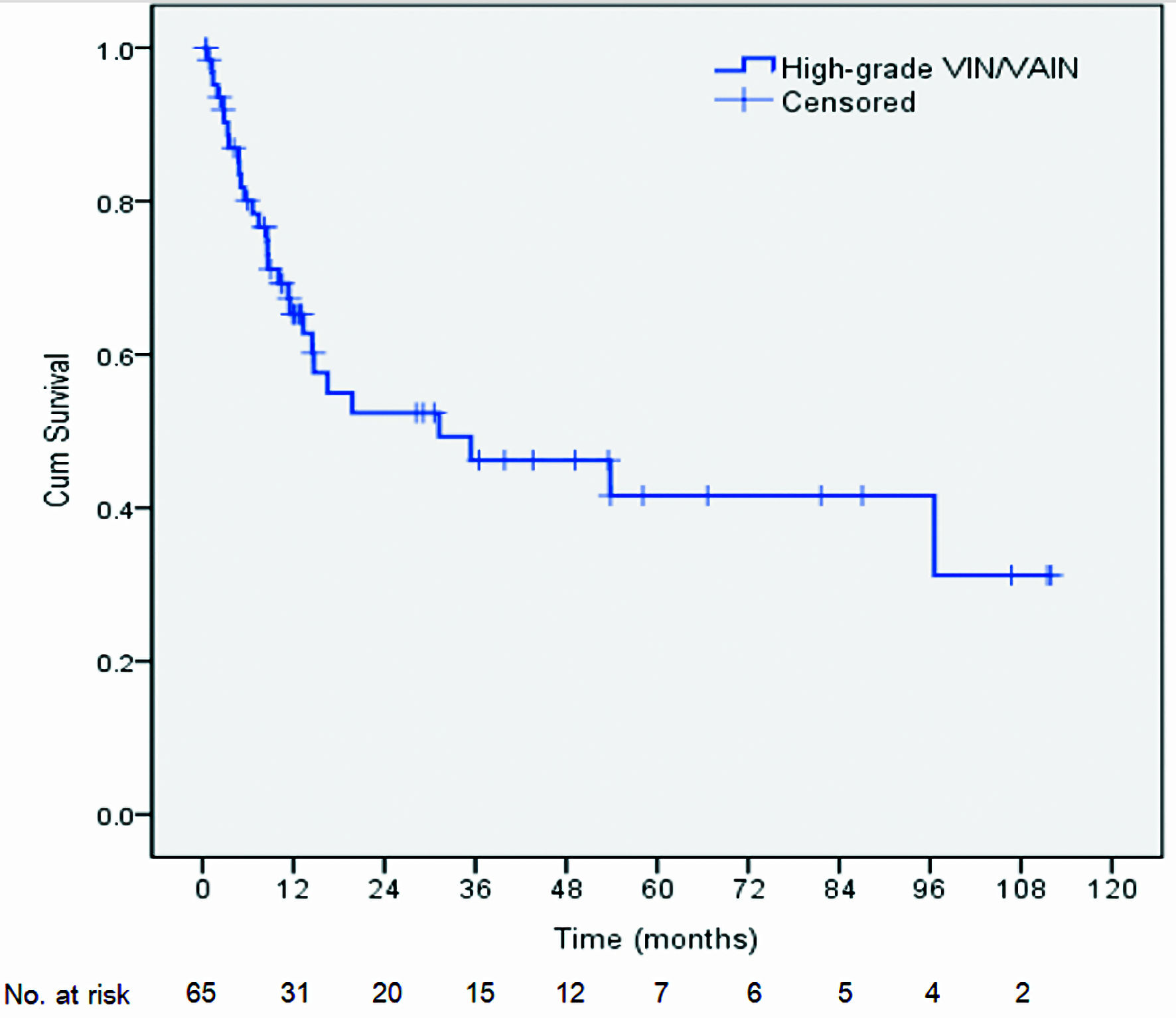
Figure 1. Kaplan-Meier life table analysis of recurrence-free survival in high-grade VIN/VAIN patients following laser vaporization. VIN: vulvar intraepithelial neoplasia; VAIN: vaginal intraepithelial neoplasia.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 1, February 2024, pages 90-99
Multicentricity and the Risk of Recurrence/Persistence After Laser Vaporization for High-Grade Vulvar and Vaginal Intraepithelial Neoplasia
Figures

Tables
| Characteristic | N (%) |
|---|---|
| aTwo patients were diagnosed with high-grade VAIN and high-grade VIN at different times. bPrevious or concurrent high-grade IN of the lower genital tract. cHigh-grade IN or worse occurring synchronously or sequentially. dAny grade. eCombination therapy was defined as laser plus additional treatment(s). Anti-HIV: antibody to human immune deficiency virus; LSIL: low-grade squamous intraepithelial lesion; ASC-H: atypical squamous cells, high-grade squamous intraepithelial lesion cannot be excluded; ASC-US: atypical squamous cells of undetermined significance; VAIN: vaginal intraepithelial neoplasia; VIN: vulvar intraepithelial neoplasia; VAIN2: vaginal intraepithelial neoplasia grade 2; VAIN3: vaginal