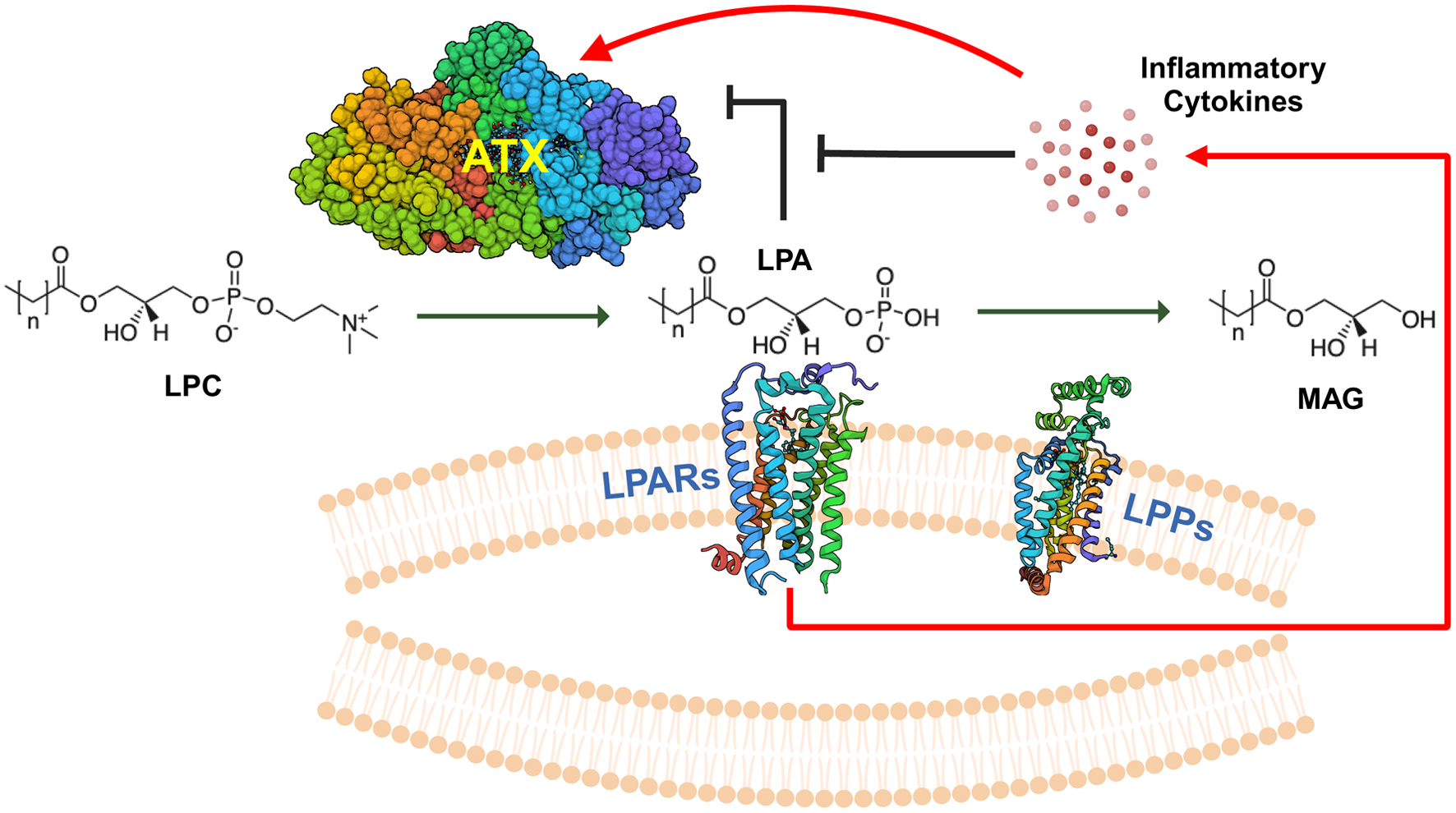
Figure 1. Overview of ATX-LPAR-LPP signaling. Autotaxin (ATX) is a secreted 125-kDa glycoprotein with lysophospholipase D activity, which generates lysophosphatidate (LPA) from lysophosphatidylcholine (LPC), the most abundant phospholipid in the plasma at concentrations of about 200 µM in human beings [32]. Plasma LPA levels are typically in the 100 - 300 nM range [33]. LPA signals through at least six G-protein coupled receptors (LPARs) to elicit intracellular effects. Signaling through these receptors may be either redundant or antagonistic, depending on the coupling between the receptor and the heterotrimeric G-protein [5, 34]. LPA is degraded by the ecto-activity of three lipid phosphate phosphatases (LPPs) [8]. Cancers can increase the tumorigenic effects of LPA signaling by increasing local concentrations of extracellular LPA through either increasing ATX secretion (either by the cancer cells themselves or induction in the tumor stroma) or by decreasing ecto-LPP activity levels, and by increasing LPAR levels [4]. LPA can exert feedback inhibition on ATX transcription to decrease further LPA production [29]. However, signaling mediated by inflammatory cytokines can increase ATX protein expression in the tumor microenvironment, which can overcome this feedback inhibition [29]. MAG: monoacylglycerols.
