| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 10, Number 2, April 2019, pages 118-122
Vestibular Schwanomma: An Experience in a Developing World
Prakash Bahadur Thapaa, Sudha Shahia, Rajiv Kumar Jhab, Deependra Shresthaa
aDepartment of Otorhinolaryngology Head and Neck Surgery, National Academy of Medical Sciences, Bir Hospital, Kathmandu, Nepal
bDepartment of Neurosurgery, National Academy of Medical Sciences, Bir Hospital, Kathmandu, Nepal
cCorresponding author: Sudha Shahi, Department of Otorhinolaryngology Head and Neck Surgery, National Academy of Medical Sciences, Bir Hospital, Kathmandu 44601, Nepal
Manuscript submitted March 7, 2019, accepted April 5, 2019
Short title: Vestibular Schwanomma
doi: https://doi.org/10.14740/wjon1195
| Abstract | ▴Top |
Background: Tumors related to the acoustic nerves represent 90% of cerebellopontine angle diseases and have been in the picture for at least 200 years. Famous as acoustic neuromas and vestibular neuromas, these are usually benign, slow-growing tumors of Schwann cells of the myelin sheath. Surgery is the treatment of choice though some authors have suggested “wait and watch” policy. The aims of our study were to study the clinical presentation and management of the tumors, and to evaluate the perioperative outcomes of the surgery.
Methods: A retrospective review of the datasheet of 33 patients diagnosed with vestibular schwanomma who had undergone surgery from January 2014 to January 2017 was performed in National Academy of Medical Sciences, Bir Hospital, Kathmandu, Nepal. Analysis of the demographic data and perioperative outcomes was performed.
Results: Hearing loss was the main presenting symptom in 72% cases followed by tinnitus, dizziness, facial numbness and sudden sensorineural hearing loss. Mean tumur size was 39.7 ± 3 mm. The mean age of the patients was 46 ± 3 years with a female preponderance (1.2:1). In particular, the retrosigmoid route was preferred in all the cases since it was the most employed approach at our center and 63% of the tumors presented to us were grade 5. The surgical techniques allowed safe preservation of the facial function which was 93%. The hearing loss did not improve after the surgery in 94% while it worsened in 6% of cases. We did not find any significant relation between outcome and size, age, gender or laterality of the tumor (P > 0.05). There was no perioperative mortality.
Conclusions: The benign and slow-growing nature of vestibular schwanomma usually poses problems for the early diagnosis and treatment especially in a poor resource setting like ours. Likewise, there are very few studies so far done in the country regarding the incidence and management of the disease. Thus, this study might be helpful in providing insight into the occurrence of the disease in the present scenario and the need for much more studies in the future.
Keywords: Neuroma; Vestibular schwannoma; Hearing loss; Management
| Introduction | ▴Top |
Vestibular schwannomas (VSs) are also known as acoustic neuromas. It can be considered a misnomer since acoustic neuromas are neither acoustic in origin nor neuromas. These are basically benign tumors of vestibular division of eighth cranial nerve and arise from Schwann cells lining the vestibular branch of the latter. It is characterized by slow growth but it may be locally destructive causing erosion of the internal auditory canal (IAC) or compression of the fifth and seventh cranial nerves. Less often, the ninth and 10th cranial nerves alone or in various combinations might be involved [1, 2]. Vestibulocochlear nerve neoplasms account for approximately 90% of all conditions affecting the cerebellopontine angle. The incidence in Denmark has been found in an increasing trend with 7.8 to 12.4 cases/million/year all possibly due to better diagnostic modalities. However, the first observation of acoustic nerve tumor was made during an autopsy in 1777 by Eduard Sandifort, Professor of Anatomy at Leiden University. The etiology of VS is unknown. The growth of the tumor is variable. The percentage of growing tumors has been reported to vary from 30-90% which might determine the therapeutic approach [3]. Sometimes its resultant effects are stagnation or shrinkage. However, progressive growth in the cerebellopontine angle and large tumors might lead to compression of the brainstem or cerebellum leading to occlusion of the fourth ventricle and subsequent incarceration and hydrocephalus [2]. Hearing loss (HL) is the most common symptom associated with tinnitus and vertigo. Computed tomography (CT) and magnetic resonance imaging (MRI) have become more affordable and are now considered to be the diagnostic method of choice in patients suspected of having VS [4, 5]. Surgery is the treatment of choice with various approaches. Retrosigmoid approach is routinely practiced at our center. Some authors have suggested “wait and watch” policy for small tumors and in the elderly [6]. There are very few studies done in our part of the world [7, 8]. The aim of our study was to evaluate the clinical presentation and management and the perioperative outcomes of the surgery. With this study, we believe that it might add up to the literature on VS management in our context.
