| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 10, Number 4-5, October 2019, pages 169-175
Serum Neurofilament Light, Glial Fibrillary Acidic Protein and Tau Are Possible Serum Biomarkers for Activity of Brain Metastases and Gliomas
Adriana Hepnera, g, Jason Porterb, Felicia Hareb, Syed Sameer Nasirb, Henrik Zetterbergc, d, e, f, Kaj Blennowc, d, Michael Gary Martinb
aMedical Oncology Service, Instituto do Cancer do Estado de Sao Paulo, Universidade de Sao Paulo, Sao Paulo, Brazil
bDivision of Hematology and Oncology, University of Tennessee Health Science Center/West Cancer Center, Memphis, TN, USA
cDepartment of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, the Sahlgrenska Academy at the University of Gothenburg, Molndal, Sweden
dClinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Molndal, Sweden
eDepartment of Neurodegenerative Disease, UCL Institute of Neurology, Queen Square, London, UK
fUK Dementia Research Institute at UCL, London, UK
gCorresponding Author: Adriana Hepner, Junior Faculty, Instituto do Cancer do Estado de Sao Paulo, Universidade de Sao Paulo, Sao Paulo, Brazil
Manuscript submitted August 31, 2019, accepted September 9, 2019
Short title: Serum NfL and GFAP for CNS Malignancies
doi: https://doi.org/10.14740/wjon1228
| Abstract | ▴Top |
Background: Primary central nervous system (CNS) tumors and brain metastases (BMs) are major causes of morbidity and mortality, accompanied by low survival rates. Efforts to early discovery of CNS malignancies are critical. However, to date, there are no biomarkers approved for detection of cancer activity in the brain. Blood levels of neurofilament light (NfL) and tau, as well as glial fibrillary acidic protein (GFAp), show promise as biomarkers for brain injury in previous studies. Therefore, we performed a cross-sectional study to investigate correlations of those biomarkers with CNS activity of gliomas and BMs.
Methods: Serum samples of 36 participants of a single centered institution were tested for NfL, GFAp and tau with Simoa immunoassay, and correlated with clinical and radiological data.
Results: NfL and GFAp levels were significantly associated with the state of intracranial disease (analysis of variance (ANOVA), PsNfL = 0.03; ANOVA, PGFAp = 0.03). Although statistically significant (P = 0.04), differences in concentrations were not clinically meaningful for tau levels. Serum NfL (sNfL) and GFAp concentrations were higher in the group of patients with CNS tumors with disease in progression versus CNS with stable disease (P = 0.03 and P = 0.01, respectively). In addition, sNfL were higher in patients with metastatic solid tumors with known BMs than in those with metastatic tumors with no BM (P = 0.0004).
Conclusion: sNfL and GFAp both apparently vary closely with presence and activity of gliomas and BMs. Further studies in larger populations are needed to expand these findings.
Keywords: Biomarker; Central nervous system; Neurofilament light; Tau protein; Glial fibrillary acidic protein
| Introduction | ▴Top |
Intracranial neoplasms consist of a diverse range of pathologic entities, including primary central nervous system (CNS) tumors and brain metastases (BMs). In 2018, the overall estimated incidence of brain and other nervous system tumors in the United States was 23,880, with a 5-year relative survival rate around 35% [1]. Nonetheless, brain metastases (BMs) comprise more than half of the adult brain tumors diagnosed. Hematogenous dissemination to the brain occurs in approximately 10-30% of patients with systemic disease [2-4] and can become symptomatic in 60-75% of the cases [5]. Higher frequencies of BM are seen in lung and breast carcinoma, followed by melanoma and kidney cancer [5].
Primary CNS tumors and BMs are major causes of morbidity and mortality. Among patients with solid tumors and BMs, retrospective studies suggest that the actuarial 3-year survival rate is less than 5% [6]. Besides psychological and physical impairments, brain tumor diagnosis imposes a potential increased economic burden for the individual, families and even to the whole society.
