Figures
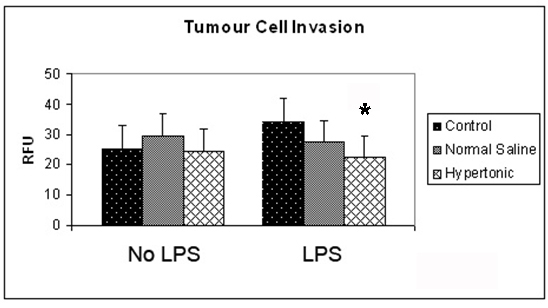
Figure 1. Effect of hypertonicity on tumor cell invasion. Tumor cells were exposed to hypertonic or isotonic conditions in the presence or absence of LPS before quantitative determination of tumor cell invasion at 12 hours. Data expressed as mean and are representative of 8 separate experiments. (*P < 0.05 versus isotonic culture medium and LPS)
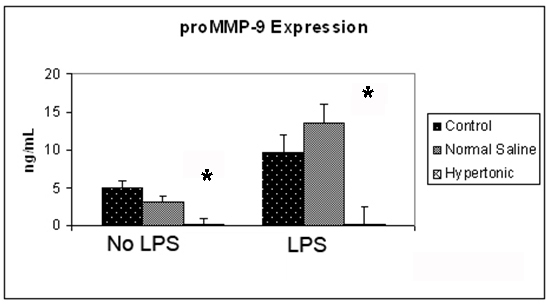
Figure 2. Effect of hypertonicity on tumor cell expression of proMMP-9. Tumor cells were exposed to hypertonic or isotonic conditions before quantitative determination of proMMP-9 expression at 12 hours. Data expressed representative of 8 separate experiments. *P < 0.05 versus isotonic culture medium.
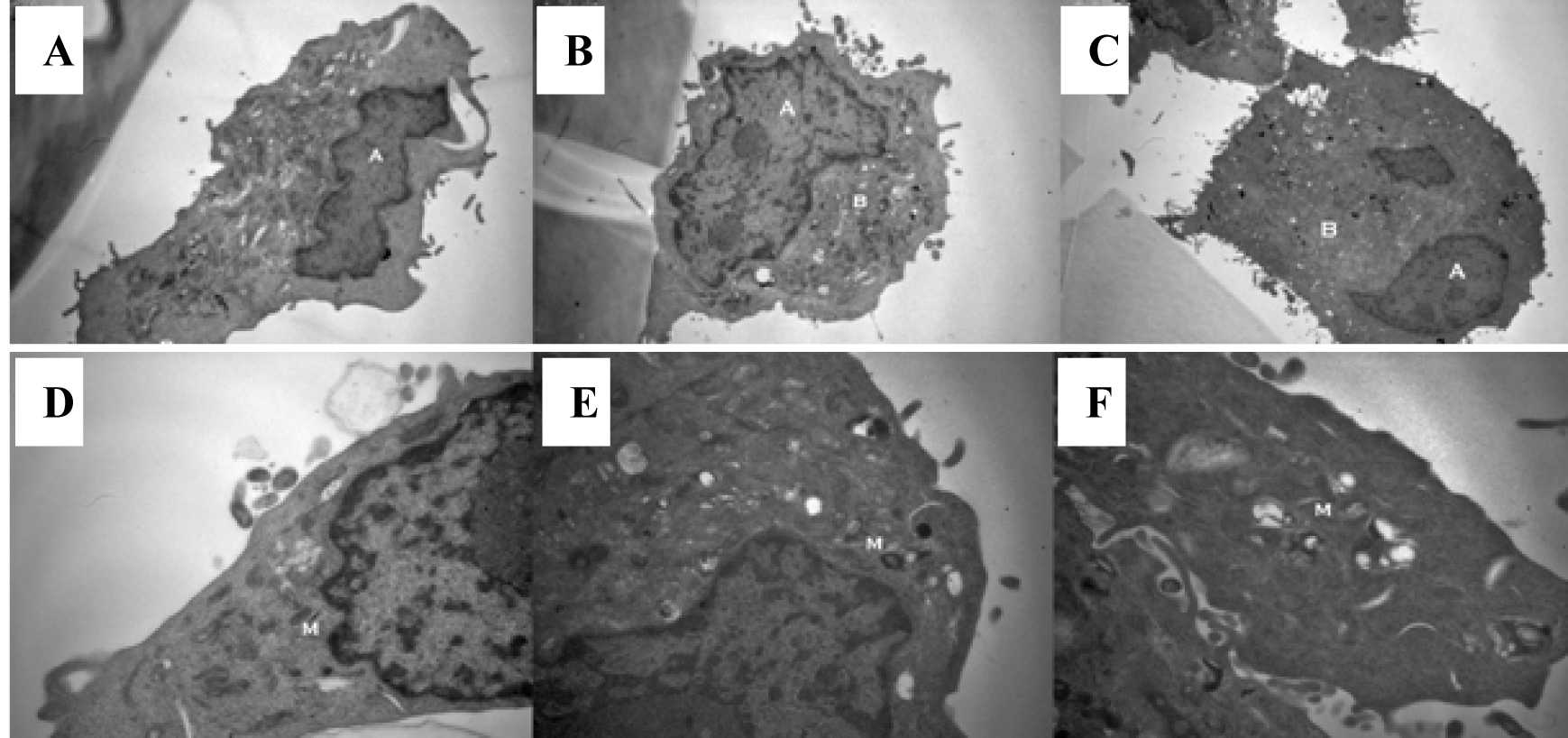
Figure 3. Exposure to a hypertonic environment (B) results in a reduced nuclear to cytoplasmic ratio, when compared to isotonic culture medium (A) or isotonic normal saline (C): magnification x 8000. Furthermore, a hypertonic environment results in a decrease in the electron density of cellular mitochondria (E) compared to those cultured in isotonic culture medium (D) or isotonic normal saline (F): magnification x 20 000.
