| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 11, Number 4, August 2020, pages 158-164
Effect of Rapamycin on the Radio-Sensitivity of Cultured Tumor Cells Following Boron Neutron Capture Reaction
Hitoshi Tatebea, c, Shin-ichiro Masunagab, Yasumasa Nishimuraa
aDepartment of Radiation Oncology, Kindai University Faculty of Medicine, Ohno-Higashi, Osaka-Sayama, Osaka, Japan
bParticle Radiation Biology, Division of Radiation Life Science, Institute for Integrated Radiation and Nuclear Science, Kyoto University, Kumatori, Osaka, Japan
cCorresponding Author: Hitoshi Tatebe, Department of Radiation Oncology, Kindai University School of Medicine, Ohno-Higashi, Osaka-Sayama, Osaka 589-8511, Japan
Manuscript submitted June 13, 2020, accepted July 15, 2020, published online August 10, 2020
Short title: Effect of RAP on Tumor Cells After BNCT
doi: https://doi.org/10.14740/wjon1296
| Abstract | ▴Top |
Background: Mammalian target of rapamycin (mTOR) signaling pathway has been implicated in multiple mechanisms of resistance to anticancer drugs and poor treatment outcomes in various human cancers. Meanwhile, clinical boron neutron capture therapy (BNCT) has been carried out for patients with malignant gliomas, melanomas, inoperable head and neck tumors and oral cancers. This study aimed to evaluate the effect of mTOR inhibition on radio-sensitivity of cultured tumor cells in BNCT, employing p-boronophenylalanine-10B (BPA) as a 10B-carrier.
Methods: Cultured SAS cells had been incubated for 48 h at RPMI medium with mTOR inhibitor, rapamycin at the dose of 1 µM, and then continuously incubated for 2 more hours at RPMI medium containing both BPA at the 10B concentration of 10 ppm and rapamycin (1 µM). Subsequently, the SAS cells received reactor neutron beams, and then surviving fraction and micronucleus frequency were determined.
Results: SAS cells incubated with rapamycin showed resistance to γ-rays compared with no treatment with rapamycin. The efficiency of delivery of 10B from BPA into cultured SAS cells was reduced through combining with rapamycin, leading to reduced sensitivity following boron neutron capture reaction.
Conclusions: Since many tumors are characterized by deregulated PI3K/AKT/mTOR pathway, rapamycin is thought to inhibit the pathway and tumor growth. However, it was revealed that rapamycin can also inhibit the transport of 10B for BNCT into tumor cells. When BNCT is combined with mTOR inhibitor, the efficiency as cancer treatment can be reduced by repression of distributing 10B in tumor cells, warranting precaution when the two strategies are combined.
Keywords: Rapamycin; Boron neutron capture therapy; Boronophenylalanine-10B
| Introduction | ▴Top |
Boron neutron capture reaction (10B (n, α)7Li) is, in principle, very effective in destroying tumors, provided that a sufficient amount of 10B can be accumulated in the target tumor and a sufficient number of very-low-energy thermal neutrons can be delivered [1, 2]. The two particles generated in this reaction have a high linear energy transfer (LET) and have a range of roughly the diameter of one or two tumor cells [1, 2]. It is theoretically possible to kill tumor cells without affecting adjacent normal cells if 10B atoms can be selectively accumulated in the interstitial space of tumor tissue and/or intracellular space of tumor cells [1, 2]. Thus, successful boron neutron capture therapy (BNCT) requires the selective delivery of large amounts of 10B to tumor cells.
Two most common 10B-carriers used in clinical BNCT, designed for the treatment of malignant gliomas, melanomas, inoperable head and neck tumors and oral cancer, are sodium mercaptoundecahydro-dodecaborate-10B (sodium borocaptate-10B, BSH, Na210B 12H11SH) and boronophenylalanine-10B (BPA, C9H1210B NO4) [3]. The delivery of 10B from BSH relies on passive diffusion from the blood to the brain tumor through a disrupted blood-brain barrier [4]. Thus, the use of BSH results in a high concentration of 10B in the blood and subsequent vascular damage during BNCT. BPA is designed to be mostly taken up by active transport across the cancer cell membrane [5]. Based on BPA import and efflux measurements in the presence of system L-specific substrates, Wongthai and colleagues reported that L-amino acid transporter-1 (LAT1) appears to be a key BPA transporter [6]. The transport mechanism is operative even in normal cells, leading to the accumulation of BPA in the normal brain. However, BPA uptake rate is lower in normal cells than in tumor cells due to their lower LAT1 expression than tumor cells [4].