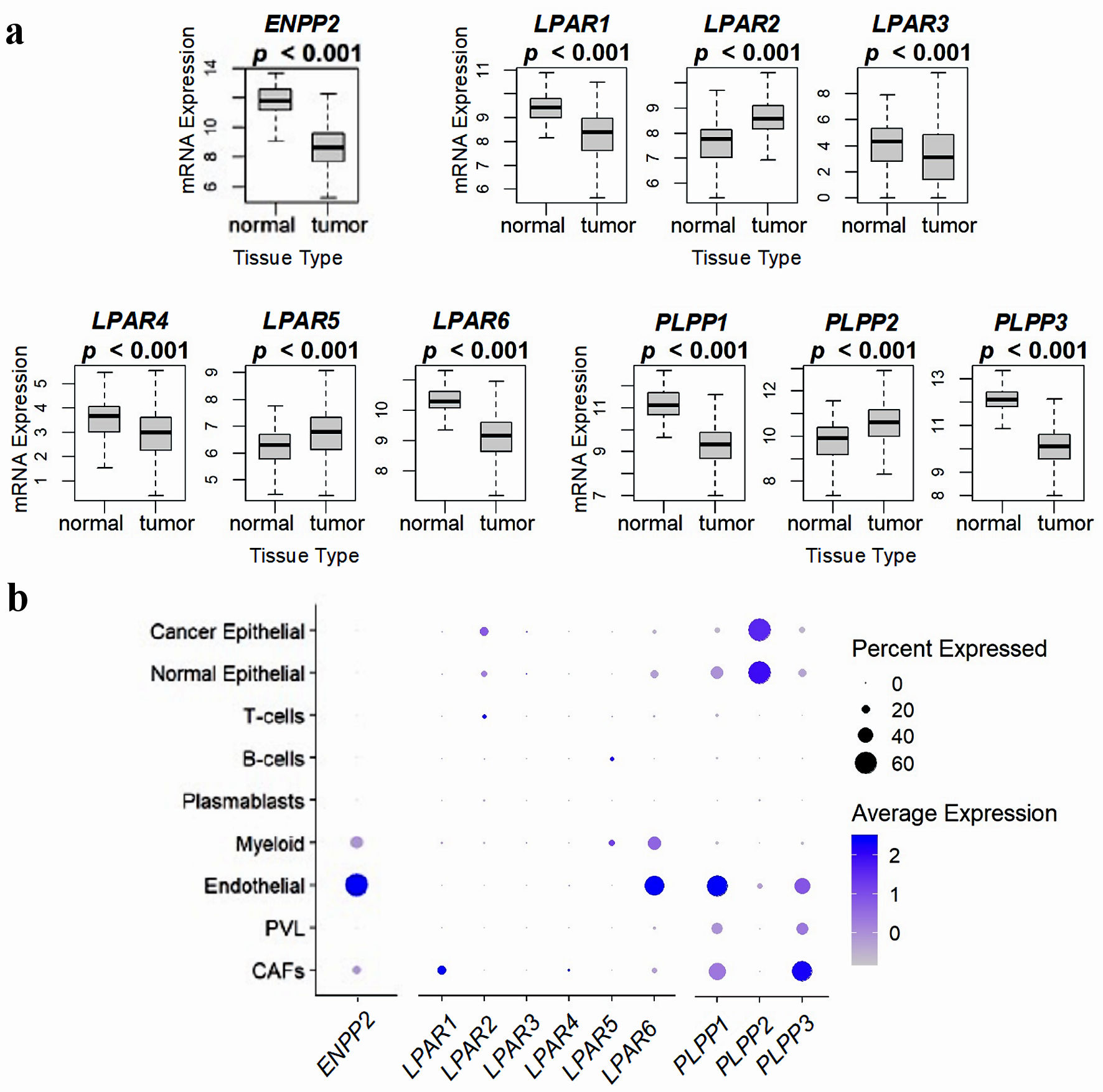
Figure 2. Comparison of ATX, LPARs, and LPPs expression in normal breast tissue and whole breast tumors, and via breast tumor single cell RNA sequencing. (a) mRNA expression from 114 normal breast tissues and 1,090 whole breast cancer tumors from the TCGA database. Results are plotted as box plots, with the bolded center bar representing the median, the lower and upper bounds the 25th and 75th percentiles, respectively, and the lower and upper tails the minimum and maximum values, respectively. (b) Single-cell RNA sequencing results from the cohort described in [42], comprised of 26 breast tumors (11 ER+ HER2-, five HER2+, and 10 TNBC), for a total of 130,246 single cells, to demonstrate which cell populations in the tumor express these genes. Percent expressed (circle size) refers to the percent of the total gene expression for the whole tumor by cell type, and color refers to the average gene expression. Figures were reproduced from [15, 40, 41] with permission. ATX: autotaxin; LPP: lipid phosphate phosphatase; ER: estrogen receptor; HER: human epidermal growth factor receptor; TNBC: triple-negative breast cancer.
