Figures
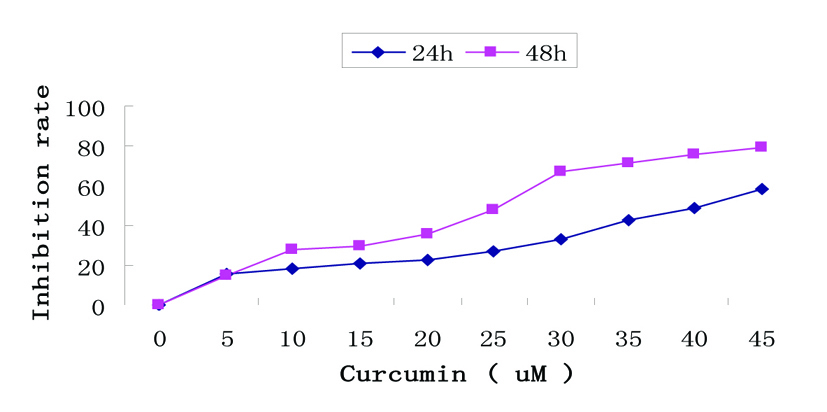
Figure 1. Growth inhibition by curcumin treatment in EJ cells. Dose- and time-dependent inhibitory effect of curcumin on EJ cells by MTT assay. Exponentially growing cells (8 × 104 cells/ml) were seeded in 96-well plates and incubated with the working curcumin solutions at final concentrations of 5 - 45 µM for 24h and 48h. Data are represented as the means ± SD of at least three independent experiments performed in duplicate.
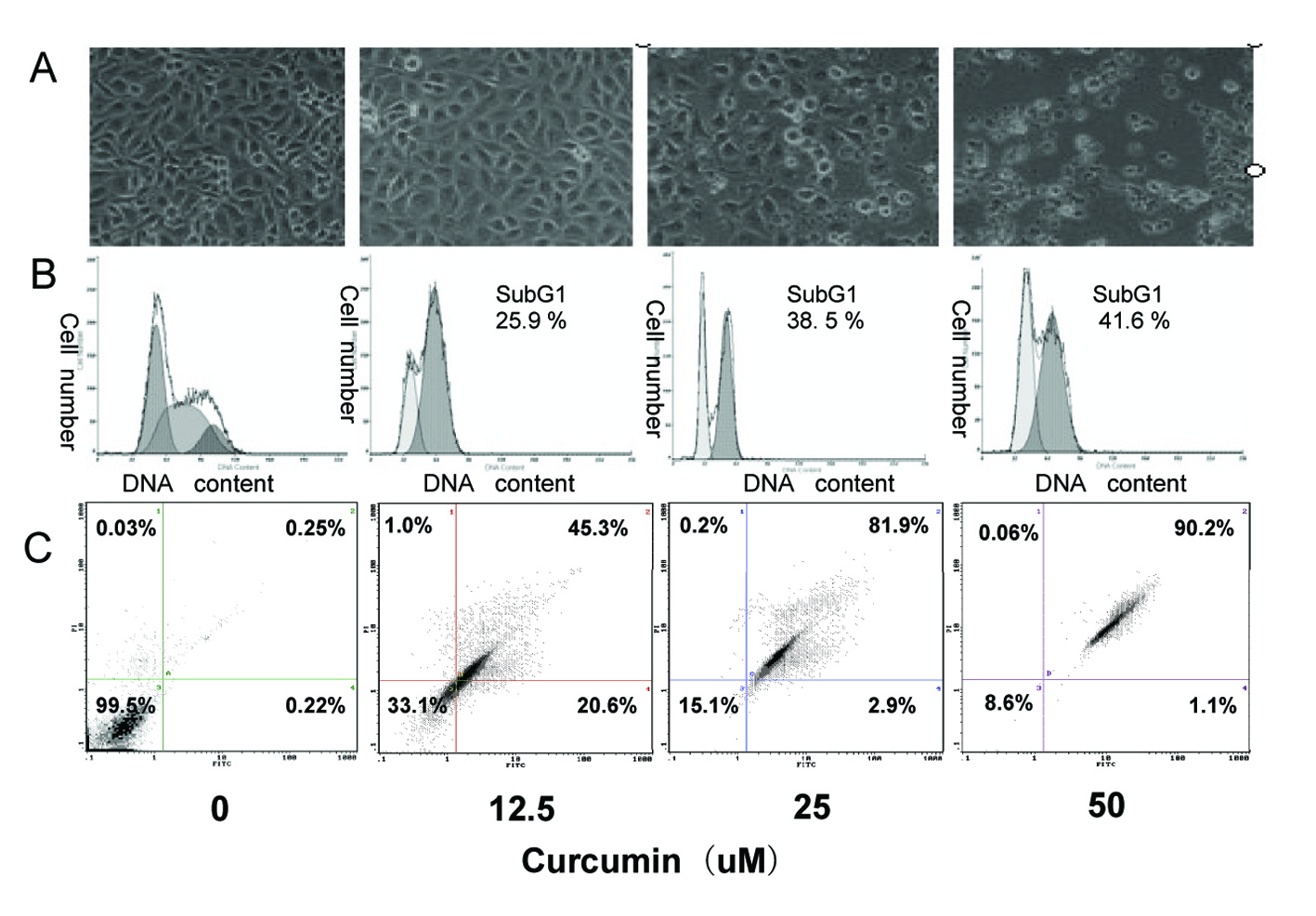
Figure 2. Induction of apoptosis in EJ cells by curcumin. EJ cells (2 × 106 cells/ml) were treated with various concentrations of curcumin (0, 12.5, 25 and 50 µM) for 24h. (A) Morphological changes of EJ cells by treatment with curcumin. After 24h incubation, morphological changes of EJ cells were observed under the inverted phase-contrast microscope and photographed. The data are representative examples for duplicate tests. Original magnification, x 200. (B) Effect of curcumin on cell DNA content in cultured EJ cells. Treated-cells were washed, fixed, stained with PI and analyzed for DNA content by flow cytometry. The histograms presented are representative of at least three separate experiments. (C) Flow cytometric analysis of annexin V-FITC stained EJ cells after treatment with curcumin. Overall cells were stained with Annexin V-FITC and analyzed by flow cytometer for apoptotic events. At least three independent experiments were performed in duplicate.
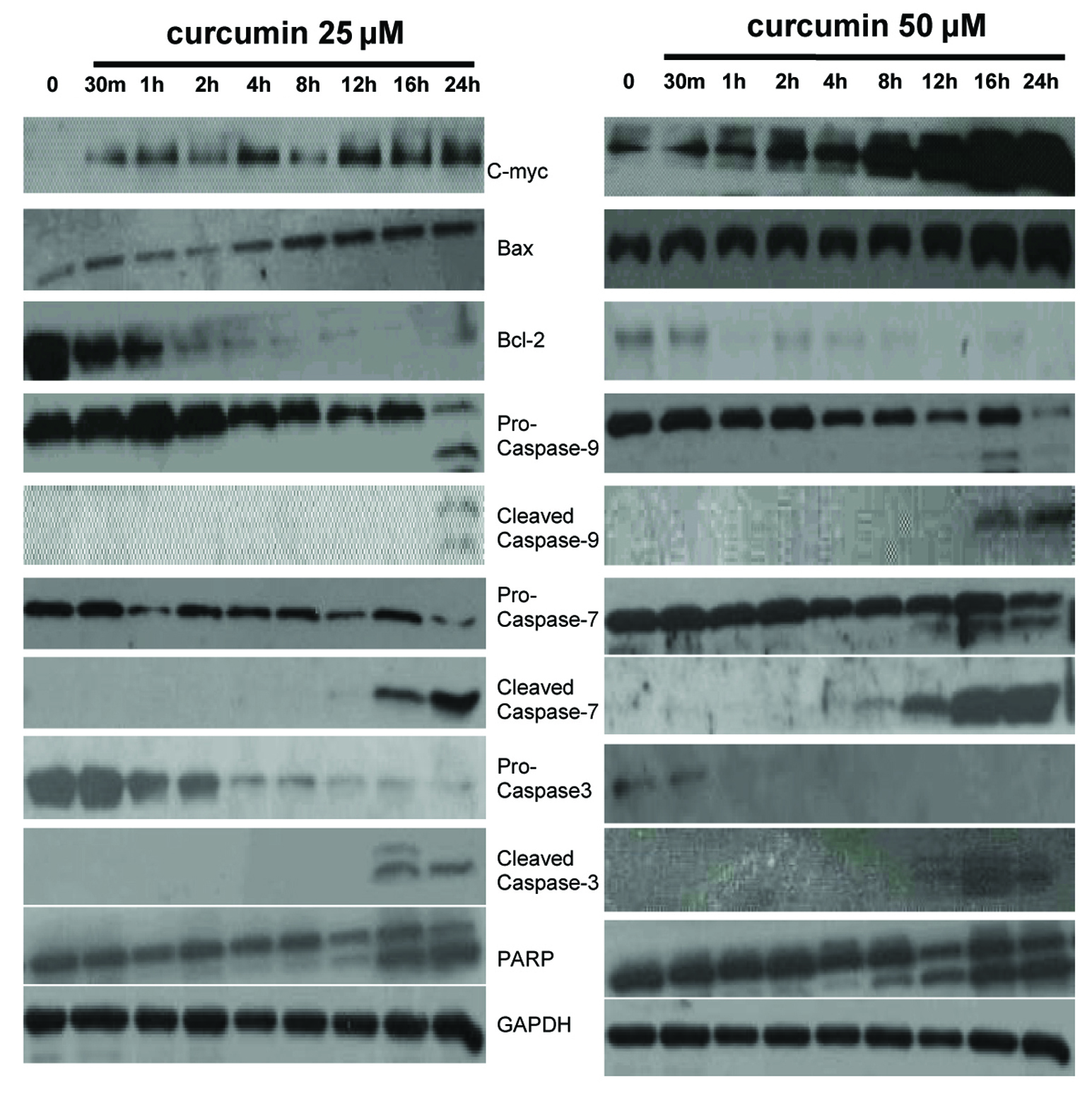
Figure 3. Expression of c-myc, Bcl-2 family members and intrinsic caspase cascade-related proteins in EJ cells. EJ cells (2 × 106 cells/ml) were cultured in the absence or presence of curcumin (25 and 50 µM) for indicated times and whole-cell extracts were prepared. Then, 50 mg of protein extracts were isolated on 10% SDS-PAGE gel and subjected to western blot analysis with antibody against c-myc, Bax, Bcl-2, pro-caspase-9, cleaved caspase-9, pro-caspase-7, cleaved caspase-7, pro-caspase-3, cleaved caspase-3 and PARP. GAPDH was examined as a loading control. Data are representative of three independent experiments with similar results.