| Materials and Methods | ▴Top |
The study was a retrospective observational study with ethical clearance. It was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. We included all the patients who had been diagnosed with Vestibular Schwanomma between January 2014 and January 2017 and were managed by a combined team of surgeons that included a team of Otorhinolaryngologists, head and neck surgeons and Neurosurgeons at National Academy of Medical Sciences, Bir Hospital, Kathmandu,Nepal. A careful retrospective review of datasheet was done. The patients were subjected to clinical history and physical examination along with the following tests at our center: pure tone audiometry, and MRI of the head with gadolinium scan. Definitive diagnosis was made on the basis of clinical presentation and findings in MRI gadolinium scan. Demographic and geographical classification of the patients was done along with additional details like chief complaint, laterality of the tumor, duration, tumor size, other signs and symptoms (HL, vertigo or dizziness, tinnitus, facial paresthesis, intracranial symptoms). Duration of the identifiable clinical symptoms was categorized as 5 - 10 years, 10 - 15 years, 15 - 20 years, and more than 20 years. Audiometric findings were classified after performing pure tone audiometry for the frequencies 250, 500, 1,000, 2,000, 4,000 and 6,000 Hz: profound HL: > 90 dB HL, severe HL: > 70 dB HL, moderate HL: 51 - 70 dB HL, mild HL: 26 - 50 dB HL, and normal HL: < 25 dB HL.
Tumor size was classified into five grades based on MRI findings [9] followed by standard surgical procedures. Preservation of hearing was emphasized in all patients who had a serviceable hearing, which has been defined as grade A or B by the American Academy of Otolaryngology-Head and Neck Surgery Committee on Hearing and Equilibrium seven guidelines: pure tone threshold less than 50 dB and speech discrimination score greater than 50%. Tumor size was measured according to the New and Modified Reporting Systems from the Consensus Meeting on Systems for Reporting Results in Vestibular Schwannoma classification of five grades [10]: grade 1, tumor size 1 - 10 mm; grade 2, tumor size 11 - 20 mm; grade 3, tumor size 21 - 30 mm; grade 4, tumor size 31 - 40 mm; grade 5, > 40 mm. Intraoperative findings were recorded. Postoperative complications were analyzed as immediate (days) and delayed (months). Facial nerve function was evaluated using the House-Brackmann grading system, during the immediate postoperative period and 3-month, 6-month and 12-month follow-up. Other symptoms like headache, vertigo, unsteadiness, and hearing status were also analyzed during follow-up. All of the continuous variables were calculated as mean and standard deviation (SD) which were compared with independent t-test or Mann-Whitney test according to the type of distribution. For categorical value, Chi-square test or Fisher’s exact test was used as feasible. Statistical software SPSS version 25.0 (Statistical Package for the Social Sciences) was used for statistical analysis. P value < 0.05 was considered statistically significant.
| Results | ▴Top |
A cohort of 30 patients, summarized in the study, had been followed retrospectively between January 2014 and January 2017 for a median follow-up of 12 months. Three (9%) were lost to follow-up and were excluded. The duration of disease was found to be 5 - 10 years in 9/33 (27%) patients, 10 - 15 years in 21/33 (63%), and 15 - 20 years in 3/33 (9%). There was a slight female preponderance 1.2:1 (18:15). Age distribution was: 3 (9%) of 21 - 30 years; 5 (15%) of 31 - 40 years; 18 (54%) of 41 - 50 years; and 7 (21%) of 51 - 60 years. The mean age was 46 ± 3 years. Laterality of tumor was determined based on CT/MRI and was found more in the right side in 16/33 (48%), left side in 14/33 (43%) and the central in 3/33 (9%) patients. Clinical presentation included symptoms like gradual HL in 24/33 (72%) patients, tinnitus in 17/33 (51%), dizziness in 7/33 (21%), facial nerve paresthesia in 3/33 (9%) and sudden SNHL in 4/33 (12%) (Fig. 1).