Continuous efforts to early discovery of CNS malignancies, including primary CNS tumors and synchronous or metachronous BMs are critical. However, to date, there are no blood biomarkers approved for the detection of cancer activity in the brain. Neurofilament light (NfL) is a brain protein that is abundantly expressed in the long myelinated subcortical white matter axons [7]. Numerous studies support that serum NfL (sNfL) is a sensitive biomarker for acute neuronal injury [8] and chronic neurodegenerative disorders such as Alzheimer’s disease [9], progressive supranuclear palsy [10] and parkinsonian disorders [11]. Although less studied, tau protein, a microtubule stabilizing protein expressed primarily in neurons, is also considered a biomarker with specificity for neuronal injury [12-14]. Glial fibrillary acidic protein (GFAp) is an intermediate filament highly expressed in astrocytes, being an important cytoskeletal protein [15, 16]. Studies have shown association of high levels of GFAp in CNS tissue damage, including neurotrauma and neurodegenerative disorders (Fig. 1) [17-21].
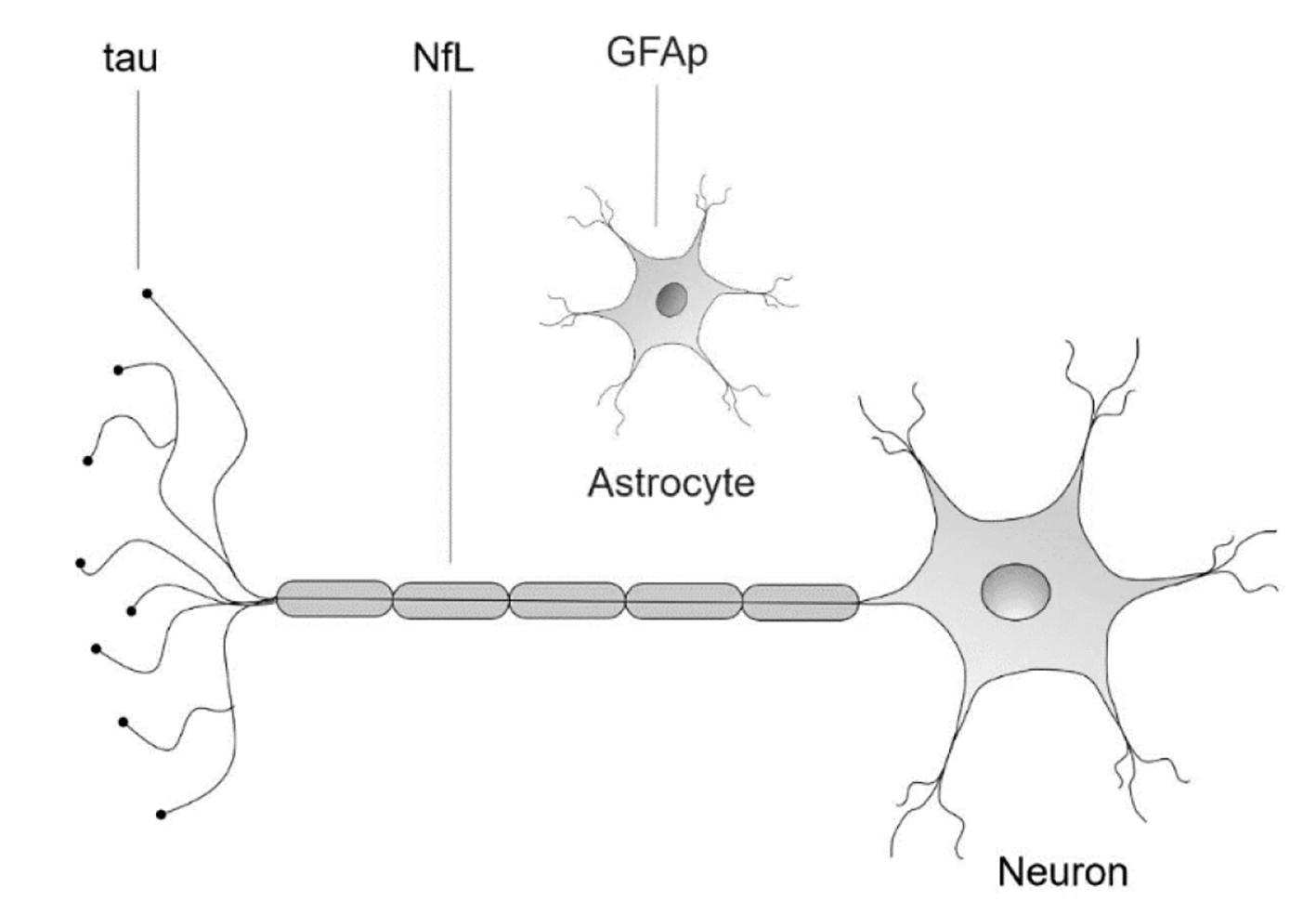 Click for large image | Figure 1. Neuronal and glial biomarkers. NfL: neurofilament light protein; GFAp: glial fibrillary acidic protein; tau: tau protein. |
Brain tumors affect function and integrity of neighboring neurons or may cause increased intracranial pressure that compromise neuronal function and cause astroglial activation. Neuron-enriched proteins, such as NfL and tau, and cytoskeletal proteins, such as GFAp, could be ideal candidate biomarkers to identify and monitor growth or spread of such malignancies. Therefore, the current cross-sectional study was designed to investigate blood levels of the sNfL, tau and GFAp as biomarkers to be utilized in those settings. We hypothesized that these proteins could additionally differentiate activity of gliomas and BMs.