intraepithelial neoplasia grade 3; VIN2: vulvar intraepithelial neoplasia grade 2; VIN3: vulvar intraepithelial neoplasia grade 3; SD: standard deviation; IN: intraepithelial neoplasia. | |
| Number of patients/number of laser-treated casesa | 63/65 |
| Age at diagnosis: mean ± SD (range) | 47.6 ± 12.2 (23 - 74) |
| Parity: median (range) | 2 (0 - 8) |
| Smoking | 1 (1.5) |
| Menopause | 41 (63.0) |
| Positive anti-HIV test | 9 (13.8) |
| Presenting symptoms | |
| Mass/lesion/rash/ulcer | 13 (19.6) |
| Itching | 2 (3.0) |
| Abnormal cytology as presentation | 47 (72.3) |
| LSIL/ASC-US | 10 (15.3) |
| HSIL/ASC-H | 33 (50.7) |
| Squamous cell carcinoma | 4 (6.1) |
| Previous or concurrent neoplasia of the lower genital tract | 44 (67.6) |
| Previous neoplasia | 37 (56.9) |
| Concurrent neoplasia | 7 (10.7) |
| Multicentric intraepithelial neoplasia of the lower genital tractb | 22 (33.8) |
| Metachronous | 17 (26.1) |
| Synchronous | 5 (7.6) |
| Previous or concurrent high-grade IN+ of the lower genital tract (multicentricity)c | 38 (58.4) |
| Metachronous | 32 (49.2) |
| Synchronous | 6 (9.23) |
| Previous or concurrent invasive cancer of the lower genital tract | 16 (24.6) |
| History of pelvic radiation | 9 (13.8) |