On the other hand, rapamycin is a macrolide originally found as an antifungal agent and is now recognized as an agent with anticancer and immunosuppressive properties. Rapamycin is a specific mammalian target of rapamycin complex 1 (mTORC1), angiogenesis inhibitor and an autophagy inducer [7-9]. The mTORC1 is a downstream effector of the PI3K/Akt pathway [7-9]. Mammalian target of rapamycin (mTOR) controls translation of specific mRNA transcripts that encode cell cycle progression and cell proliferation proteins [7-10]. Therefore, mTOR is an important target of a new line of anticancer drugs. Based on this function of mTOR, mTOR inhibitors are thought to reduce cell proliferation. In fact, mTOR inhibitor is a type of anticancer drug used in combination chemotherapy, for example, chemotherapy for prostate cancer. Also in BNCT, it has been thought that the distribution of 10B from 10B-carrier to the lesion tumor may be decreased when combined with an mTOR inhibitor. Thus, the effect of mTOR inhibitor on 10B delivery to tumor cells in BNCT should be more clearly evaluated.
| Materials and Methods | ▴Top |
No ethics approval needed for this study; and this study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Cell culture
The human head and neck squamous cell carcinoma cell line SAS (provided by JCRB, Tokyo) was cultured at 37 °C in RPMI containing 12.5% fetal bovine serum in a conventional humified 5% CO2 incubator.
Rapamycin treatment, 10B compound and measuring 10B concentration
Cultured SAS cells were preincubated with rapamycin at a dose of 1 µM for 48 h in RPMI (containing 12.5% fetal bovine serum) medium, followed by adding BPA at a 10B concentration of 10 ppm. Then, SAS cells were continuously incubated for 2 h in the presence of both rapamycin and BPA.
10B-enriched (> 98%) BPA was purchased from Katchem spol. s.r.o. (Czech Republic). BPA was converted to a fructose complex to increase its solubility as previously reported [11]. The concentration of the aqueous suspension of BPA was 250 mg/mL. The 10B concentrations in the suspensions of a 10B-carrier were measured by prompt γ-ray spectrometry using a thermal neutron guide tube installed at the Kyoto University Reactor (KUR).
Irradiation
As mentioned above, before neutron beam exposure, cultured cells had been incubated in flasks with a culture area of 75 cm2 and treated with BPA at a 10B concentration of 10 ppm in a cell culture medium for 2 h. Subsequently, the cells were exposed to reactor neutron beams in the presence of BPA in the cell culture medium at an operation power of 1 MW. As control conditions, the cultured cells incubated with rapamycin only or BPA only, or without rapamycin or BPA were exposed to neutron beams. Other cell cultures untreated with BPA were irradiated with γ-rays using a cobalt-60 γ-ray irradiator (made by TOKYO SHIBAURA ELECTRIC CO., LTD. in Japan) at a dose rate of approximately 2.0 Gy/min after incubation with or without rapamycin. Cadmium ratio of employed reactor neutron beams was 9.4.
Neutron fluence was measured by the radioactivation of gold foils on the front and back of the flasks, as described in previous studies. Contaminating γ-ray, including secondary γ-ray, doses were measured with a thermoluminescence dosimeter (TLD) powder. The TLD used was beryllium oxide (BeO) enclosed in a quartz glass capsule. BeO itself has a fairly strong sensitivity to thermal neutrons. The TLD is usually used together with gold activation foil for neutron-sensitivity correction.
To estimate neutron energy spectra, eight types of activation foil and 14 kinds of nuclear reaction were used. The absorbed dose was calculated using the flux-to-dose conversion factor. The average neutron flux and Kerma rates of the beams used were 1.0 × 109 n/cm2/s and 48.0 cGy/h for the thermal neutron range (less than 0.6 eV), 1.6 × 108 n/cm2/s and 4.6 cGy/h for the epithermal neutron range (0.6 through 10 keV), and 9.4 × 106 n/cm2/s and 32.0 cGy/h for the fast neutron range (more than 10 keV), respectively. The Kerma rate for the boron dose per Φ n/cm2/s of the thermal neutron flux for 1 µg/g of 10B was 2.67 × 10-8 Φ cGy/h. The dose rate of γ-rays, including contaminating γ-rays in reactor neutron beams and γ-rays resulting from capture of thermal neutrons by hydrogen atoms (1H(n, γ)2H) was 66.0 cGy/h.
Colony formation assay
Following irradiation, colony formation was performed. SAS cells were plated onto 60 or 100 mm dishes at a cell density yielding approximately 100 - 1,000 or 3,000 - 50,000 cells per dish, respectively. SAS cells were cultured for 10 days, fixed in ethanol, and stained with 1% crystal violet. The surviving cell fraction was determined as percentage of number of colonies in the treated culture compared to the non-irradiated control culture.
