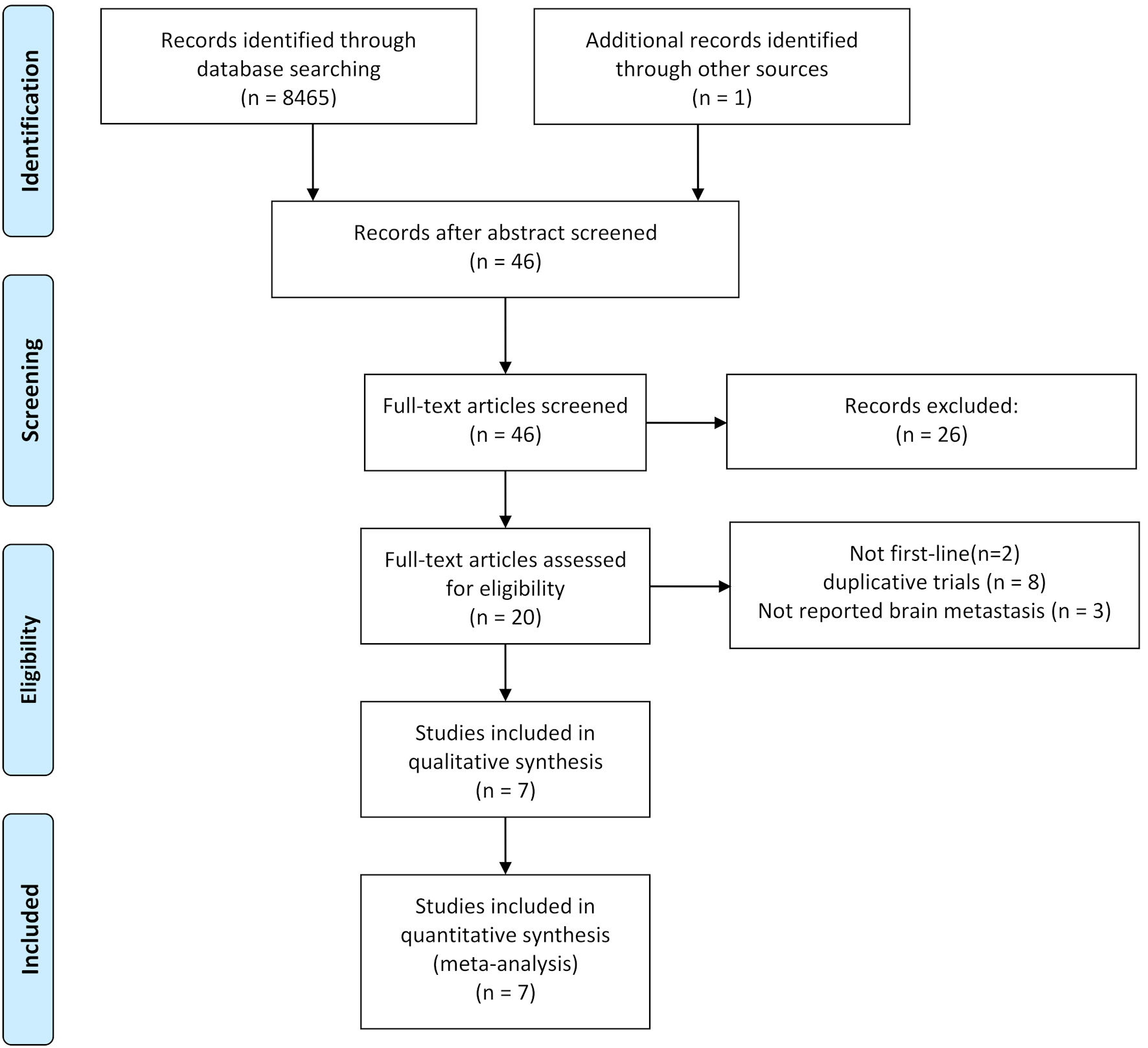
Figure 1. Flowchart illustrating trial selection.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 6, December 2023, pages 529-539
Efficacy of First-Line Immunotherapy Combined With Chemotherapy in Extensive-Stage Small Cell Lung Cancer Patients With Different Brain Metastases Status: A Systematic Review and Meta-Analysis
Figures