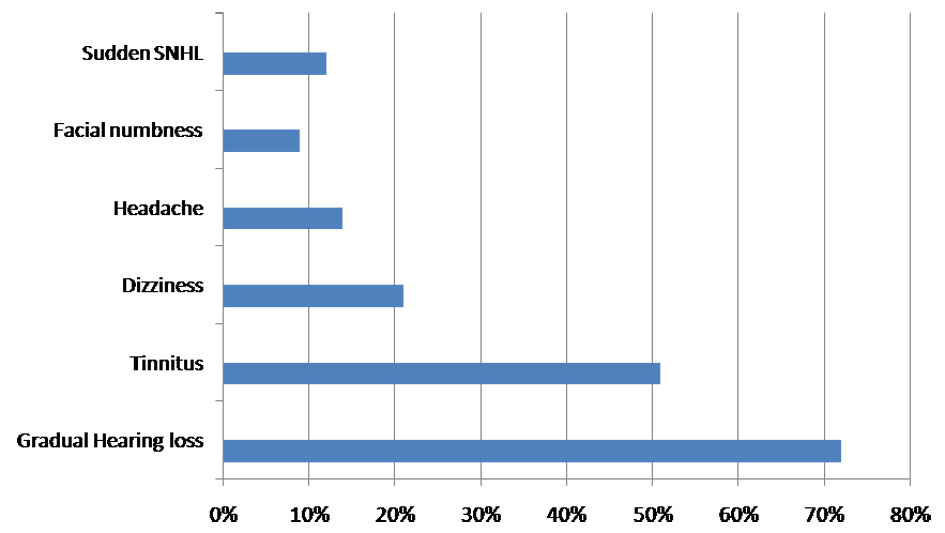 Click for large image | Figure 1. Chart showing the frequency of presentation of symptoms. |
The median tumor size was graded according to the size and extension in MRI findings which was found out to be: grade 3, 15% (20 - 30 mm within the CPA); grade 4, 22% (30 - 40 mm within the CPA); and grade 5, 63% (> 40 mm) (Table 1).
 Click to view | Table 1. Table Showing % of the Grade of the Tumor |
Preoperative HL was categorized as normal (< 25), mild (26 - 50 dB), moderate (51 - 70 dB), severe (> 70), and profound (> 90). Follow-up audiometric results were available in 30 out of the 33 patients who continued with follow-up. Postoperative hearing status of the patients did not show any significant change (<% 5 dB change) in 93% (28/30 patients, five of the normal pure tone audiogram (PTA) preoperatively included) while two from the patients with HL preoperatively (8%, 2/25) had deterioration of HL by > 10 dB in 6-month follow-up. The status remained unchanged in 12 months follow-up. The percentage distribution of mean has been shown in Table 2.
 Click to view | Table 2. Table Showing % of Mean PTA in Patients |
The surgical approach that was followed was retrosigmoid approach. All the patients underwent the surgery via the same approach given the size of the tumor at the time of presentation where all the cases were grade 3 or above and hearing preservation and facial nerve preservation were one of the goals. Also, it was the most employed approach in our center. Postoperatively, the patient was kept in intraveous (IV) antibiotics with regular dressing and discharged after 1 week. In the immediate postoperative period, facial nerve palsy, grade 6, was seen in 7% (2/30) patients who were managed with rehabilitation. Sixteen out of 30 (53%) patients and 12/30 (40%) patients had grade 2 and grade 3 facial palsy graded according to House-Brackman grading system [11]. However the latter resolved spontaneously in the subsequent follow-up. Likewise, lower cranial nerve palsies were found in 3/30 (10%) patients, hydrocephalus in 4/30 (13%), and pseudomeningocele in 2/30 (7%). Nevertheless, none required surgical intervention and were managed conservatively. We were fortunate enough to have no incidence of infection and mortality. The complications of the patients had been documented in Table 3. Similarly, improvement of tinnitus was seen in 20% of patients, worsening in 7%, postoperatively. Dizziness was persistent in 3/7 patients with preoperative dizziness and was kept under regular follow-up. The symptoms were better on subsequent follow-up.