Micronucleus (MN) assay
The SAS cells that were not used for colony formation assay were further incubated for 1 day in tissue culture dishes with 1.0 µg/mL of cytochalasin-B to inhibit cytokinesis while allowing nuclear division, and cultures were then trypsinized and cell suspensions were fixed. The MN frequency was defined as the ratio of the number of micronuclei in the binuclear cells to the total number of binuclear cells observed. The ratios were counted for all treatment conditions.
Data analysis and statistics
The γ-ray irradiation experiment was repeated fourth and neutron beam experiment was performed once. Other experiments without irradiation were carried out in triplicates. To examine the differences between pairs of values, the Student’s t-test and χ2-test were used when variances of the two groups could be assumed to be equal; otherwise, the Welch t-test was used. P values were from two-sided tests.
| Results | ▴Top |
Cell survival curves of SAS cells under γ-ray irradiation combined with or without rapamycin are shown in Figure 1. SAS cells treated with mTOR inhibitor rapamycin (1 µM or 2 µM) showed resistance to γ-rays compared with untreated control (Fig. 1). The radio-resistance induced by rapamycin against γ-rays was about two-fold higher than that of control at surviving fraction of 0.3. Regarding the concentration of rapamycin coexisting in the culture medium, the degree of reduction in sensitivity to γ-rays was almost the same at 1 µM and 2 µM. Thus, it was considered that the effect of rapamycin under γ-ray irradiation was saturated at 1 µM. Therefore, at the subsequent irradiation experiments other than this experiment in which changes in sensitivity to γ-ray irradiation were detected using colony forming assay, as the conditions under which rapamycin coexisted in the culture medium, the only conditions under which 1 µM rapamycin coexisted in the culture medium was selected.
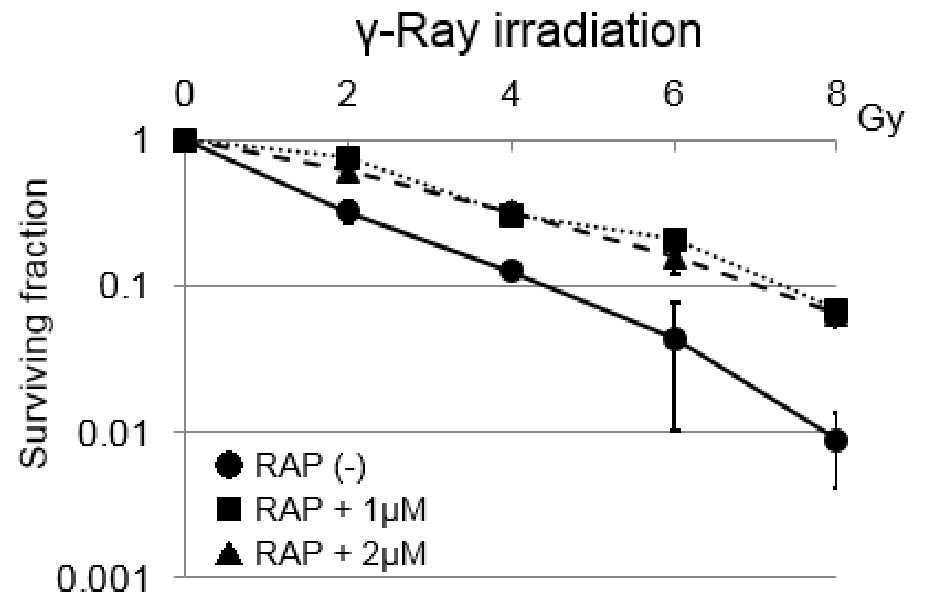 Click for large image | Figure 1. Cell survival curves of SAS tumor cells under γ-ray irradiation combined with or without rapamycin. Error bars indicate standard deviations (SDs) calculated from three independent experiments. Both 1 and 2 µM rapamycin induced significant radio-resistance to γ-rays in SAS cells. The differences between control and RAP (1 µM) were significant (P < 0.05). The same tendency was observed with control and RAP (1 µM). RAP: rapamycin. |
Dose-response curves of MN frequencies under γ-ray irradiation are shown in Figure 2. Clear difference in MN frequency was detected between with and without rapamycin. With rapamycin, MN frequency was reduced through combining with rapamycin treatment.