| History of hysterectomy | 38 (58.4) |
| Concurrent VAIN/VINd | 10 (15.3) |
| Concurrent high-grade VAIN/VIN | 5 (7.6) |
| Multiple lesions | 22 (33.8) |
| Number of lesions: median (range) | 1 (1 - 6) |
| Final histopathologic diagnosis | |
| VAIN2 | 7 (10.7) |
| VAIN3 | 44 (67.6) |
| VIN2 | 4 (6.1) |
| VIN3 | 15 (23.0) |
| Line of therapy | |
| Laser as first-line therapy | 59 (90.7) |
| Laser for recurrence after non-laser therapy | 6 (9.2) |
| No previous laser therapy | 56 (86.1) |
| Monotherapy or combination therapy | |
| Laser as monotherapy | 58 (89.2) |
| Laser as part of combination therapye | 7 (10.7) |
| RFS time | RFS rate (%) | 95% CI |
|---|---|---|
| CI: confidence interval. | ||
| 6 months | 80.1 | 69.9 - 90.2 |
| 1 year | 65.3 | 52.9 - 77.6 |
| 2 years | 52.4 | 38.0 - 66.7 |
| 3 years | 46.2 | 31.3 - 61.0 |
| 5 years | 41.6 | 25.7 - 57.4 |
| Factor | Number of cases of recurrence or persistence/total number of cases (%) | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | ||
| at-test. bFisher’s exact test. cPrevious or concurrent high-grade IN of the lower genital tract. dHigh-grade IN or worse occurring synchronously or sequentially. eIn the case of multicentricity, the final diagnosis was based on the disease