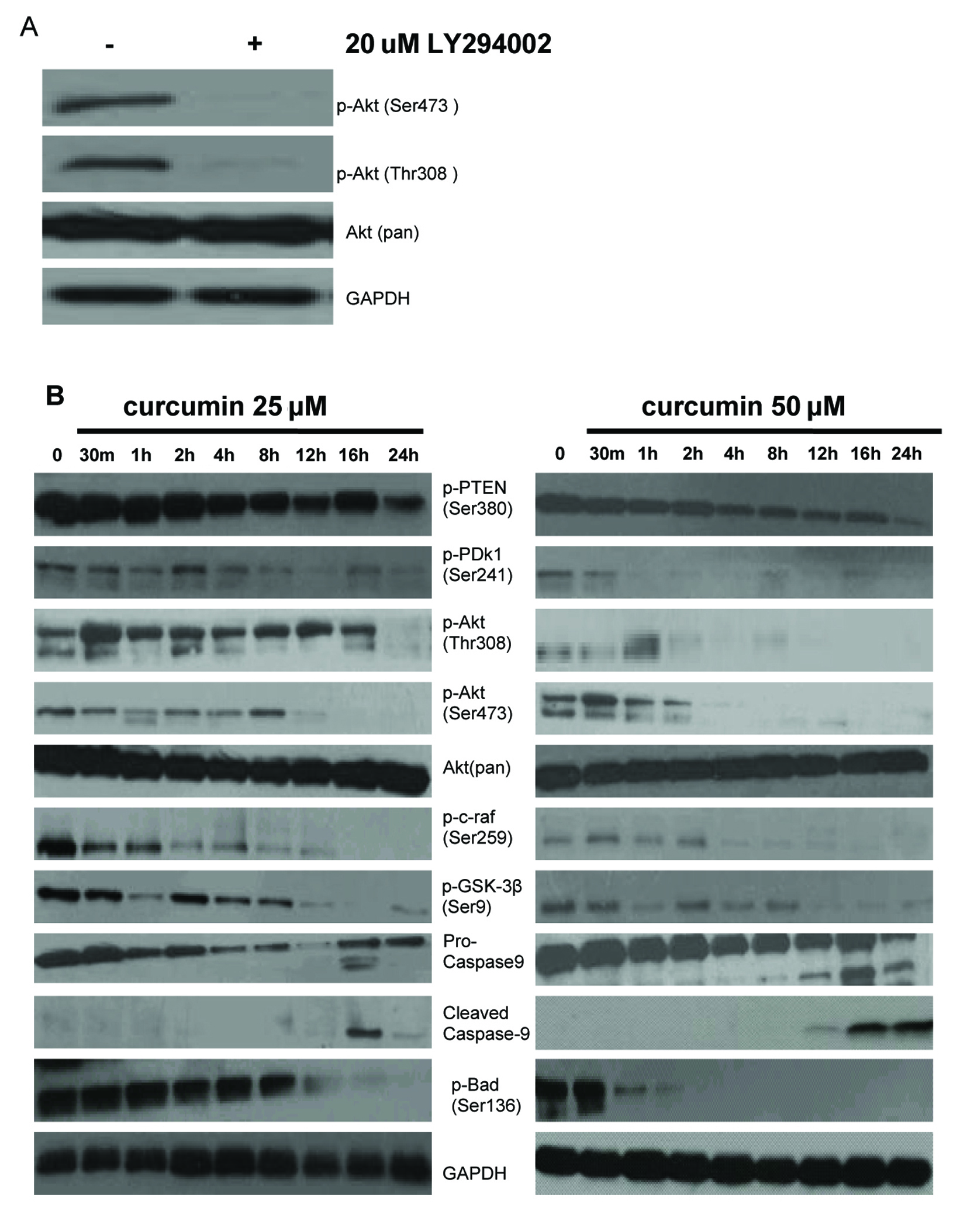
Figure 4. Effect of curcumin on PI-3K/Akt signaling pathway in EJ cells. (A) PI3K is required for constitutive Akt activation in EJ cells. EJ cells were treated with (+) or without (–) 20 µM LY294002 for 1h. Whole cell lysates were prepared and analyzed by western blot with antiphospho-Akt (Ser473), antiphospho-Akt (Thr308) and anti-Akt (Pan) antibodies. GAPDH was examined as a loading control. Data are representative of three independent experiments with similar results. (B) Effect of curcumin on the expressing of PI-3K/Akt signaling pathway proteins in EJ cells. EJ cells (2 × 106 cells/ml) were treated with or without curcumin (25, 50 µM) for the indicated times and the cellular protein was analyzed by 10% SDS-PAGE gel and western blotting with antibodies to p-PTEN (Ser380), p-PDk1 (Ser241), p-Akt (Thr308), p-Akt (Ser473), Akt (pan), p-c-raf (Ser259), p-GSK-3β (Ser9), pro-caspase-9, cleaved caspase-9, and p-Bad (Ser136). GAPDH was examined as a loading control. Data are representative of three independent experiments with similar results.