| Materials and Methods | ▴Top |
Patients
Thirty-six participants were recruited from the West Cancer Center, Methodist Healthcare-Memphis Hospitals and the University of Tennessee Health Science Center (UTHSC) in Memphis, Tennessee. Approval for the study was granted by the UTHSC institutional ethics committee and all participants provided written consent. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. Subjects were included in the study by convenience sampling into six groups, according to institution’s standard of care diagnose and follow-up routine, specific for each primary disease. Patients were distributed into the following groups: CNS tumors with progressive disease, CNS with stable disease, patients with cancer (except primary CNS) with no BM, patients with metastatic solid tumors with known BM, patients currently without evidence of measurable disease, but with prior history of treatment of metastatic brain lesions and healthy controls. Radiological assessments were performed in all patients with known CNS tumors before enrollment and evaluation was performed by local radiologists according to RECIST guidelines (version 1.1). Participants were excluded if they had history of head trauma or had been through any surgery to CNS in the last month. Controls were not allowed to take any medication before or during study protocol.
Specimen characteristics
From each participant, two tubes of blood were obtained for protocol analysis. Serum samples were processed, batched into aliquots and frozen at -80 °C, according to standardized procedures.
Measurement of serum concentrations of NfL, tau and GFAp
Serum concentration of sNfL was measured using an in house single molecule array (Simoa) method on an HD-1 analyzer (Quanterix, Lexington, MA), as previously described in detail by Gisslen [22, 23]. Serum concentrations of tau and GFAp were measured using commercially available Simoa assays (Quanterix, Lexington, MA). All samples were measured as duplicates. The mean coefficient of variation of duplicate concentrations was 4.3%. In addition, a quality control sample was measured in duplicate on each of the seven runs used to complete the study. The intra-assay coefficient of variation for this sample was < 10%. All measurements were performed by board-certified laboratory technicians. The laboratorial analysis was conducted in a blinded fashion, as the assays were done without knowledge of the patient’s identity or diagnosis. Only one round of experiments was performed, using one batch of reagents.
Statistical analysis
The sNfL, GFAp and tau proteins concentrations were initially compared between all six groups. As normality of the distribution was shown, analysis of variance (ANOVA) was performed for the comparisons. As the groups were small and with probable unequal sample sizes, the Brown-Forsythe test was used to compare mean sNfL, GFAp and tau concentrations across each of the clinical subgroups. In addition, planned analysis was performed for the three variables in patients with CNS tumors with stable versus progressive disease and in patients with metastatic cancer with and without known BM. Both analyses were done with unpaired t-test. Assumptions were satisfied if the two-tailed t-test assumed a significance alpha level inferior to 0.05. The GraphPad Prism7 software was used for all analysis.