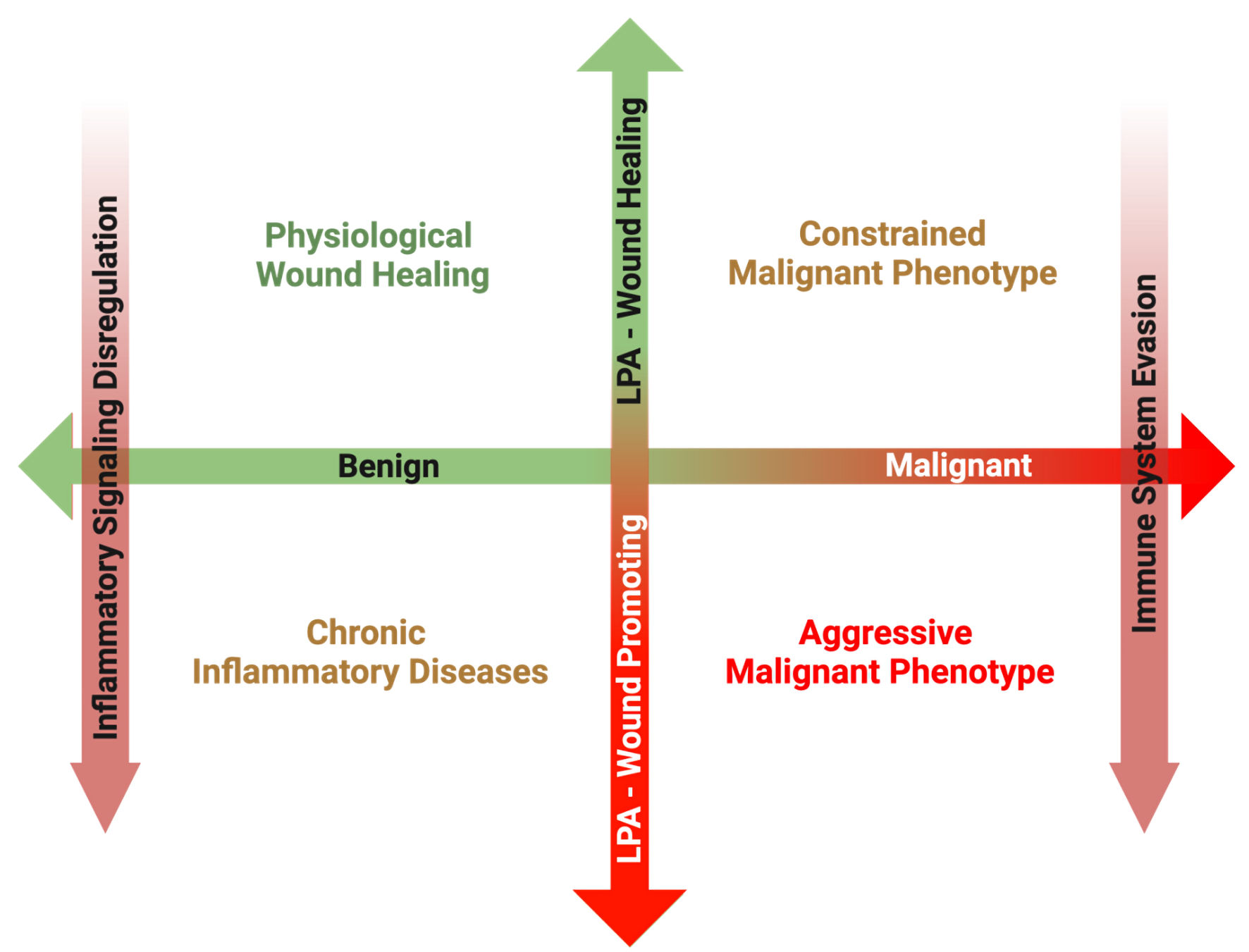
Figure 3. Overview of the proposed matrix between disease transformation and LPA subversion from a physiological wound healing mediator to a pathological wound promoting ligand. In the post-natal organism, a primary role of LPA signaling is to promote physiological wound healing processes in response to acute inflammation following tissue damage. Once the tissue is repaired, inflammation subsides, and increased LPA signaling is returned to basal levels. However, in the case of chronic inflammatory conditions, inflammatory pathways remain upregulated, resulting in increased ATX production and sustained LPA signaling. Some cancers can arise secondary to the sequalae of chronic inflammation, and in these cases, LPA signaling from tumor initiation perpetuates the chronicity of sustained inflammation, promoting tumorigenesis. However, during the early phases of some cancer initiation, LPA signaling from the surrounding host tissue can attempt to suppress development through its physiological wound healing mechanisms. In some cases, this LPA signaling is hijacked, and through a combination of inflammatory signaling dysfunction and immune system evasion, LPA signaling begins to mediate a pro-growth and pro-survival tumor microenvironment. LPA: lysophosphatidate; ATX: autotaxin.