that was most severe. fRecurrence after non-laser therapy. Anti-HIV: antibody to human immune deficiency virus; IN2: intraepithelial neoplasia grade 2; IN3: intraepithelial neoplasia grade 3; VAIN: vaginal intraepithelial neoplasia; VIN: vulvar intraepithelial neoplasia. | |||||||
| Age | 0.66a | ||||||
| Smoking | |||||||
| Yes | 0/1 (0) | - | - | 1.00b | |||
| No | 22/49 (44.9) | ||||||
| Menopause | |||||||
| Yes | 21/41 (51.2) | 2.10 | 0.73, 5.98 | 0.16 | |||
| No | 8/24 (33.3) | ||||||
| Anti-HIV test | |||||||
| Reactive | 5/9 (55.6) | 1.66 | 0.40, 6.87 | 0.49b | |||
| Non-reactive | 24/56 (42.9) | ||||||
| Previous or concurrent neoplasia of lower genital tract | |||||||
| Yes | 24/44 (54.5) | 3.84 | 1.19, 12.32 | 0.02 | |||
| No | 5/21 (23.8) | ||||||
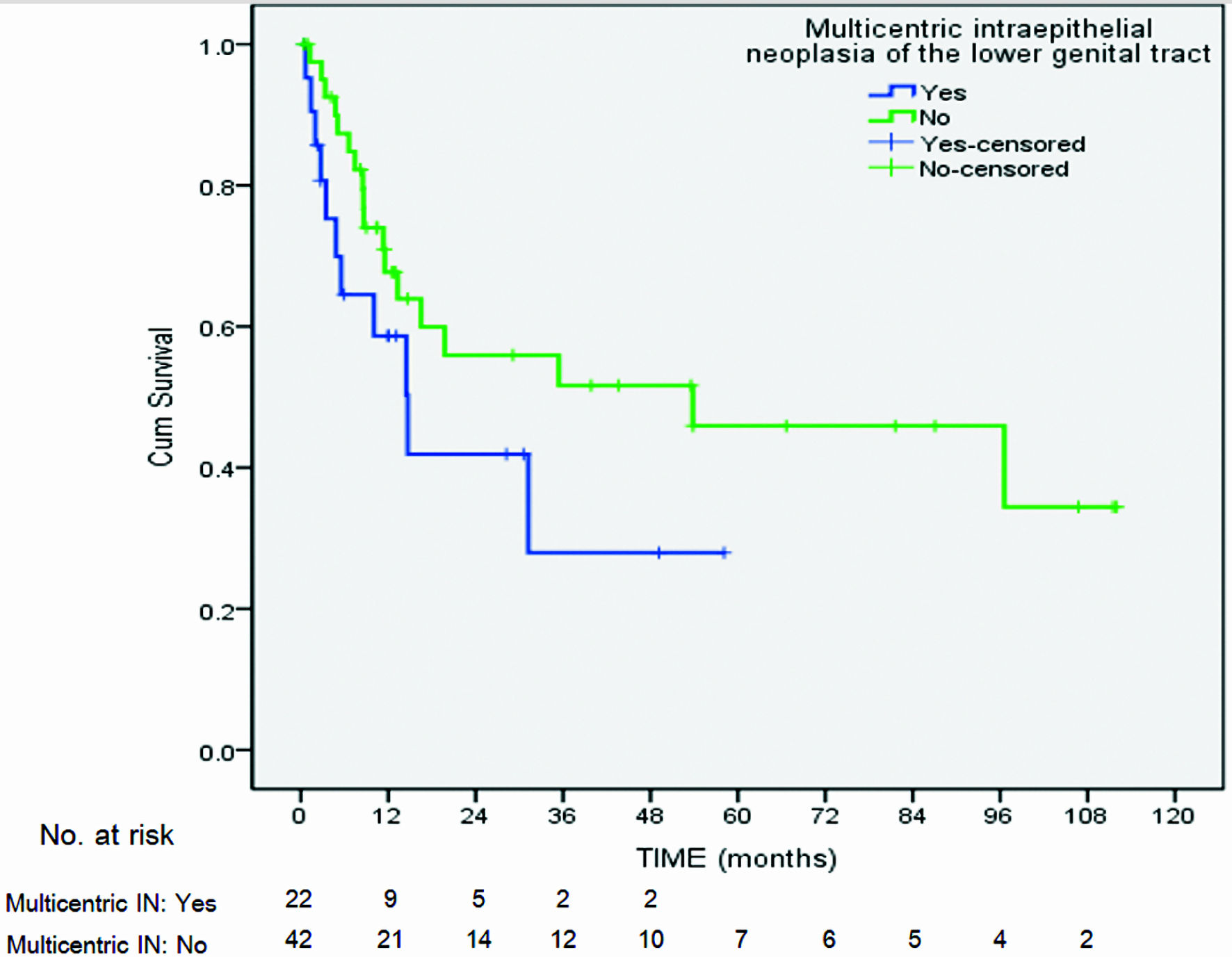
| Multicentric intraepithelial neoplasia of lower genital tractc | |||||||
| Yes | 11/22 (50) | 1.33 | 0.47, 3.75 | 0.58 | |||
| No | 18/42 (42.8) | ||||||
| Time to occur | |||||||
| Metachronous | 10/17 (58.8) | 5.71 | 0.52, 62.65 | 0.31b | |||
| Synchronous | 1/5 (20.0) | ||||||
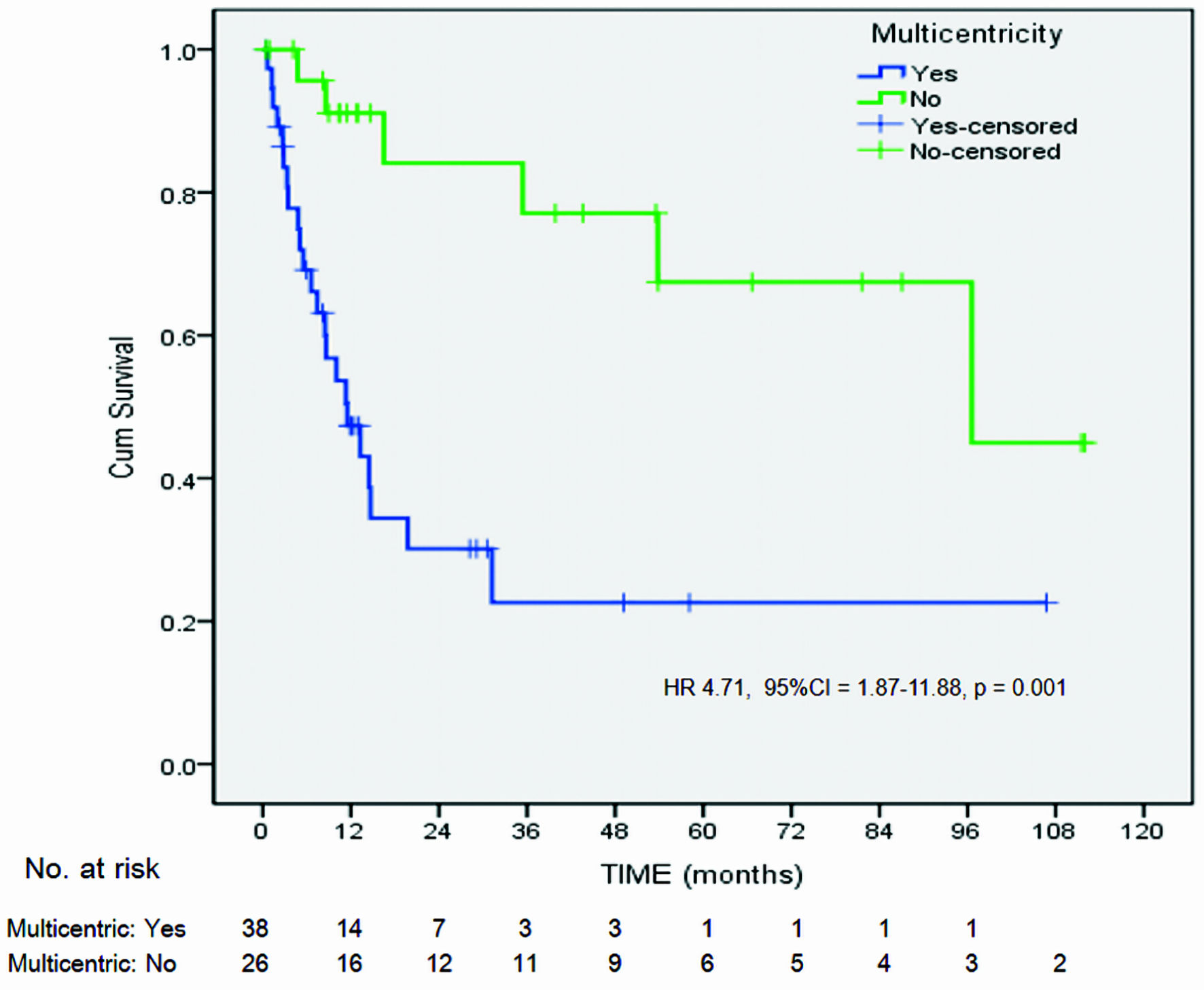
| Previous or concurrent high-grade IN+ of lower genital tract (multicentricity)d | |||||||