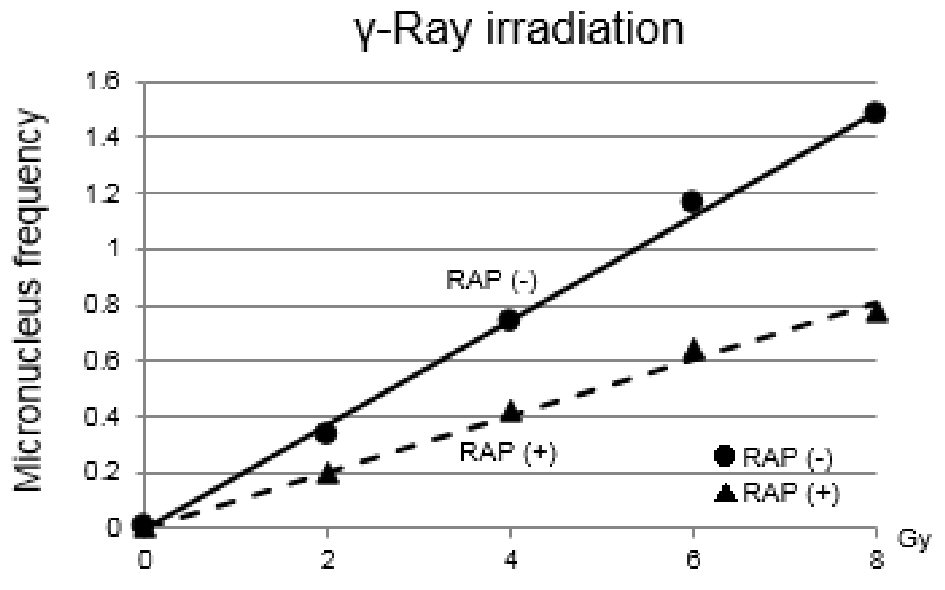 Click for large image | Figure 2. Dose-response curves of micronucleus frequencies under γ-ray irradiation are shown. Clear difference in micronucleus frequency under γ-ray irradiation was detected between with and without rapamycin. With rapamycin, micronucleus frequency was reduced through combining with rapamycin treatment. The differences between the two values were significant (P < 0.05). RAP: rapamycin. |
Figure 3 shows 10B concentration in SAS cell suspensions. 10B concentration in SAS cells was determined with prompt γ-ray analysis. 10B concentration from BPA into SAS cells was reduced more remarkably through combining with rapamycin treatment at higher concentration.
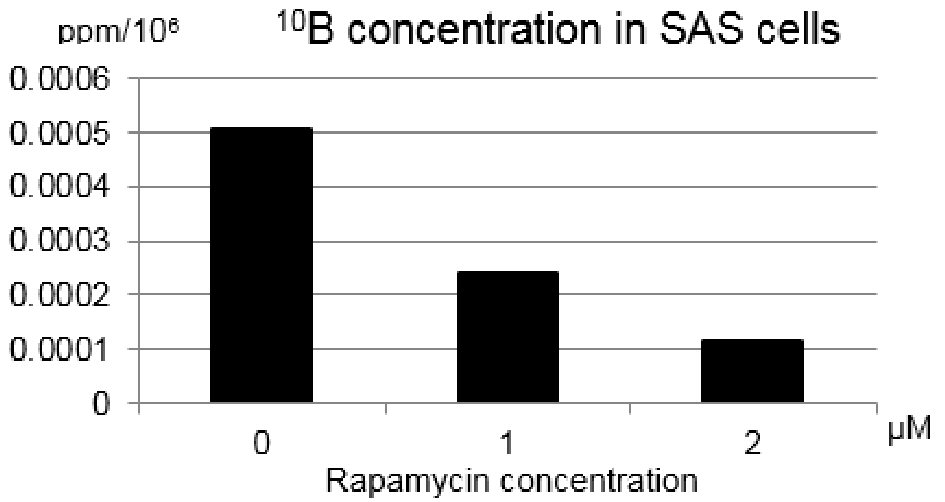 Click for large image | Figure 3. Changes in the 10B concentrations of single cell suspensions at the density of 200,000 SAS cells per 1.0 mL of 10B free cell culture medium after treatment with rapamycin (1 µM or 2 µM) for 24 h. The concentration of 10B from boronophenylalanine-10B into SAS cells was reduced through combining with rapamycin. |
The cell survival curves for reactor neutron beams without or with BPA are shown in Figure 4a. Reactor neutron beams include both neutrons and γ-rays. Thus, the cell survival following reactor neutron beam irradiation was normalized with the cell survival after γ-ray irradiation only by dividing the data for neutron beams by the data for γ-ray irradiation only in order to obtain the data on cell survival for irradiation with neutrons only, that is named as “neutron beams-γ-rays” (Fig. 4b). Under neutron beam irradiation without BPA, the surviving fraction was not significantly different between with and without rapamycin (1 µM). However, with BPA, the surviving fraction was significantly higher in combination with rapamycin treatment than without rapamycin. But, under neutrons only irradiation (“neutron beams-γ-rays”), with or without BPA, no clear differences in cell survival were detected between with and without rapamycin.