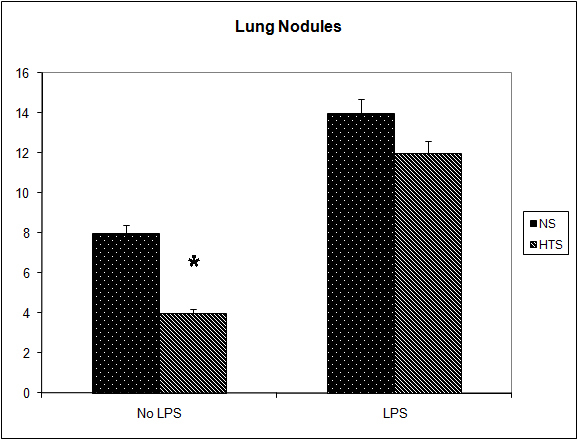
Figure 4. Effect of hypertonicity on lung nodules. Animals were injected with 5 x 104 4T1 tumor cells. Two minutes later mice received an intravenous bolus of 2 mL/kg normal saline (0.9% sodium chloride; NS) or 2 mL/kg hypertonic saline (7.5% sodium chloride; HTS) via lateral tail vein. Animals were sacrificed after 14 days and lung nodules counted. In a second experiment Effect of hypertonicity on lung/body weight ratio (g/kg) following LPS stimulation. Animals were injected with 5 x 104 4T1 tumor cells. Seven days later mice received an intraperitoneal (i.p.) injection of 10 µg lipopolysaccharide. Two minutes later the animals received either an intraperitoneal bolus of 2 mL/kg normal saline (0.9% sodium chloride; NS) or 2 mL/kg hypertonic saline (7.5% sodium chloride; HTS) via lateral tail vein. Animals were sacrificed after 14 days and lung nodules counted. Data are expressed as mean and are representative of n = 20 animals. *P < 0.05 versus NS control.
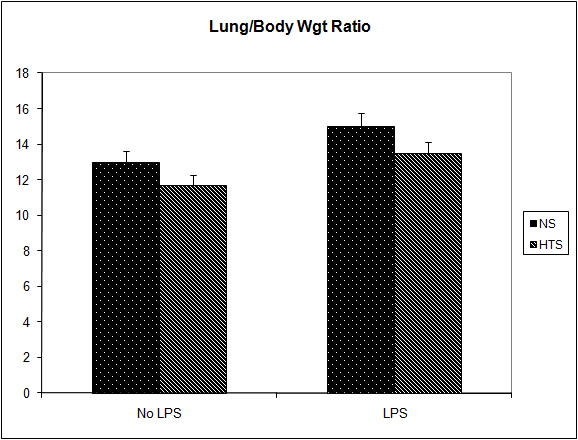
Figure 5. Effect of hypertonicity on lung/body weight ratio (g/kg). Animals were injected with 5 x 104 4T1 tumor cells. Two minutes later mice received an intravenous bolus of 2 ml/kg normal saline (0.9% sodium chloride; NS) or 2 mL/kg hypertonic saline (7.5% sodium chloride; HTS) via lateral tail vein. Animals were weighed and sacrificed after 14 days and lungs weighed to calculate lung/body weight ratio. In a second experiment to determine the effect of hypertonicity on lung/body weight ratio (g/kg) following LPS stimulation, animals were injected with 5 x 104 4T1 tumor cells. Seven days later mice received an intraperitoneal (i.p.) injection of 10 µg lipopolysaccharide. Two minutes later the animals received either an intraperitoneal bolus of 2 mL/kg normal saline (0.9% sodium chloride; NS) or 2 mL/kg hypertonic saline (7.5% sodium chloride; HTS) via lateral tail vein. 7 days later animals were weighed and sacrificed and their lungs weighed to calculate lung/body weight ratio. Data are expressed as mean and are representative of n = 20 animals. *P < 0.05 versus NS control.
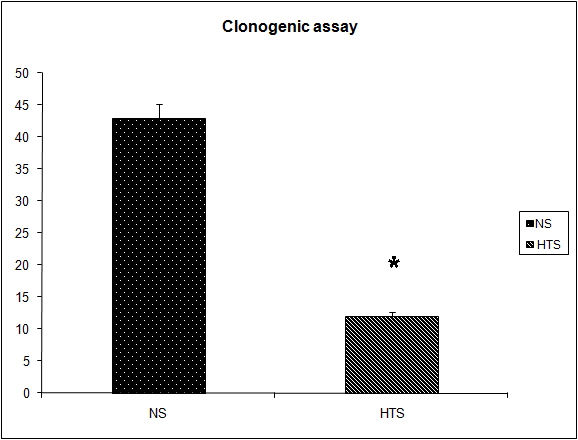
Figure 6. Effect of hypertonicity on clonogenic assay. Animals were injected with 5 x 104 4T1 tumor cells. Two minutes later mice received an intravenous bolus of 2 mL/kg normal saline (0.9% sodium chloride; NS) or 2 mL/kg hypertonic saline (7.5% sodium chloride; HTS) via lateral tail vein. Animals were sacrificed after 14 days and clonogenic assay performed. Data are expressed as mean and are representative of n = 20 animals. *P < 0.05 versus NS control.