 Click to view | Table 3. Postoperative Complications |
The overall result of the study revealed a better surgical outcome.
| Discussion | ▴Top |
Vestibular schwanommas are important clinicopathological entities for the last 200 years. The study of the tumor and its management has been an important concern for both neurosurgeons and otolaryngologists. It is because of the number of studies that have been conducted in different parts of the world and have widened the area of knowledge. The patients usually present to both otolaryngologist and neurologist with subtle symptoms which might go undiagnosed. Sometimes the dilemma of whether to go for a more expensive diagnostic modality is usually a problem in an underdeveloped country like ours on the basis of presumptive diagnosis. And given the benign and slow-growing nature of the tumor, it is always a dilemma even after the diagnosis is made whether or not to proceed with surgery or proceed with “wait and watch” policy or other modalities like radiosurgery. Though many still believe that “wait and watch” policy is more suitable for patients where tumor size is relatively smaller and more especially in an elderly patient, there are studies that have stated that long-term expectant management might result in poor perioperative outcomes. Thus, it is of immense value to study the growth patterns of VS which may help in the understanding of the natural history, the clinical applicability of the growth studies in the management of the disease [3, 6].
When we look upon the literature, the studies are more from the western world. The reason behind this might be a lack of disease awareness of the patients and the access to health facilities in the context of developing countries. Our study, however, includes patients who were able to reach to our center despite all the odds. This study especially focuses on the patients with a diagnosis of VS and the demographic distribution, the otological symptoms, and the surgical outcomes. Mean age of our patients was 46 ± 3 similar to other studies mentioned in the literature [7]. Most of the patients who were diagnosed had the history for 10 - 15 years (63.3%), and the mean duration of symptoms was 11.9 years. The long duration of the presentation was possibly due to poor access to health care facilities, and poor referral systems as discussed above. Besides these, poor economic conditions and difficult geographical situations are also important factors in our context similarly reported by Awan et al [7]. The disease was also more lateralized to the right side (48%). The average preoperative PTA was found to be 78.9 ± 18 SD of the mean. The main symptom is HL, often associated with tinnitus. Other signs and symptoms may also be present, such as vertigo, dizziness, headache, hypoesthesia, and palsies. In our patients, we found HL as the most common symptom followed by tinnitus and dizziness comparable to other studies. However, the clinical presentation has not always been found to be proportional to tumor size according to some studies [12-15].
In the past, the diagnosis of VS was challenging and expensive but with the newly improved modalities of diagnosis like auditory brainstem response tests, imaging systems such as contrast-enhanced CT and MRI with gadolinium diethylenetriamine pentaacetic acid (DTPA), diagnosis has become easier, leading to increasing numbers of detection of even smaller and less symptomatic VS [5, 16]. In our case diagnosis was done based upon clinical findings and MRI gadolinium scan. The treatment of VS depends upon various factors like the size of the tumor, its position, surgeon’s preference and expertise, and the hearing status on the affected side. The standard treatment for VS is surgical excision. There are many approaches like: translabyrinthine approach, tumor exposure is gained by drilling the mastoid and vestibular system; posterior cranial fossa approach which includes the suboccipital and retrosigmoid approaches; the middle cranial fossa approach, which provides a good opportunity for hearing preservation; combined approach observation with neuro-radiological follow-up, microsurgical resection, and stereotactic radiotherapy are other alternatives preferred in situation were surgery is deemed not possible [17]. As stated above, various studies have suggested that the choice of surgery is influenced by tumor size. Hearing preservation and facial nerve function are considered as realistic goals while in large sized tumors it might be a difficult choice and thus surgical approach is usually not influenced by the choice of hearing preservation [18, 19]. In our case, we used retrosigmoid approach in all of the cases since most of the cases presenting to us were grade 3 tumors (2 cm) and above, and also it is the routinely applied approach at our center. Despite all, there are some studies done in different parts of the world that have suggested that small tumors without any signs and symptoms and tumors in the elderly can be managed conservatively [20, 2]. There was no intraoperative problem. Tumor resection was complete in 94% and partial in 6% of cases. Similarly, our study did not find any significant relation between outcome and size, age, gender or laterality of the tumor (P > 0.05). The findings were comparable to other studies [22-24].