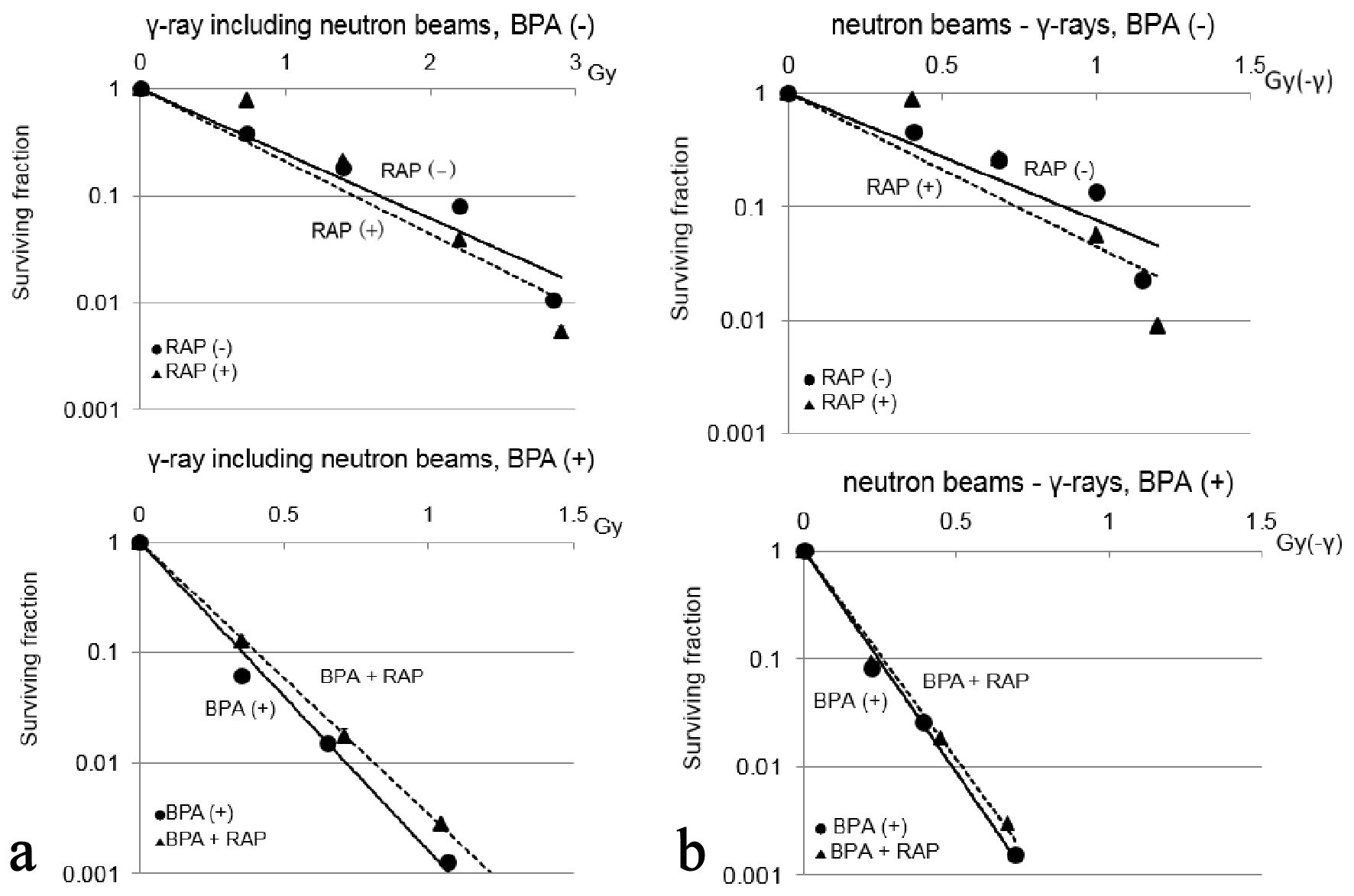 Click for large image | Figure 4. Cell survival curves for neutron beams without and with BPA are shown in (a) and (b), respectively. Error bars indicate standard deviations (SDs) calculated from three independent experiments. The quantification of cell survival following reactor neutron beam irradiation was normalized with the cell survival for γ-ray irradiation alone by dividing the data for neutron beams by the data for γ-ray irradiation alone in order to obtain the data on cell survival for irradiation with “neutron beams-γ-rays” (b). Under irradiation with γ-ray including neutron beams without BPA, the cell survival did not show significant difference between with and without rapamycin. With BPA, significantly higher survival fraction was observed with rapamycin treatment compared to untreated control (P < 0.05). However, under “neutron beams-γ-rays”, with or without BPA, no clear differences in cell survival were detected between treatments with and without rapamycin. BPA: boronophenylalanine-10B; RAP: rapamycin. |
We further investigated MN frequency after irradiation with γ-ray including neutron beams with or without BPA (Fig. 5). MN formation is a hall mark of genotoxicity, and the MN assay is an important method for genotoxicity screening. As shown here, under the neutron beam irradiation without BPA, clear difference in MN frequency was not detected between with and without rapamycin. With BPA, MN frequency was remarkably reduced through combining with rapamycin treatment.