Data availability
Individual deidentified participant data, related documents and statistical analysis will be shared on request from any qualified investigator for 2 years after the date of publication.
| Results | ▴Top |
A total of 36 patients were registered between August 2017 and October 2017. Patients were enrolled into the following groups: eight patients with CNS tumors with progressive disease (CNSPD); seven patients with CNS with stable disease (CNSSD); nine patients with metastatic cancer with no brain metastasis (CNBM); seven patients with metastatic solid tumors with known brain metastasis (BM); four healthy controls (C); one patient without evidence of measurable disease, but with prior treatment of a metastatic brain lesion, more than 2 years before inclusion in the study (remote BM) (Table 1). All patients irrespective of disease or line of treatment received approved therapies according to National Comprehensive Cancer Network (NCCN) guidelines. Exclusion criteria included patients with recent history of brain concussion or trauma, personal history of cerebrovascular accident, dementia or neurodegenerative disorders. The most relevant characteristics of the trial population are shown in Tables 1 and 2.
 Click to view | Table 1. Conditions Per Group |
 Click to view | Table 2. Characteristics of the Study Participants |
Female participants represented 42% of the population studied. Most of the patients were still on first line of systemic treatment when included into the study. All patients with a glioma had received radiotherapy previously. In addition, all patients in the group BM had received radiotherapy. Five patients underwent whole brain radiotherapy (WBRT) for multiple brain lesions and two patients received only treatment for single lesions, one delivered by volumetric modulated arc (VMAT) and the other by stereotactic body radiotherapy (SBRT). The patient who had a remote BM also underwent WBRT at the time of diagnosis, more than 3 years ago. The single epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer (NSCLC) received erlotinib in first line. Also in first line, two patients received immunotherapy combinations: one patient with metastatic melanoma had ipilimumab and nivolumab, and another patient with NSCLC had a combination of carboplatin, pemetrexed and pembrolizumab.
The sNfL, GFAp and tau concentrations in the control and in each clinical subgroup are shown in Table 3. The lowest sNfL median concentration in the study was found in the control group (7.2 pg/mL) and the highest in CNS tumors with progressive disease (239.0 pg/mL). The minimum and maximum median levels of GFAp followed the same pattern, with 74.5 pg/mL in C and 2,092.1 pg/mL in CNSPD. Differently, absolute tau levels did not differ much between groups, and the highest values were seen in CNBM (12.5 pg/mL).
 Click to view | Table 3. Median Serum NfL, Tau and GFAp Concentrations Per Group |
Compared with controls, sNfL and GFAp were higher in patients with BM, CNBM, remote BM and CNSPD (P = 0.003 and P = 0.03, respectively) (Fig. 2). The sNfL concentrations were significantly higher in the total CNSPD group versus CNSSD (mean 364.3 pg/mL (SEM 123.0 pg/mL) and 41.9 (SEM 16.4 pg/mL), respectively; mean difference: 322.3 pg/mL, 95% confidence interval: 34.9 - 609.7; P = 0.03). GFAp levels, similarly, were significantly higher in the total CNSPD group versus CNSSD (mean 1,523 pg/mL (SEM 397 pg/mL) and 226.4 pg/mL (SEM 68.4 pg/mL) respectively; mean difference: 1,296 pg/mL, 95% confidence interval: 365 - 2228; P = 0.01). The sNfL concentrations were also significantly higher in patients with metastatic solid tumors with known BM (BM) (mean 161.7 pg/mL (SEM 34.1 pg/mL)) than in those with no BM (CNBM) (22.6 (SEM 5.8 pg/mL); mean difference: 139.0 pg/mL, 95% confidence interval: 73.5 - 204.5; P = 0.0004). GFAp concentrations were in BM (mean 1,625 pg/mL (SEM 836.7 pg/mL) and in CNBM 113.5 pg/mL (SEM 24.8 pg/mL); mean difference: 1,511 pg/mL, 95% confidence interval: 56.4 - 3,079; P = 0.05). Regarding tau levels, there was a statistically significant difference between groups (P = 0.0445), but no difference in CNSPD group versus CNSSD (P = 0.39) (Fig. 2).