All of our patients had facial nerve paralysis postoperatively out of which 93% resolved completely on subsequent follow-up. The findings were comparable to studies by Yamakami et al (84%) [25] and Patni and Kartush (95%) [26]. Similarly, in a study by Lanman et al [27], they had reported 21% lower cranial nerve palsies which were slightly greater than our results (10%). The incidence of hydrocephalus was found in 4/30 (13%), and pseudomeningocele in 2/30 (7%). The findings were comparable to a study done by Shrestha et al, where they had reported the incidence of hydrocephalus in 14% and pseudomeningocele in 16% of their patients [8]. In our cases, however, none required surgical intervention and were managed conservatively. Meanwhile in the previous study by Shrestha et al 14% of patients underwent ventriculoperitoneal shunting for hydrocephalus. We had no incidence of infection and mortality which is seemingly a better outcome as compared to 3.5% patients in the study reported by Shrestha et al [8].
However, for every study, there are certain limitations. The main limitation of our study was that it was a retrospective study. Also, it was a single institution-based study and follow-up of the cases was short (12 months). Another important limitation was the limited number of patients enrolled in the study which might have been due to poor referral systems. So we believe the number of patients that presented to our center is just the tip of the iceberg. Thus more awareness and access to the equipped health centers might be helpful in the diagnosis and management of more cases in the future. We also acknowledge the fact that we were unable to compare the outcome of retrosigmoid approach with different other approaches. Therefore, for the proper validation of our research finding, we recommend further well-designed prospective studies with a larger sample size in the setting of a developing country like ours.
Conclusions
The benign and slow-growing nature of VS usually poses problems for the early diagnosis and treatment of the disease. Adding to it are the subtle symptoms that make it difficult for the surgeon to decide the diagnostic modality especially in the context of a developing country like Nepal. And the delay in diagnosis might be due to one of the reasons above. There are very few studies so far done in the country regarding the incidence and management of the disease. Thus, this study might be helpful in providing insight into the occurrence of the disease in the present scenario and the need for much more studies in the future. It also gives a brief idea about the outcomes of management in our context.
Acknowledgments
We would like to acknowledge the patients who were followed up during the course of our study. We also like to thank Dr.Tika Ram Bhandari for his contribution in manuscript writing.
Financial Diclosure
None.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Inforemed Consent
Both written and informed consent was taken from patients for the participation in the study and no harm was done.
Author Contributions
PBT and SS contributed to concept design, writing, patient care, data collection, and analysis; RKJ and DS participated in review of the article, patient care, and analysis.
Abbreviations
VS: vestibular schwanomma; IAC: internal auditory canal; CT: computed tomography; MRI: magnetic resonance imaging; DTPA: diethylenetriamine pentaacetic acid; SD: standard deviation; PTA: pure tone audiogram; HL: hearing loss; IV: intravenous
| References | ▴Top |
- Kleihues P, Burger PC, Scheithauer BW. Histological typing of tumours of the central nervous system. Springer Science & Business Media. 2012.
- Tos M, Charabi S, Thomsen J. Clinical experience with vestibular schwannomas: epidemiology, symptomatology, diagnosis, and surgical results. Eur Arch Otorhinolaryngol. 1998;255(1):1-6.
doi pubmed - Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol. 2006;27(4):547-552.
pubmed - Borg E. Correlation between auditory brainstem response (ABR) and speech discrimination scores in patients with acoustic neurinoma and in patients with cochlear hearing loss. Scand Audiol. 1982;11(4):245-248.
doi pubmed - Wayman JW, Dutcher PO, Jr., Manzione JV, Nelson CN, Kido DK. Gadolinium-DTPA-enhanced magnetic resonance scanning in cerebellopontine angle tumors. Laryngoscope. 1989;99(11):1167-1170.
doi pubmed - Hughes M, Skilbeck C, Saeed S, Bradford R. Expectant management of vestibular schwannoma: a retrospective multivariate analysis of tumor growth and outcome. Skull Base. 2011;21(5):295-302.
doi pubmed - Awan MS, Qureshi HU, Sheikh AA, Ali MM. Vestibular schwannomas: clinical presentation, management and outcome. J Pak Med Assoc. 2001;51(2):63-67.
