| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 2, April 2022, pages 69-83
Long-Term Cardiac Disease- and Cancer-Associated Mortalities in Patients With Non-Metastatic Stomach Adenocarcinoma Receiving Resection and Chemotherapy: A Large Competing-Risk Population-Based Cohort Study
Lei Huanga, b, c, g, h , Yan Shia, g, Ya Jie Zhaob, d, Lei Wangb, d, Wei Guo Hub, d, h, Zheng Gang Zhua, e, h, Jun Zhanga, e, f, h
aDepartment of Oncology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China
bMedical Center on Aging of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China
cGerman Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ), Heidelberg 69120, Germany
dDepartment of Geriatrics, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China
eDepartment of General Surgery, Shanghai Key Laboratory of Gastric Neoplasms, Shanghai Institute of Digestive Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200025, China
fState Key Laboratory of Oncogenes and Related Genes, Shanghai Jiao Tong University, Shanghai 200025, China
gThese authors contributed equally to this work.
hCorresponding Authors: Jun Zhang,; Lei Huang,; Wei Guo Hu,; Zheng Gang Zhu,
Manuscript submitted January 10, 2022, accepted February 21, 2022, published online April 12, 2022
Short title: Heart- and Cancer-Specific Mortalities in nmGaC Patients
doi: https://doi.org/10.14740/wjon1445
| Abstract | ▴Top |
Background: The survival of patients with non-metastatic gastric adenocarcinoma (nmGaC), who are receiving more and more frequently chemotherapy, has improved throughout the last decades, while treatment-caused cardiotoxicity remains a major concern. This study aimed to investigate competing causes of mortality and prognostic factors within a large cohort of patients with resected nmGaC, and to describe the heart-specific mortalities of patients undergoing resection and chemotherapy and of all resected patients.
Methods: In this population-based cohort study, data on patients diagnosed with nmGaC from 2004 through 2016, managed with resection with or without chemotherapy, followed up until the end of 2016, and surviving ≥ 1 month were retrieved from the US Surveillance, Epidemiology, and End Results-18 Program. Cumulative mortality functions were calculated. Prognostic factors for heart- and cancer-specific mortalities were evaluated using both multivariable-adjusted Fine-Gray subdistribution and cause-specific hazard functions.
Results: Together 21,257 patients with resected nmGaC were eligible for analysis with an accumulated follow-up of 73,711 person-years, where 10,718 (50%) also underwent chemotherapy. Mortalities were overestimated when using the Kaplan-Meier method. Heart diseases were the most common non-cancer cause of mortality. Compared with all resected patients, heart-specific mortality of those also receiving chemotherapy was lower overall and especially at older ages. In the total group of patients, the 8-year cumulative mortalities from heart diseases were 4.4% and 2.0% in resected patients and those also receiving chemotherapy, respectively; in patients ≥ 80 years, the heart disease-specific mortalities were as high as 11.1% and 6.5%, respectively. In overall patients undergoing resection, older ages, black ethnicity, and location at gastric antrum/pylorus were associated with increased heart-specific mortality, while more recent period, female sex, Asian/Pacific Islanders, invasion of serosa, and more positive lymph nodes were associated with lower heart-specific mortality; among those further receiving chemotherapy, only the associations with period of diagnosis, age, and ethnicity were significant. Associations with older ages were stronger for heart-specific mortality than for cancer-associated mortality.
Conclusions: Among survivors with resected nmGaC receiving chemotherapy, heart-specific mortality, the most common one among non-cancer causes of mortality, is not higher compared to overall resected patients in this observational study, suggesting that chemotherapy may be relatively safely administered to selected patients under strict indications. Age and ethnicity were major factors associated with heart-specific mortality in both overall resected patients and those further receiving chemotherapy. Overall and stratified cause-specific cumulative incidences of mortality are provided, which can be more clinically useful than the Kaplan-Meier estimates. Our study provides clinically useful evidence for tailored patient management.
Keywords: Cardio-oncology; Gastric adenocarcinoma; Cardiovascular; mortality; Competing risk; Cumulative incidence function; Population-based cohort study
| Introduction | ▴Top |
Gastric cancer, the majority of which is adenocarcinoma, ranks fifth in cancer incidence and is the fourth leading cause of cancer-related mortality globally, with about 1,089,000 new cases and around 769,000 associated deaths in 2020 [1-4]. While resection remains the mainstay and cornerstone of curative treatment for most non-metastatic gastric adenocarcinoma (nmGaC) [5, 6], increasing numbers of patients with nmGaC also receive chemotherapy, based on evidence from major clinical trials [7-10].