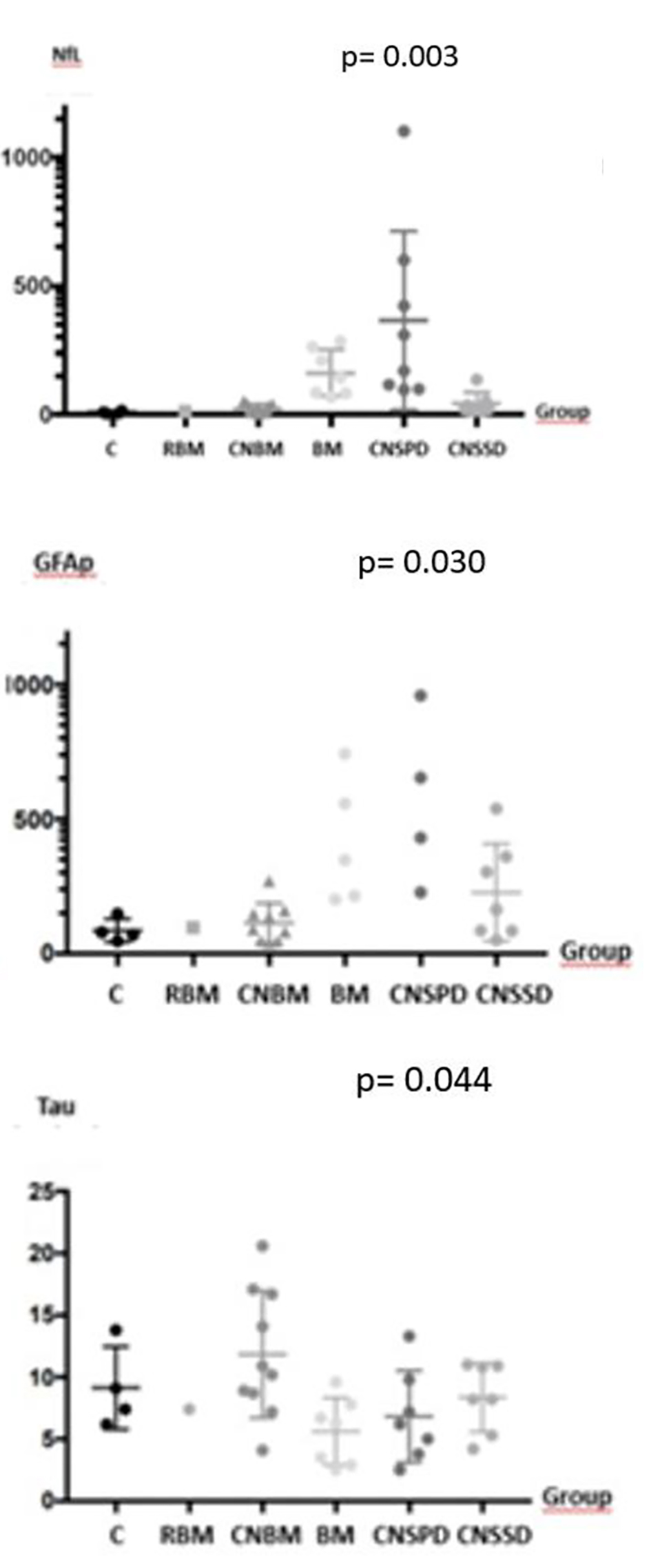 Click for large image | Figure 2. sNfL, GFAp and tau concentrations per intracranial neoplasm group. There is statistically significant difference in sNfL, GFAp and tau levels between groups, although concentrations were not clinically meaningful for tau. CNSPD: patients with CNS tumors with progressive disease; CNSSD: patients with CNS with stable disease; CNBM: patients with metastatic cancer with no brain metastasis; BM: patients with metastatic solid tumors with known brain metastasis; C: healthy controls; remote BM: patient without evidence of measurable disease, but with prior treatment of a metastatic brain lesion, more than 2 years before inclusion in the study. sNfL: serum neurofilament light chain; GFAp: glial fibrillary acidic protein; tau: tau protein; CNS: central nervous system. |
| Discussion | ▴Top |
Intracranial neoplasms comprise a group of heterogeneous entities. Diagnosis and assessment of disease burden for staging and follow-up mainly rely on standard imaging techniques, such as computed tomography (CT) scans and magnetic resonance imaging (MRI). Those techniques are able to only indirectly reflect the extent of the neoplasms, as they are limited to just represent measurable disease, and treatment-related changes can further compromise interpretation of the results. Consequently, alternative testing methods are being evaluated. NfL and tau proteins are major components of the neuronal cytoskeleton [24] and studies have shown that their release in cerebrospinal fluid (CSF) is an indicator of axonal damage [5, 6, 25-30]. Biofluid levels of GFAp, a protein responsible for the cytoskeleton structure of glia cells, could reflect glioma formation and expansion, according to Zang et al [17]. In this pilot study, we clearly showed that patients with BM or CNS neoplasms either on progression or on current response had higher serum concentrations of NfLs and GFAp than healthy controls, patients with cancer and no BM or than a patient with remote treated BMs.