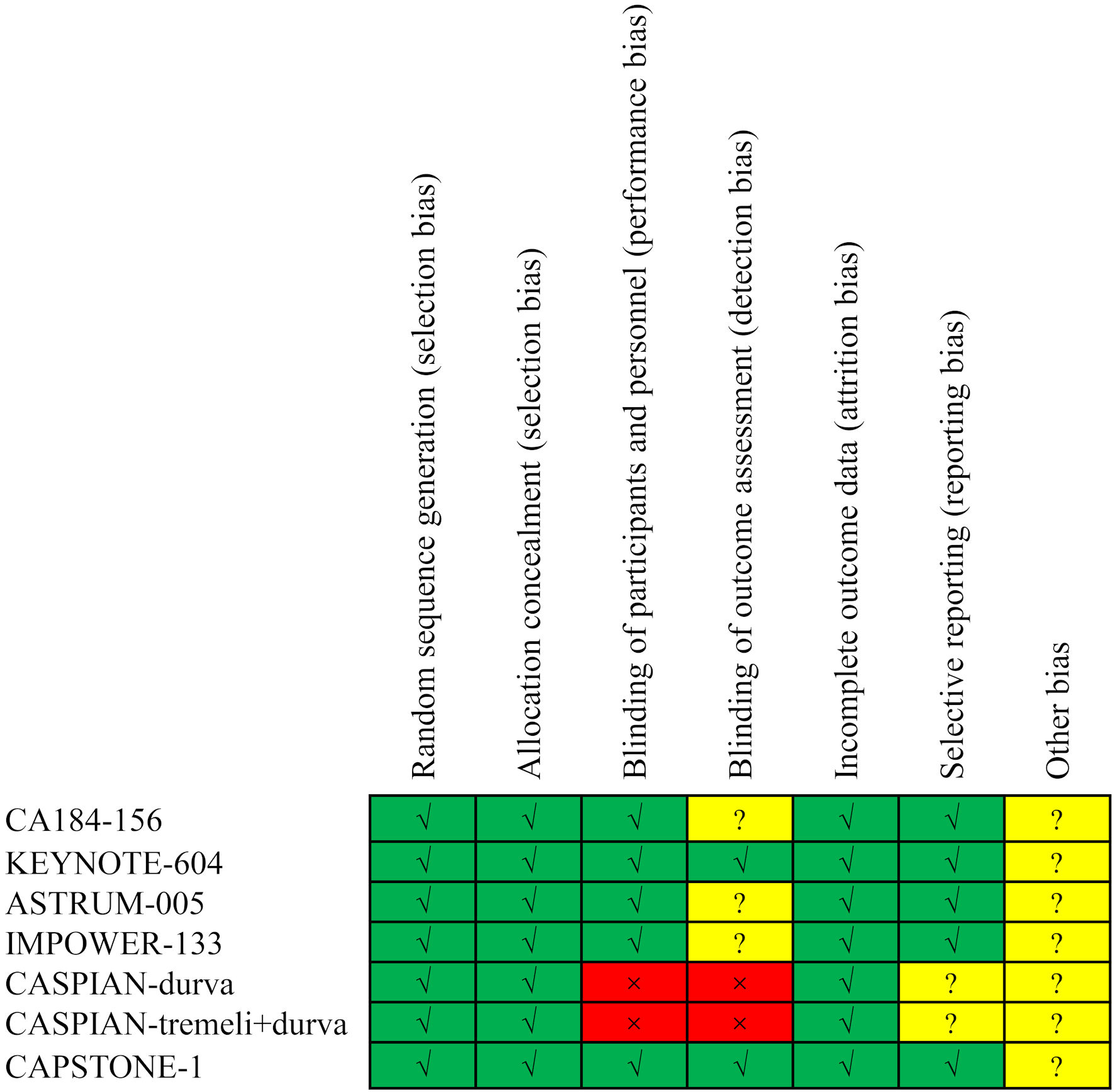
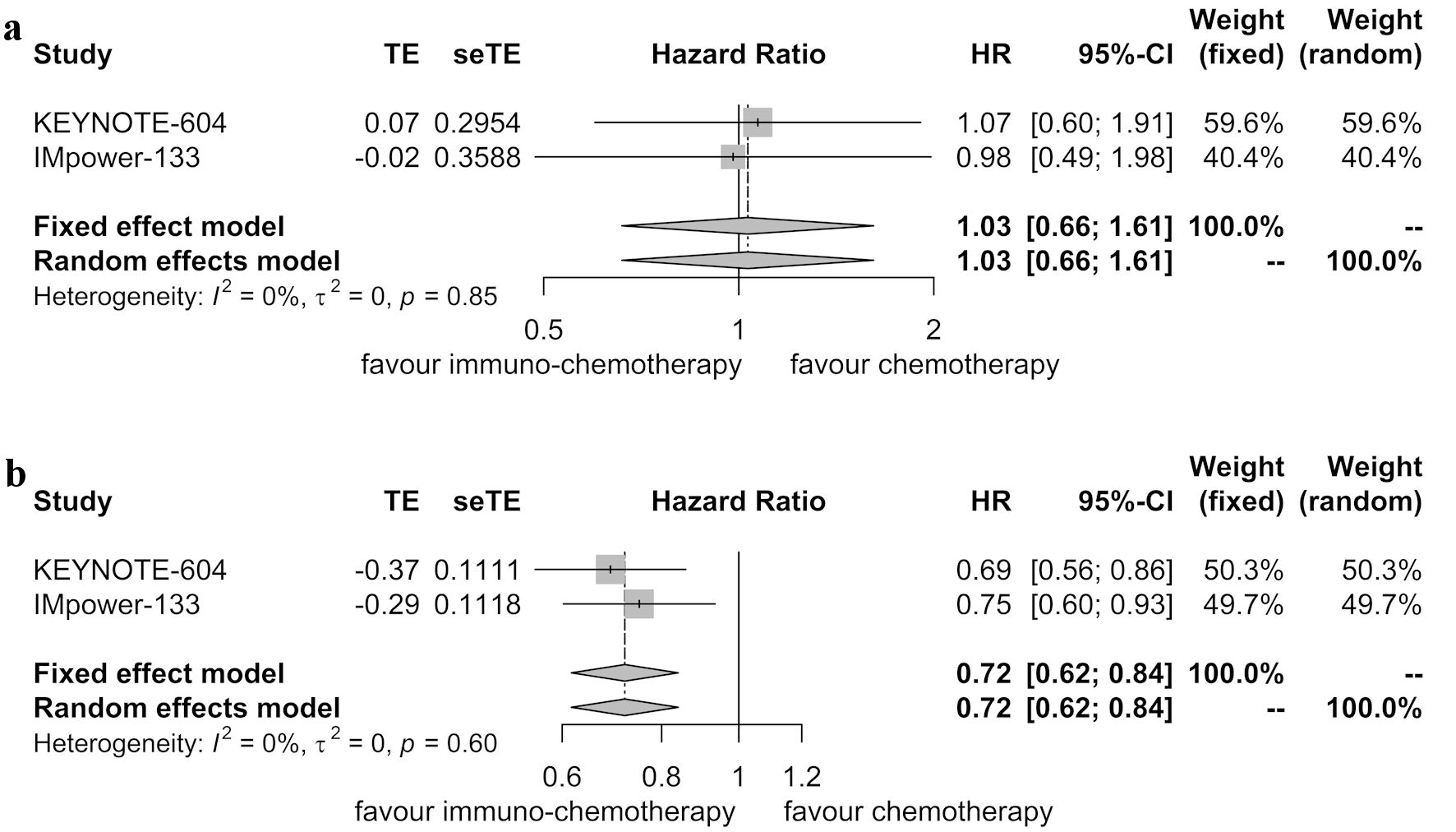
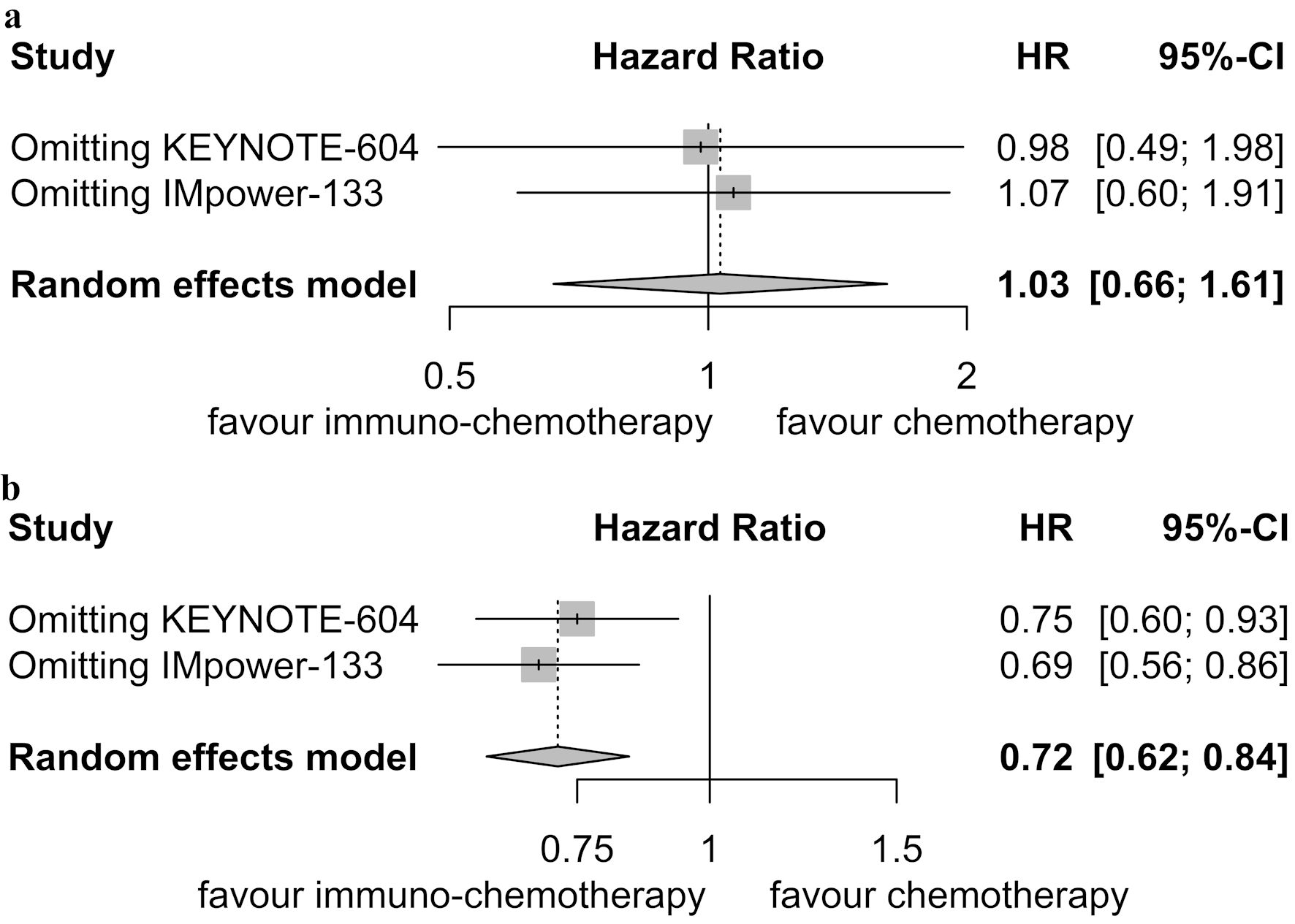
Table
| Trials | Year | Area | Phase | Treatments | Brain metastases | |
|---|---|---|---|---|---|---|
| Yes | No | |||||
| EP: etoposide + cisplatin; EC: etoposide + carboplatin; q3w: once every 3 weeks. | ||||||
| CA184-156 | 2016 | Worldwide | 3 | Ipilimumab q3w × 4 + EP/EC q3w × 4 | 55 | 423 |
