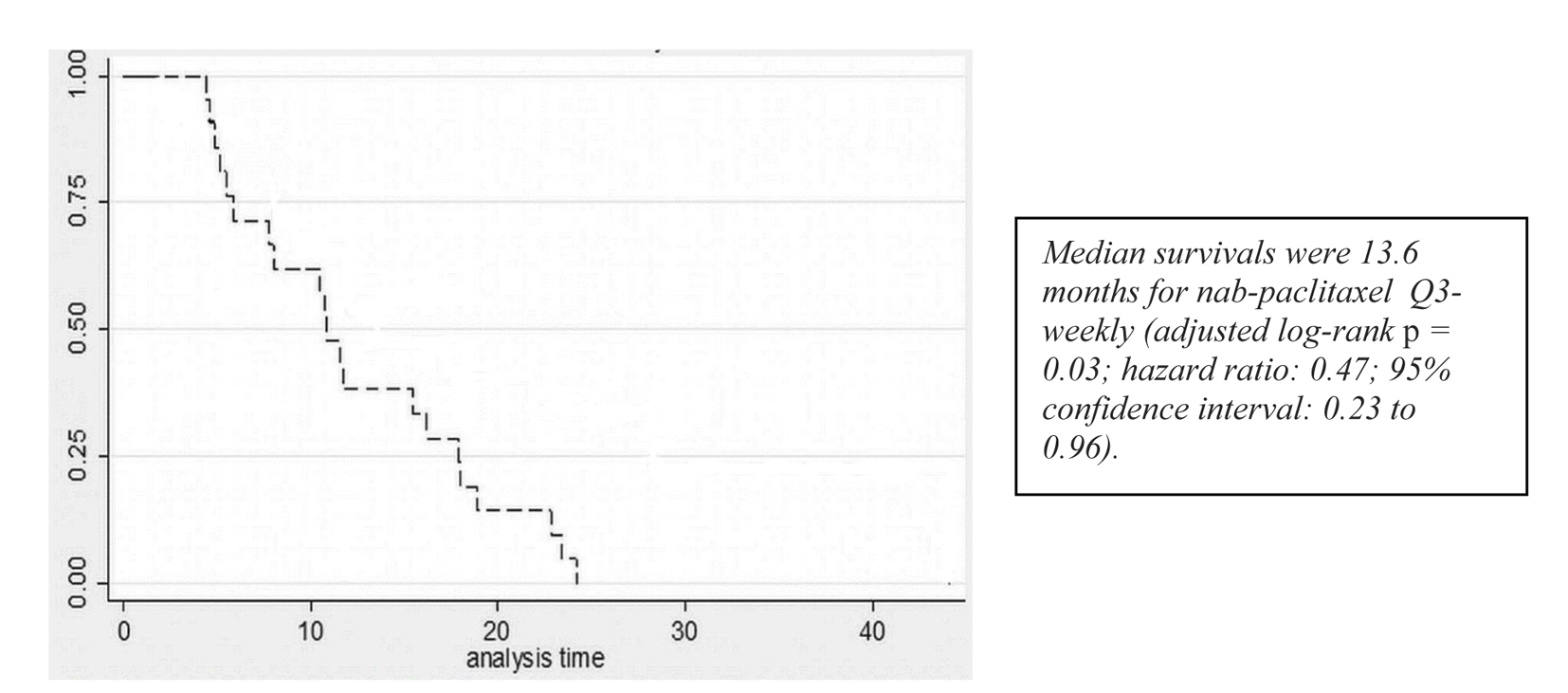
Figure 1. Survival curves for patients receiving q3w nab-paclitaxel.
| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 5, Number 5-6, December 2014, pages 204-209
A Retrospective Study of Efficacy and Safety of Albumin-Bound Paclitaxel in Metastatic Breast Cancer
Figures

Tables
| Variable value | |
|---|---|
| ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; OS: overall survival. | |
| Patients (n) | 14 |
| Age (years) | |
| Mean | 58.0 |
| Range | 39 - 70 |
| Receptor status (%) | |
| ER-positive | 71.42 |
| PR-positive | 57.14 |
| HER2-positive | 14.28 |
| Sites of metastasis (%) | |
| Bone only | 14.28 |
| Liver only | 7.14 |
| Lung only | 7.14 |
| Multiple sites | 71.44 |
| Current line of chemotherapy for advanced disease (median (range)) | 3 (1 - 6) |
| Previous taxane exposure (n (%)) | 10 (71.42) |
| Adjuvant | 2 (20) |
| Metastatic | 7 (70) |
| Adjuvant and metastatic | 1 (10) |
| Median duration (months) | 4.5 |
| Variable | Nab-paclitaxel schedule q3w (260 mg/m2) |
|---|---|
| Patients (n) | 14 |
| Overall response (%) | 43 |
| Complete | 7.14 |
| Partial | 35.71 |
| Development of neuropathy (%) | 35.7 |
| Overall survival (months) | |
| Median | 11.9 |
| Interquartile range | 7.7 - 21.8 |
| Variable | HR | 95% CI | P-value | Impact on survival |
|---|---|---|---|---|
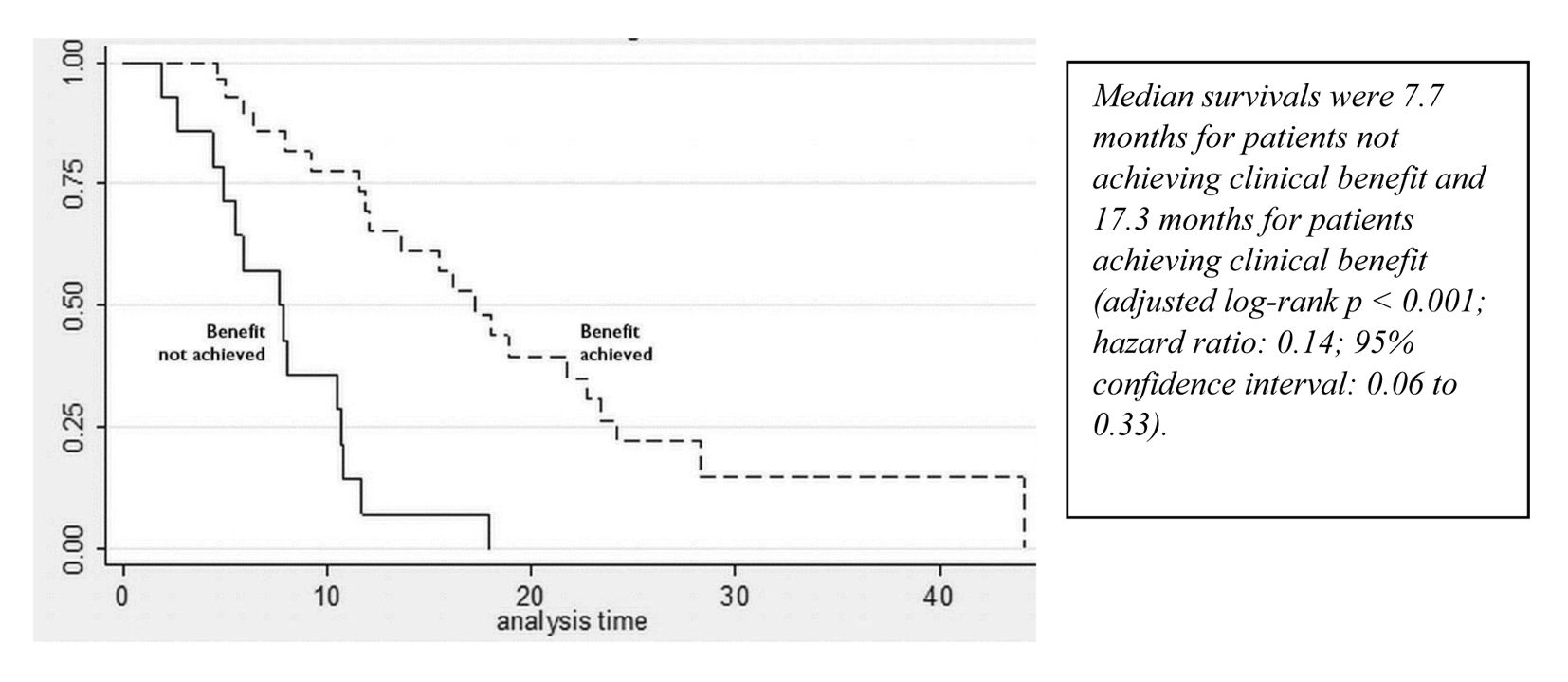
| Line of chemotherapy | 1.39 | 1.05 - 1.84 | 0.021 | ↑ Risk by 39% per line |
| Achievement of clinical benefit | 0.14 | 0.06 - 0.33 | 0.001 | ↓ Risk by 86% |