Due to concerns with the blood-brain barrier permeability, rationally, CSF analysis appears to be more attractive than a blood-based test as a source of biomarkers for CNS tumors [30]. However, the development of ultrasensitive immunoassays, such as Simoa technology used in this study, allows the detection of extremely low concentration of those biomarkers in the peripheral circulation [22]. For instance, Gisslen et al showed strong correlation between CSF and plasma concentrations of NfL in HIV patients with ongoing CNS injury (r = 0.89, P < 0.0001) [23]. Likewise, sNfL had similar sensitivity and specificity as CSF NfL for the diagnosis of genetic and sporadic Creutzfeldt-Jakob disease [29], and the same diagnostic performance was also found for Alzheimer’s disease [9]. Further, sNfL closely followed CSF NfL concentrations in a study of controlled neurosurgical trauma [31]. Although less studied, serum GFAp appears to be useful as a biomarker for traumatic brain injury [32] and in tracking progression and outcome of ischemic stroke [33]. Blood-based tests have clear advantages in comparison to CSF tests, especially regarding accessibility, safety (in patients with tumors who may have increased intracranial pressure), comfort to the patient and the possibility of more easily multiple testing [34].
Rohrer et al described a positive correlation of sNfL with intensity of disease in frontotemporal dementia (FTD) [35]. A hypothesis for the highest value of median sNfL to be found in the group with CNS with disease in progression, 239 pg/mL (96.7 - 1,101.4 pg/mL), is that higher concentrations of sNfL in patients with intracranial neoplasms could also reflect higher tumor burden, due to increased axonal lesion. As GFAp levels have a strong brain-specificity [17], concentrations in our study assumed similar disposition as for NfL levels, with the highest value also found in the group CNSPD, 2,092.1 pg/mL (227.8 - 3,435.6 pg/mL).
In our study, there were statistically significant differences in tau levels between groups, although concentrations were not clinically meaningful. The highest values were seen in CNBM group (12.5 pg/mL; 4.1 - 20.6 pg/mL). Two patients in this group, one with esophageal cancer taking a combination of a platin and a taxane, and the other with cholangiocarcinoma, receiving pembrolizumab, had the highest values found in the whole study (17.9 and 20.6 pg/mL). Despite known tau and NfL levels elevation in acute neuroaxonal injury, tau has very different dynamics than NfL. Tau increases within hour and has a half-life of around 10 h in plasma [13]. NfL on the other hand, increases with a maximum sometimes between 7 and 10 days, perhaps even longer after an injury and has a half-life of several weeks to a month or two [25, 36]. So, this could explain the differences in correlation between tau and NfL levels seen in this study. Additionally, as tau half-life is short, the highest levels found in CNBM could be derived from treatment-related side effects, such as neurotoxicity. Similarly, Mattsson et al concluded that plasma tau alone is insufficient as a biomarker for Alzheimer’s disease [14, 37].
Moreover, our findings of median NfL in the control patients were similar to the concentration of the patient who had a remote BM 7.3 pg/mL (3.2 - 14.8 pg/mL) and 13.9 pg/mL, respectively. Those values are comparable to the concentrations found in the healthy control group studied against the patients with FTD [35], suggesting that our data are reproducible in a bigger scale population.
It is known that the prevalence of cancer patients with brain BM is rising, probably in consequence of a better control of the systemic disease and longer overall survival. Nonetheless, it is still an open question whether NfL, GFAp and tau could be complementary tools for CNS staging in patients with cancer without BMs. Some limitations of the study are the convenience sampling, the lack of prospective data and the diverse and small sample size. However, in conclusion, our intriguing data suggest that both NfL and GFAp are potential clinical biomarkers for CNS tumors and BM. Further studies are needed to validate our encouraging early results.
Acknowledgments
None to declare.