| EP/EC q3w × 6 | 45 | 431 | ||||
| KEYNOTE-604 | 2020 | Worldwide | 3 | Pembrolizumab + EP/EC q3w × 4 | 33 | 195 |
| EP/EC q3w × 4 | 22 | 203 | ||||
| ASTRUM-005 | 2022 | Worldwide | 3 | Serplulimab + EC q3w × 4 | 50 | 339 |
| EC q3w × 4 | 28 | 168 | ||||
| IMpower-133 | 2018 | Worldwide | 3 | Atezolizumab + EC q3w × 4 | 17 | 184 |
| EC q3w × 4 | 18 | 184 | ||||
| CASPIAN-durva | 2019 | Worldwide | 3 | Durvalumab + EP/EC q3w × 6 | 28 | 240 |
| EP/EC q3w × 6 | 27 | 242 | ||||
| CASPIAN-tremeli + durva | 2020 | Worldwide | 3 | Durvalumab + tremelimumab + EP/EC q3w × 6 | 38 | 230 |
| EP/EC q3w × 6 | 27 | 242 | ||||
| CAPSTONE-1 | 2022 | China | 3 | Adebrelimab + EC q3w × 4 - 6 | 5 | 225 |
| EC q3w × 4 - 6 | 5 | 227 | ||||