 Click for large image | Figure 5. Dose-response curves of after irradiation with γ-ray including neutron beams without and with BPA are shown in (a) and (b), respectively. Clear difference in micronucleus frequency under neutron beam irradiation without BPA was not detected between with and without rapamycin. With BPA, micronucleus frequency was reduced through combining with rapamycin treatment (P < 0.05). This suggested that the delivery of 10B from BPA into cultured SAS cells was reduced through the treatment with rapamycin. BPA: boronophenylalanine-10B; RAP: rapamycin. |
To analyze the effect of rapamycin on the surviving fractions in SAS cells, the dose-modifying factors in SAS cells for rapamycin treatment relative to without rapamycin under “γ-ray including neutron beams” and γ-rays were calculated, at the surviving fractions of 0.3 and 0.03 (Table 1). Further, at the endpoint of MN frequency of 0.6, the dose-modifying factors for rapamycin treatment relative to without rapamycin in SAS cells was also calculated (Table 1). Under γ-ray including neutron beam irradiation without BPA, rapamycin treatment did not show any significant difference in sensitivity. Actually, when combined with rapamycin, SAS cells became slightly radiosensitive in terms of the cell survival analysis. However, in contrast, they showed slight radio-resistance in terms of the MN frequency analysis. This may be partly because the release of mTOR inhibition may have caused a rebound phenomenon in intracellular processes such as autophagy control. With regard to irradiation with BPA, the values of the dose-modifying factor for “with BPA” were larger than those for “without BPA”. This suggested that the delivery of 10B from BPA into cultured SAS cells was reduced through the treatment with rapamycin. Concerning γ-ray irradiation, 2 and 1.8 were significantly larger than 1 and therefore the sensitivity to γ-ray was significantly reduced in combination with rapamycin. This was thought to be due to the fact that rapamycin had an effect of suppressing cell growth and proliferation, leading to reducing sensitivity of SAS cells to γ-rays [12].
 Click to view | Table 1. Dose-Modifying Factorsa for SAS Cells Combined With Rapamycin Compared Without Rapamycin Under the Irradiation With γ-Rays Only or γ-Ray Including Neutron Beams |
To evaluate the relative biological effectiveness (RBE) for “neutron beams - γ-rays” compared with γ-rays, the data for “neutron beams - γ-rays” at the endpoint of surviving fraction of 0.3 are shown in Table 2. Overall, all values of the RBE were significantly larger than 1.0 (P < 0.05), meaning cultured SAS cells were much more sensitive to neutron beam irradiation than γ-ray irradiation with or without BPA or rapamycin. This is probably because the employed neutron beam consists of a high proportion of high linear energy transfer (LET) radiation. Moreover, with or without rapamycin, the values for “with BPA” were significantly higher than those “without BPA” (P < 0.05). This indicates that the contribution of boron dose purely derived from neutron capture reaction between 10B and thermal neutrons is significantly larger than any dose other than the boron dose among the doses that have to be considered when reactor neutron beams were irradiated. However, at “neutron beams-γ-rays” in the use of BPA, the value for “incubation with rapamycin” was lower than those without rapamycin, although not significantly. This is again thought to be due to the reduction of the delivery of 10B from BPA into cultured SAS cells through combining with rapamycin.
 Click to view | Table 2. Relative Biological Effectivenessa Under the Irradiation With “Neutron Beams-γ-Rays” Compared With γ-Rays Only at the Surviving Fraction of 0.3 |
| Discussion | ▴Top |
The mTOR signaling pathway has been implicated in multiple mechanisms of resistance to anticancer drugs and poor treatment outcomes in various human cancers [13-16]. However, significance of mTOR in BNCT and its effect on outcome of BNCT remains unknown. Therefore, the current study was undertaken to examine the effects of mTOR inhibition on BNCT using BPA in cultured SAS cells. Actually, clinical BNCT has been carried out for patients with malignant gliomas, melanomas, inoperable head and neck tumors and oral cancer [1, 17, 18]. The use of mTOR inhibitors has been approved for the treatment of advanced renal cell carcinoma, subependymal giant cell astrocytoma associated with tuberous sclerosis, pancreatic neuroendocrine tumors, and in combination with exemestane in advanced hormone receptor-positive breast cancer [13-16]. Several preclinical studies have also suggested mTOR inhibitors can enhance the efficacy of different chemotherapeutic agents in various cancers [15, 19]. However, little is still known about the significance of employing an mTOR inhibitor as a combined agent with BNCT.