Cardiovascular diseases are a leading non-cancer cause of competing mortality among cancer survivors and can cause early death [11, 12]. While overall the application of chemotherapy has contributed to improvement of survival in some patients with resected nmGaC throughout the last decades, not all patients benefit from chemotherapy use [13-16], and the long-term mortality due to persistent deterioration in left ventricular ejection fraction and incidental heart diseases remains a major threat to cancer survivors [17]. The commonly used chemotherapeutic agents for nmGaC include fluorouracil-based (e.g., capecitabine, 5-fluorouracil, S-1, and tegafur) and platinum-based drugs (e.g., oxaliplatin and cisplatin); and their potential cardiotoxicity has long attracted oncologists’ attention [18-22]. On the other hand, to receive further chemotherapy after resection, patients usually undergo careful assessment and selection based on performance status and others, and can be generally medically fitter and more tolerable than overall resected patients. Older patients are frailer and more often have comorbidities. Older age is a natural driver for the incidence of heart diseases and related mortality, and heart diseases may compete with cancer as a major cause of mortality especially for older patients [23]. Various other competing risk factors for mortality exist in patients with nmGaC, and survival estimates may be incorrect without them taken into account. It is of utmost importance to analyze prognostic factors for patients with nmGaC with careful consideration of competing risks [24].
In the light of potential treatment-induced cardiotoxic effects, herein we aimed to investigate the long-term heart- and cancer-specific mortalities within a large cancer cohort involving 21,257 US patients with resected nmGaC in the presence of competing risks, and to investigate the cause-specific mortalities and factors associated with risk of mortality in patients receiving both resection and chemotherapy and in all resected patients.
| Materials and Methods | ▴Top |
Patients
In this population-based cohort study, individual-level data on patients with nmGaC were retrieved from the Surveillance, Epidemiology, and End Results (SEER)-18 Program, which consists of 18 population-based registries collecting cancer incidence and mortality data in the USA [25]. Access to the data was approved after sending a formal request to SEER and signing the required data usage agreements.
Patients of nmGaC were defined as patients with microscopically-confirmed first primary invasive adenocarcinoma of the stomach (International Classification of Diseases for Oncology, Third Edition (ICD-O-3) code: C16 [26]) without distant metastasis. Only those who underwent resection in January 2004 through December 2016 were included (Supplementary Material 1, www.wjon.org). Cancers of other histology types including squamous cell carcinoma, gastrointestinal stromal tumor or sarcoma, neuroendocrine tumor or carcinoid, lymphoma, and germ-cell tumor were ineligible (Supplementary Material 2, www.wjon.org), as were patients with non-gastric cancers involving the stomach, with benign or in situ tumors, or with other malignancies before gastric cancer. We further excluded patients with diagnosis based on death certificate only or autopsy, with missing follow-up period, survival status, or cause of death, or with unknown metastasis status. Cancers with distant metastasis were excluded since resection is not routinely recommended for them, and competing effects of other death causes than gastric cancer could be too small to be analyzable for them. Data before 2004 were not included, as the TNM stage information was unavailable. To minimize the effect of perioperative events on survival, we excluded patients surviving < 1 month.
Data on patient (year of diagnosis, sex, age, ethnicity, follow-up time, vital status, and cause of death), tumor (morphology, topography, stage, positive lymph node count, differentiation grade, and size), and treatment variables (resection type, chemotherapy, and radiotherapy) were retrieved. Tumor morphology and topography followed the ICD-O-3, where information on tumor pathology, differentiation, and location could be obtained. Tumor local invasion, lymph node involvement, and distant metastasis were derived from the American Joint Committee on Cancer/Union for International Cancer Control TNM staging, and were reclassified into categories consistent across the investigated period when the sixth or seventh edition was in effect (the seventh edition is identical with the newest eighth edition). Information on chemotherapy and radiotherapy was under-ascertained in SEER-18 [27], and it is not possible to differentiate between neoadjuvant and adjuvant chemotherapy. Cause of death was coded according to the International Classification of Diseases Tenth Revision (ICD-10). The major focus of our study was on mortality caused by diseases of the heart as a possible long-term risk of death competing with cancer, and heart-specific causes of mortality were defined following the SEER Recode 50060 (ICD-10 codes, I00-I09, I11, I13, I20 - I51) [28]. Gastric cancer-specific mortality followed the SEER Recode 21010 and 21020. Considering the unexpectedly high proportion of mortality due to esophageal cancer (SEER Recode 21010; 24.0% among cancer causes of mortality in patients who received both resection and chemotherapy; Supplementary Material 3, www.wjon.org) and the controversy in classifying cancers located in gastric cardia [29], SEER Recode 21010 was also regarded as gastric cancer-specific mortality.
The Institutional Review Board approval was not required. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Statistics
We summarized the distributions of all baseline characteristics by computing the mean, standard deviation, median, and interquartile range (IQR) for continuous variables, and the count and frequency for categorical variables. Follow-up was until December 31, 2016. Survival time was calculated until death or last follow-up, whichever occurred first. The follow-up time was calculated using the reverse Kaplan-Meier method [8].