Financial Disclosure
HZ is a Wallenberg Academy Fellows supported by grants from the Swedish Research Council, the European Research Council, Swedish State Support for Clinical Research (ALFGBG), and the Olav Thon Foundation. KB holds the Torsten Soderberg Professorship of Medicine and is supported by grants from the Swedish Research Council, the Swedish Alzheimer Foundation, the Brain Foundation and Swedish State Support for Clinical Research (ALFGBG).
Conflict of Interest
AH honoraria Novartis. Travel expenses Roche. JP has no conflict of interest. FH has no conflict of interest. SN has no conflict of interest. HZ has served at scientific advisory boards for Eli Lilly, Roche Diagnostics, Wave, Samumed and CogRx, has received travel support from Teva and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. KB has served as a consultant or at advisory boards for Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Merck, Novartis, Pfizer, and Roche Diagnostics, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. MGM has no conflict of interest.
Informed Consent
All participants provided written consent.
Author Contributions
AH: formal analysis, visualization, writing of original draft, review and editing. JP: resources, writing of review and editing. FH: resources, writing of review and editing. SN: methodology, writing of review and editing. HZ: funding acquisition, methodology, resources, supervision, validation, writing of review and editing. KB: funding acquisition, methodology, resources, supervision, validation, writing of review and editing. MGM: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing of review and editing.
| References | ▴Top |
- American Cancer Society. Cancer Facts & Figures 2018. Available from URL: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html [accessed June 26, 2018].
- Schouten LJ, Rutten J, Huveneers HA, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94(10):2698-2705.
doi pubmed - Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579-592.
- Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Arch Neurol. 1988;45(7):741-744.
doi pubmed - Soffietti R, Cornu P, Delattre JY, Grant R, Graus F, Grisold W, Heimans J, et al. EFNS Guidelines on diagnosis and treatment of brain metastases: report of an EFNS Task Force. Eur J Neurol. 2006;13(7):674-681.
doi pubmed - Hall WA, Djalilian HR, Nussbaum ES, Cho KH. Long-term survival with metastatic cancer to the brain. Med Oncol. 2000;17(4):279-286.
doi pubmed - Zetterberg H, Smith DH, Blennow K. Biomarkers of mild traumatic brain injury in cerebrospinal fluid and blood. Nat Rev Neurol. 2013;9(4):201-210.
doi pubmed - Shahim P, Gren M, Liman V, Andreasson U, Norgren N, Tegner Y, Mattsson N, et al. Serum neurofilament light protein predicts clinical outcome in traumatic brain injury. Sci Rep. 2016;6:36791.
doi pubmed - Zetterberg H, Skillback T, Mattsson N, Trojanowski JQ, Portelius E, Shaw LM, Weiner MW, et al. Association of Cerebrospinal Fluid Neurofilament Light Concentration With Alzheimer Disease Progression. JAMA Neurol. 2016;73(1):60-67.
doi pubmed - Rojas JC, Karydas A, Bang J, Tsai RM, Blennow K, Liman V, Kramer JH, et al. Plasma neurofilament light chain predicts progression in progressive supranuclear palsy. Ann Clin Transl Neurol. 2016;3(3):216-225.
doi pubmed - Hansson O, Janelidze S, Hall S, Magdalinou N, Lees AJ, Andreasson U, Norgren N, et al. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88(10):930-937.
doi pubmed - Avila J, Lucas JJ, Perez M, Hernandez F. Role of tau protein in both physiological and pathological conditions. Physiol Rev. 2004;84(2):361-384.
doi pubmed - Randall J, Mortberg E, Provuncher GK, Fournier DR, Duffy DC, Rubertsson S, Blennow K, et al. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation. 2013;84(3):351-356.
doi pubmed - Mattsson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, Palmqvist S, et al. Plasma tau in Alzheimer disease. Neurology. 2016;87(17):1827-1835.
doi pubmed - Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7-35.
doi pubmed - Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem Res. 2000;25(9-10):1439-1451.
doi pubmed - Yang Z, Wang KK. Glial fibrillary acidic protein: from intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015;38(6):364-374.
doi pubmed - Guez M, Hildingsson C, Rosengren L, Karlsson K, Toolanen G. Nervous tissue damage markers in cerebrospinal fluid after cervical spine injuries and whiplash trauma. J Neurotrauma. 2003;20(9):853-858.