| Yes | 23/38 (60.5) | 5.11 | 1.66, 15.67 | 0.003 | 4.16 | 1.56, 11.06 | 0.004 |
| No | 6/26 (23.1) | ||||||
| Time to occur | |||||||
| Metachronous | 21/32 (65.6) | 3.81 | 0.60, 24.22 | 0.18b | |||
| Synchronous | 2/6 (33.3) | ||||||
| Previous or concurrent invasive cancer of lower genital tract | |||||||
| Yes | 12/16 (75.0) | 5.47 | 1.52, 19.61 | 0.009 | |||
| No | 17/48 (35.4) | ||||||
| History of pelvic radiation | |||||||
| Yes | 8/9 (88.9) | 13.33 | 1.55, 114.24 | 0.008b | 6.33 | 2.39, 16.72 | 0.000 |
| No | 21/56 (37.5) | ||||||
| Concurrent VAIN/VIN | |||||||
| Yes | 5/10 (50.0) | 1.29 | 0.33, 4.97 | 0.74b | |||
| No | 24/55 (43.6) | ||||||
| Multiple lesions | |||||||
| Yes | 10/22 (45.5) | 1.01 | 0.33, 3.03 | 0.98 | |||
| No | 14/31 (45.2) | ||||||
| Final histologic diagnosise | |||||||
| IN3 | 26/56 (46.4) | 1.73 | 0.39, 7.63 | 0.72b | |||
| IN2 | 3/9 (33.3) | ||||||
| Line of therapy | |||||||
| Laser for recurrencef | 6/6 (100.0) | 2.56 | 1.86, 3.53 | 0.006b | 5.34 | 2.00, 14.22 | 0.001 |
| Laser as first-line therapy | 23/59 (39.0) | ||||||
| Monotherapy or combination therapy | |||||||
| Laser as monotherapy | 25/58 (43.1) | 0.56 | 0.11, 2.77 | 0.69b | |||
| Laser as part of combination therapy | 4/7 (57.1) | ||||||