Here, we found that mTOR inhibitor rapamycin reduced the effect of BPA-BNCT. Our hypothesis was that the addition of mTOR inhibitors prevents the proliferation of tumor cells and consequently decreases 10B-loading into tumor cells, resulting in reducing the antitumor effect of BPA-BNCT. The boron dose in boron neutron capture reaction accounts for most of the biological effect in BNCT. The other dose components are contributed to background doses consisted of high- and low-LET radiation components delivered to both tumor and normal tissues [20].
It has been demonstrated that growth-arrested cells are relatively radioresistant, compared with actively cycling cells [21]. Since mTOR inhibitor can suppress tumor cell proliferation, SAS cells with mTOR inhibitor (rapamycin 1 µM or 2 µM) showed resistance to γ-rays in terms of cell survival compared with no treatment with rapamycin (Fig. 1). In addition, a clear difference in MN frequency under γ-ray irradiation was detected between with and without rapamycin (Fig. 2). Namely, MN frequency was reduced through combining with rapamycin treatment. But, irradiation using the γ-ray including neutron beams only without BPA suppressed this decrease in sensitivity even when combined with rapamycin (Fig. 4a, upper). This may be because the employed reactor neutron beams mainly consist of high-LET neutrons although including low-LET γ-rays [22]. However, when neutron beams were delivered after BPA was administered, the decrease in sensitivity through combination with rapamycin became clearer than irradiation without BPA (Fig. 4b). This may be because the distribution of 10B from BPA to tumor cells was suppressed by rapamycin. In fact, the 10B concentration from BPA into tumor cells was reduced through combination with rapamycin (Fig. 3).
In recently performed clinical BNCT for brain tumors, refractory recurrent head and neck tumors and malignant melanoma, BPA is always employed as a 10B-carrier combined with or without BSH [20]. As shown in this study, in BNCT, especially in BPA-BNCT, when mTOR inhibitor is employed as one of chemotherapeutic agents, there is a possibility that the distribution of 10B into tumor cells can be suppressed, resulting in reducing therapeutic effect of BNCT. In other words, the period for chemotherapy using mTOR inhibitor should not overlap with that for BNCT. However, it was previously reported that in Glioma models no evidence of increased radio-sensitivity through combination with rapamycin was observed in vitro. However, in another report, significantly increased radio-sensitivity was shown in vivo [23]. In addition to its direct role in repressing proliferation of tumor cells, since rapamycin is thought to be able to inhibit angiogenesis and tumor vasculature, the tumor cells might be significantly sensitized to radiotherapy in vivo. On the other hand, it was already clarified that 10B from BSH shows different bio-distribution characteristics in solid tumors from that from BPA [4, 5]. Therefore, when BSH is employed as a 10B-carrier in BNCT, significance and usefulness of combined treatment with mTOR inhibitor in BSH-BNCT also has to be evaluated in the future.
The present study has several limitations. First, only cultured SAS cells were used for experimentation in vitro. Under in vivo conditions, solid tumors may behave differently from in vitro cultured cells even after totally similar treatment. Second, it was difficult to repeat these experiments using reactor neutron beams because neutron beams for experiment are available exclusively at the reactor institute, KUR. Therefore, further studies are needed to examine the effects of mTOR inhibition when combined with BNCT in SAS cells in vivo. Finally, in cancer therapy including BNCT, through combined treatment with mTOR inhibitor, resistance to γ-rays and repression of distributing drugs into tumor cells have to be carefully taken into account.
Acknowledgments
This study was supported in part by a grant-in-aid for Scientific Research (C) (19K08171) from the Japan Society for the Promotion of Science.
Financial Disclosure
No funding to disclose.
Conflict of Interest
All authors declare that they have no conflict of interest concerning this manuscript.
Informed Consent
Not applicable.
Author Contributions
HT, SM and YN were responsible for conception and design. HT and SM were responsible for analysis, interpretation, drafting and critical revision of the article. HT, SM and YN were responsible for final approval of the article.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Barth RF, Coderre JA, Vicente MG, Blue TE. Boron neutron capture therapy of cancer: current status and future prospects. Clin Cancer Res. 2005;11(11):3987-4002.
doi pubmed - Masunaga S, Tatebe H, Nishimura Y, Tano K, Sanada Y, Moriwaki T, Sakurai Y, et al. Effect of oxygen pressure during incubation with a (10)B-carrier on (10)B uptake capacity of cultured p53 wild-type and mutated tumor cells: dependency on p53 status of tumor cells and types of (10)B-carriers. Int J Radiat Biol. 2016;92(4):187-194.
doi pubmed - Miyatake S, Kawabata S, Kajimoto Y, Aoki A, Yokoyama K, Yamada M, Kuroiwa T, et al. Modified boron neutron capture therapy for malignant gliomas performed using epithermal neutron and two boron compounds with different accumulation mechanisms: an efficacy study based on findings on neuroimages. J Neurosurg. 2005;103(6):1000-1009.
doi pubmed - Soloway AH, Aronow S, Kaufman C, Balcius JF, Whitman B, Messer JR. Penetration of brain and brain tumor. VI. Radioactive scanning agents. J Nucl Med. 1967;8(11):792-799.