doi pubmed - Zeitlberger AM, Thomas-Black G, Garcia-Moreno H, Foiani M, Heslegrave AJ, Zetterberg H, Giunti P. Plasma markers of neurodegeneration are raised in Friedreich's Ataxia. Front Cell Neurosci. 2018;12:366.
doi pubmed - Norgren N, Sundstrom P, Svenningsson A, Rosengren L, Stigbrand T, Gunnarsson M. Neurofilament and glial fibrillary acidic protein in multiple sclerosis. Neurology. 2004;63(9):1586-1590.
doi pubmed - Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, Holtta M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15(7):673-684.
doi - Wilson DH, Rissin DM, Kan CW, Fournier DR, Piech T, Campbell TG, Meyer RE, et al. The Simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom. 2016;21(4):533-547.
doi pubmed - Gisslen M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, Fuchs D, et al. Plasma concentration of the Neurofilament Light Protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2016;3:135-140.
doi pubmed - Perrot R, Berges R, Bocquet A, Eyer J. Review of the multiple aspects of neurofilament functions, and their possible contribution to neurodegeneration. Mol Neurobiol. 2008;38(1):27-65.
doi pubmed - Bergman J, Dring A, Zetterberg H, Blennow K, Norgren N, Gilthorpe J, Bergenheim T, et al. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol Neuroimmunol Neuroinflamm. 2016;3(5):e271.
doi pubmed - Soylu-Kucharz R, Sandelius A, Sjogren M, et al. Neurofilament light protein in CSF and blood is associated with neurodegeneration and disease severity in Huntington's disease R6/2 mice. Nature Scientific Reports. 2017;7:1-8.
doi pubmed - Jakobsson J, Bjerke M, Ekman CJ, Sellgren C, Johansson AG, Zetterberg H, Blennow K, et al. Elevated concentrations of neurofilament light chain in the cerebrospinal fluid of bipolar disorder patients. Neuropsychopharmacology. 2014;39(10):2349-2356.
doi pubmed - Douglas-Escobar M, Yang C, Bennett J, Shuster J, Theriaque D, Leibovici A, Kays D, et al. A pilot study of novel biomarkers in neonates with hypoxic-ischemic encephalopathy. Pediatr Res. 2010;68(6):531-536.
doi pubmed - Steinacker P, Blennow K, Halbgebauer S, Shi S, Ruf V, Oeckl P, Giese A, et al. Neurofilaments in blood and CSF for diagnosis and prediction of onset in Creutzfeldt-Jakob disease. Sci Rep. 2016;6:38737.
doi pubmed - Hulme CH, Brown SJ, Fuller HR, Riddell J, Osman A, Chowdhury J, Kumar N, et al. The developing landscape of diagnostic and prognostic biomarkers for spinal cord injury in cerebrospinal fluid and blood. Spinal Cord. 2017;55(2):114-125.
doi pubmed - Ljungqvist J, Zetterberg H, Mitsis M, Blennow K, Skoglund T. Serum neurofilament light protein as a marker for diffuse axonal injury: results from a case series study. J Neurotrauma. 2017;34(5):1124-1127.
doi pubmed - Vos PE, Jacobs B, Andriessen TM, Lamers KJ, Borm GF, Beems T, Edwards M, et al. GFAP and S100B are biomarkers of traumatic brain injury: an observational cohort study. Neurology. 2010;75(20):1786-1793.
doi pubmed - Zhang J, Zhang CH, Lin XL, Zhang Q, Wang J, Shi SL. Serum glial fibrillary acidic protein as a biomarker for differentiating intracerebral hemorrhage and ischemic stroke in patients with symptoms of acute stroke: a systematic review and meta-analysis. Neurol Sci. 2013;34(11):1887-1892.
doi pubmed - Holdhoff M, Yovino SG, Boadu O, Grossman SA. Blood-based biomarkers for malignant gliomas. J Neurooncol. 2013;113(3):345-352.
doi pubmed - Rohrer JD, Woollacott IO, Dick KM, Brotherhood E, Gordon E, Fellows A, Toombs J, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87(13):1329-1336.
doi pubmed - Zetterberg H, Blennow K. from cerebrospinal fluid to blood: the third wave of fluid biomarkers for Alzheimer's disease. J Alzheimers Dis. 2018;64(s1):S271-S279.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