pubmed - Shrestha P, Kutu N, Lohani S, Devkota UP. Facial nerve preservation and surgical outcomes of retrosigmoid approach to large vestibular schwannoma-an eight-year single institution experience. Nepal J Neurosci. 2017;14(3):19-25.
doi - Pinna MH, Bento RF, Neto RV. Vestibular schwannoma: 825 cases from a 25-year experience. Int Arch Otorhinolaryngol. 2012;16(4):466-475.
doi pubmed - Kanzaki J, Tos M, Sanna M, Moffat DA, Monsell EM, Berliner KI. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannoma. Otol Neurotol. 2003;24(4):642-648; discussion 648-649.
doi pubmed - House JW. Facial nerve grading systems. Laryngoscope. 1983;93(8):1056-1069.
doi pubmed - Tato JM, Venturini N, Gananca M, Anicet A, Antoni-Candela F, Linden A. Diagnostico e tratamento do neurinoma do acustico. Rev Bras Otorrinolaringol. 1970;36:107-117.
- Souza OG, Inacio AA, Cabral FG, Carneiro FA, Nunes CA. Neurinoma do Acustico. Rev Bras Otorrinolaringol. 1974;40:157-161.
- Bento RF, Caropreso CA, Miniti A. A via translabirintica na cirurgia do neuroma do acustico. Rev Bras Otorrinolaringol. 1989;55(2):57-63.
- Harun A, Agrawal Y, Tan M, Niparko JK, Francis HW. Sex and age associations with vestibular schwannoma size and presenting symptoms. Otol Neurotol. 2012;33(9):1604-1610.
doi pubmed - Charabi S, Hindmarsh T, Kylen P. [The value of magnetic resonance scanning in the diagnosis of small acoustic neurinomas]. Ugeskr Laeger. 1990;152(51):3867-3869.
pubmed - Wiegand DA, Ojemann RG, Fickel V. Surgical treatment of acoustic neuroma (vestibular schwannoma) in the United States: report from the Acoustic Neuroma Registry. Laryngoscope. 1996;106(1 Pt 1):58-66.
doi pubmed - Briggs RJ, Luxford WM, Atkins JS, Jr., Hitselberger WE. Translabyrinthine removal of large acoustic neuromas. Neurosurgery. 1994;34(5):785-790; discussion 790-781.
- Frohlich AM, Sutherland GR. Epidemiology and clinical features of vestibular schwannoma in Manitoba, Canada. Can J Neurol Sci. 1993;20(2):126-130.
doi - Hajioff D, Raut VV, Walsh RM, Bath AP, Bance ML, Guha A, Tator CH, et al. Conservative management of vestibular schwannomas: third review of a 10-year prospective study. Clin Otolaryngol. 2008;33(3):255-259.
doi pubmed - Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339(20):1426-1433.
doi pubmed - Bederson JB, von Ammon K, Wichmann WW, Yasargil MG. Conservative treatment of patients with acoustic tumors. Neurosurgery. 1991;28(5):646-650; discussion 650-641.
- Flint D, Fagan P, Panarese A. Conservative management of sporadic unilateral acoustic neuromas. J Laryngol Otol. 2005;119(6):424-428.
doi pubmed - Walsh RM, Bath AP, Bance ML, Keller A, Rutka JA. Consequences to hearing during the conservative management of vestibular schwannomas. Laryngoscope. 2000;110(2 Pt 1):250-255.
doi pubmed - Yamakami I, Uchino Y, Kobayashi E, Yamaura A, Oka N. Removal of large acoustic neurinomas (vestibular schwannomas) by the retrosigmoid approach with no mortality and minimal morbidity. J Neurol Neurosurg Psychiatry. 2004;75(3):453-458.
doi pubmed - Patni AH, Kartush JM. Staged resection of large acoustic neuromas. Otolaryngol Head Neck Surg. 2005;132(1):11-19.
doi pubmed - Lanman TH, Brackmann DE, Hitselberger WE, Subin B. Report of 190 consecutive cases of large acoustic tumors (vestibular schwannoma) removed via the translabyrinthine approach. J Neurosurg. 1999;90(4):617-623.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