- Wittig A, Sauerwein WA, Coderre JA. Mechanisms of transport of p-borono-phenylalanine through the cell membrane in vitro. Radiat Res. 2000;153(2):173-180.
doi - Wongthai P, Hagiwara K, Miyoshi Y, Wiriyasermkul P, Wei L, Ohgaki R, Kato I, et al. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Sci. 2015;106(3):279-286.
doi pubmed - Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27(13):2278-2287.
doi pubmed - Kuwahara Y, Mori M, Kitahara S, Fukumoto M, Ezaki T, Mori S, Echigo S, et al. Targeting of tumor endothelial cells combining 2 Gy/day of X-ray with Everolimus is the effective modality for overcoming clinically relevant radioresistant tumors. Cancer Med. 2014;3(2):310-321.
doi pubmed - Edwards E, Geng L, Tan J, Onishko H, Donnelly E, Hallahan DE. Phosphatidylinositol 3-kinase/Akt signaling in the response of vascular endothelium to ionizing radiation. Cancer Res. 2002;62(16):4671-4677.
- Schmidt-Ullrich RK, Contessa JN, Lammering G, Amorino G, Lin PS. ERBB receptor tyrosine kinases and cellular radiation responses. Oncogene. 2003;22(37):5855-5865.
doi pubmed - Ryynanen PM, Kortesniemi M, Coderre JA, Diaz AZ, Hiismaki P, Savolainen SE. Models for estimation of the (10)B concentration after BPA-fructose complex infusion in patients during epithermal neutron irradiation in BNCT. Int J Radiat Oncol Biol Phys. 2000;48(4):1145-1154.
doi - Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471-484.
doi pubmed - Kirova YM, Servois V, Chargari C, Amessis M, Zerbib M, Beuzeboc P. Further developments for improving response and tolerance to irradiation for advanced renal cancer: concurrent (mTOR) inhibitor RAD001 and helical tomotherapy. Invest New Drugs. 2012;30(3):1241-1243.
doi pubmed - Buijsen J, van den Bogaard J, Jutten B, Belgers E, Sosef M, Leijtens JW, Beets GL, et al. A phase I-II study on the combination of rapamycin and short course radiotherapy in rectal cancer. Radiother Oncol. 2015;116(2):214-220.
doi pubmed - Li SH, Lin WC, Huang TL, Chen CH, Chiu TJ, Fang FM, Huang WT, et al. Significance of mammalian target of rapamycin in patients with locally advanced stage IV head and neck squamous cell carcinoma receiving induction chemotherapy with docetaxel, cisplatin, and fluorouracil. Head Neck. 2016;38(Suppl 1):E844-852.
doi pubmed - Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6(9):729-734.
doi pubmed - Barth RF, Vicente MG, Harling OK, Kiger WS, 3rd, Riley KJ, Binns PJ, Wagner FM, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol. 2012;7:146.
doi pubmed - Miyatake S, Kawabata S, Kajimoto Y, Kuroiwa T, Ono K. [Boron neutron capture therapy without craniotomy for malignant gliomas]. Nihon Rinsho. 2005;63(Suppl 9):447-451.
- Jiang BH, Liu LZ. Role of mTOR in anticancer drug resistance: perspectives for improved drug treatment. Drug Resist Updat. 2008;11(3):63-76.
doi pubmed - Hopewell JW, Morris GM, Schwint A, Coderre JA. The radiobiological principles of boron neutron capture therapy: a critical review. Appl Radiat Isot. 2011;69(12):1756-1759.
doi pubmed - Hwang HS, Davis TW, Houghton JA, Kinsella TJ. Radiosensitivity of thymidylate synthase-deficient human tumor cells is affected by progression through the G1 restriction point into S-phase: implications for fluoropyrimidine radiosensitization. Cancer Res. 2000;60(1):92-100.
- Masunaga SI, Sakurai Y, Tano K, Tanaka H, Suzuki M, Kondo N, Narabayashi M, et al. Effect of bevacizumab combined with boron neutron capture therapy on local tumor response and lung metastasis. Exp Ther Med. 2014;8(1):291-301.
doi pubmed - Eshleman JS, Carlson BL, Mladek AC, Kastner BD, Shide KL, Sarkaria JN. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res. 2002;62(24):7291-7297.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


