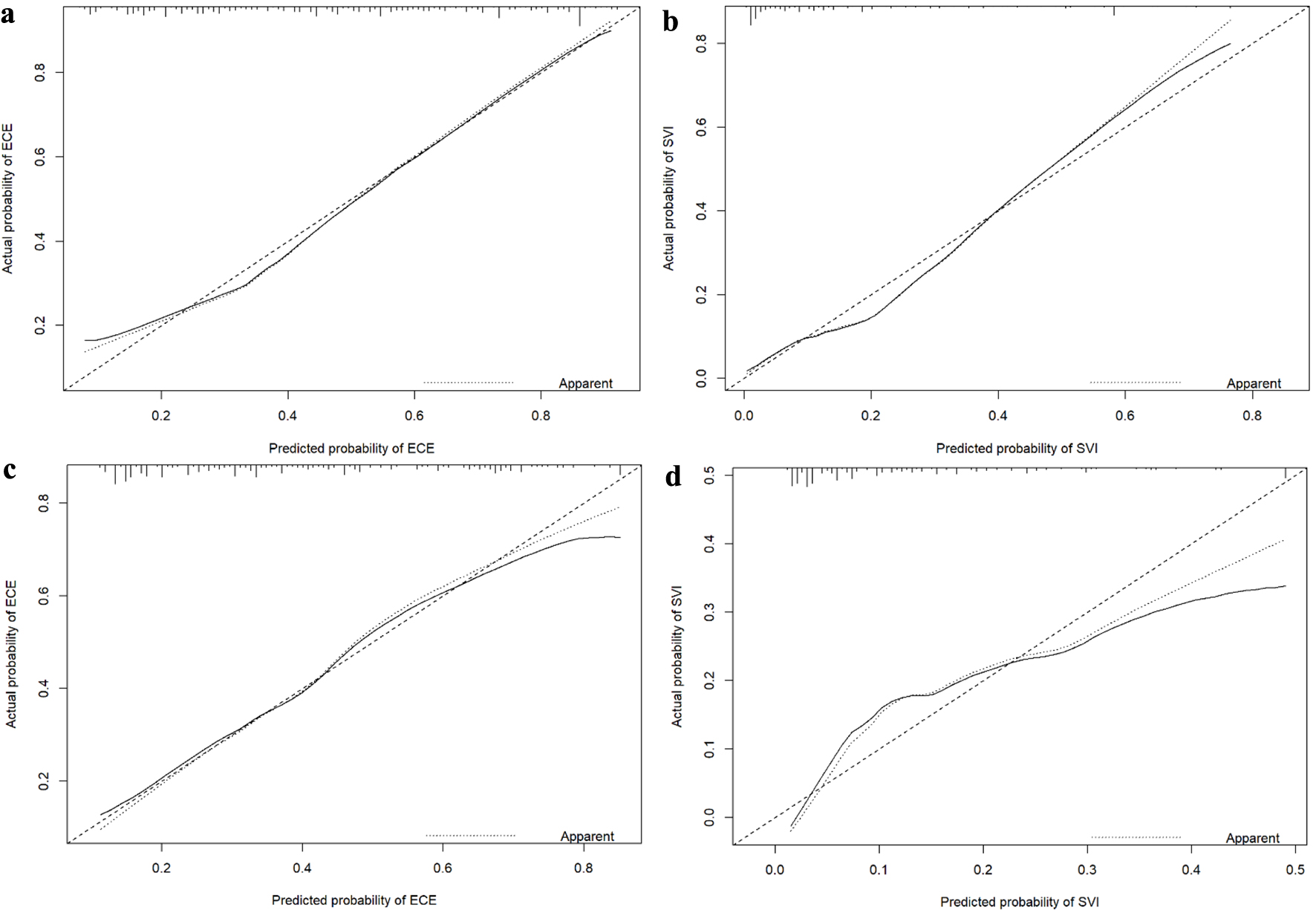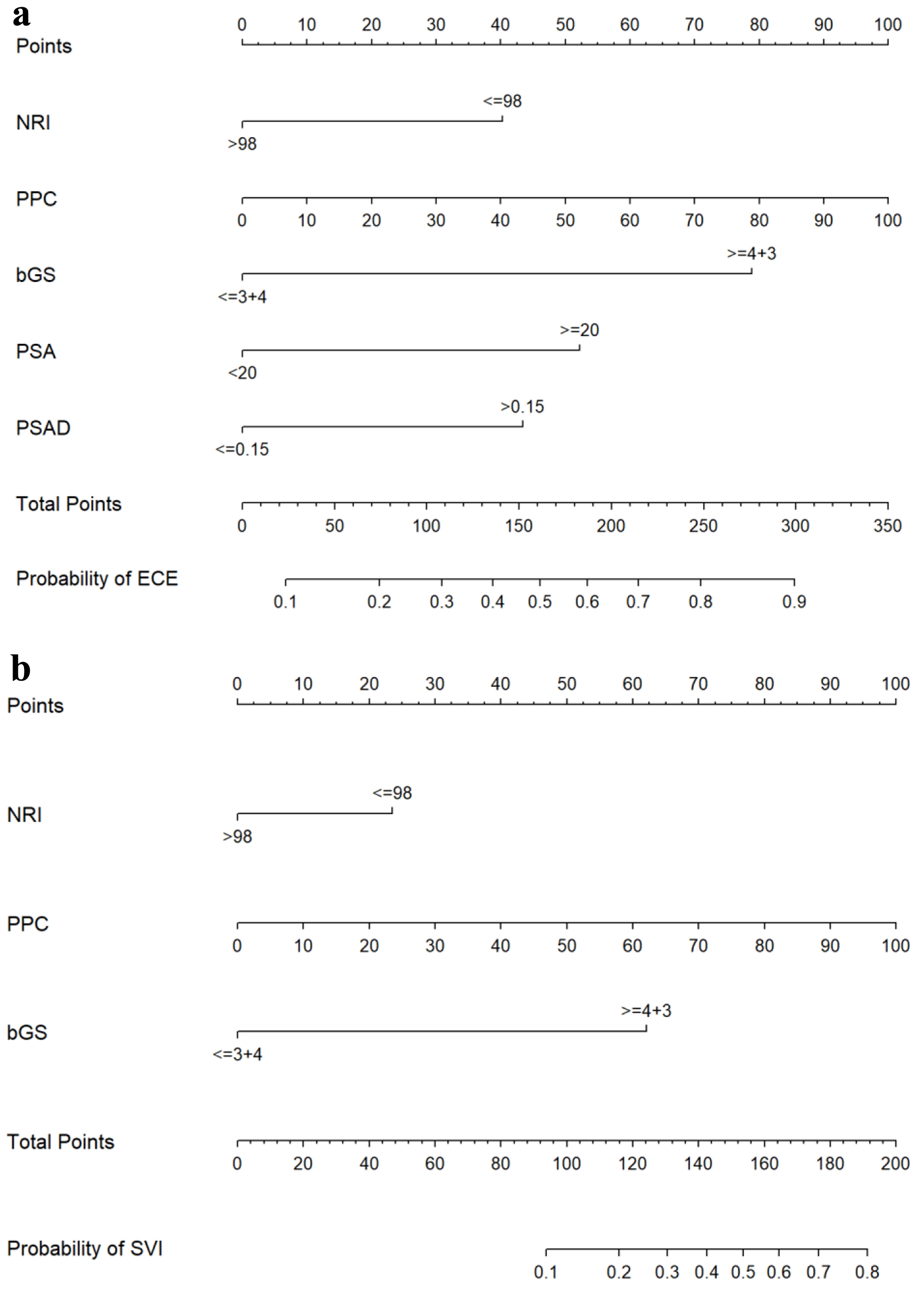
Figure 1. Nomograms for predicting ECE (a) and SVI (b). bGS: biopsy Gleason score; ECE: extracapsular extension; NRI: nutritional risk index; PPC: percentage of positive biopsy cores; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density; SVI: seminal vesicle invasion.