Cumulative incidence functions (CIFs) [24], which allow for estimation of the incidence of the occurrence of an event while taking competing risk into account, were computed and plotted for cause-specific mortalities (heart disease and gastric cancer) to describe the probability of experiencing a specific endpoint in the presence of competing risks within overall resected patients and those further receiving chemotherapy, respectively. Stratification analyses by age group were further performed. As standard Kaplan-Meier analysis treats failures from competing events as censored, this approach would lead to an overestimation of the absolute risk of the event of interest (Supplementary Material 4, www.wjon.org) because competing events would then violate the assumption of independent or non-informative censoring [24]. Moreover, the Kaplan-Meier estimate would reflect mortality from the event of interest in a hypothetical world without competing events, which is less clinically relevant. Thus, to calculate the probability of death (cumulative mortality) from a specific cause, patients with nmGaC who died due to competing causes of death were retained in the underlying risk set rather than being censored.
Adjusted Fine-Gray subdistribution (HRSD) and cause-specific hazard ratios (HRCS) were both calculated for all resected patients and for the subset of patients who were also known to have received chemotherapy, to investigate the relative association of individual risk and prognostic factors with both heart- and cancer-related mortality, using the corresponding hazard function regressions mutually adjusted for year of diagnosis, sex, age, ethnicity, tumor location, local invasion, positive lymph node count, differentiation, and resection type as potential confounding factors. Considering the low sensitivity of the non-surgical variables [27] and the unavailability of time intervals between resection and non-surgical management, chemotherapy or radiotherapy was not further included in the multivariable models as a prognostic factor, and we compared the findings in patients receiving resection and chemotherapy to all patients receiving resection regardless of chemotherapy application rather than those undergoing resection alone. The proportional hazards assumption was verified both graphically using the log-log plot and analytically using the scaled Schoenfeld residuals test before performing survival analyses [9]. To make the calendar year groups more comparable in follow-up opportunity, further sensitivity analysis was conducted by analyzing patients diagnosed before 2012 with follow-up time restricted to the first 5 years. Patients with missing data on the above-mentioned covariates were excluded from multivariable modelling analyses. To check if findings held similar when missing covariates were imputed, analyses were repeated and estimates from 40 multiple-imputed datasets were combined.
Analyses were performed using the R 3.5.1 software (https://cran.r-project.org), with results considered statistically significant at two-sided P < 0.05.
| Results | ▴Top |
Patient characteristics
Among 169,620 identified patients with gastric cancer, we identified 21,257 patients with nmGaC undergoing cancer-directed resection in 2004 through 2016 eligible for analysis, including 10,718 patients who were also known to have received chemotherapy (Supplementary Material 1, www.wjon.org).
Compared to the total group of patients who underwent resection, those further receiving chemotherapy were more likely to be diagnosed in 2010 or later (57% vs. 52%), male (68% vs. 63%), and younger at diagnosis (mean age, 62 vs. 66 years), with a smaller proportion of patients ≥ 70 years (17% vs. 43%) (Table 1). Compared to overall group, cancers among patients who also received chemotherapy were more frequently located at gastric cardia (37% vs. 30%), had local invasion less often limited to lamina propria/submucosa (9% vs. 28%), had more positive lymph nodes (mean positive nodes, 5 vs. 3; 0 positive nodes, 33% vs. 50%), were more often poorly-differentiated/undifferentiated (73% vs. 65%), and were averagely larger (mean size, 5.3 vs. 4.6 cm), with a greater proportion of cancers ≥ 4 cm (63% vs. 53%). Partial/subtotal gastrectomy was most commonly performed (67% and 63%, respectively).
 Click to view | Table 1. Demographic and Clinicopathologic Characteristics at Diagnosis of Patients With Non-Metastatic Gastric Adenocarcinoma Undergoing Resection (and Chemotherapy), 2004 - 2016a |
The median follow-up times were 73 and 66 months, and the accumulated follow-up was 73,711 and 34,265 person-years, for overall resected patients and those who also received chemotherapy, respectively. The proportion of patients alive at the end of follow-up was similar between all resected patients (47%) and those who also received chemotherapy (46%). Among all named causes of death for deceased patients, gastric cancer was the most common cancer cause, and diseases of heart the most frequent non-cancer cause. Among deceased patients, gastric cancer was more commonly reported as the cause of death among patients who had also received chemotherapy compared with the total group (83% vs. 72%) whereas the opposite was observed for disease of heart (3% vs. 7%).
Heart diseases (among non-cancer causes: resection group, 31%; resection and chemotherapy group, 22%) and gastric cancer (among cancer causes: resection group, 92%; resection and chemotherapy group, 94%) were the most common non-cancer and cancer causes of mortality, respectively (Supplementary Material 3, www.wjon.org).
Cumulative mortality
The cumulative mortalities for different causes of death within all patients with nmGaC undergoing resection and those further receiving chemotherapy overall and stratified by age group are illustrated in Figures 1, 2 and 3, respectively; and the point estimates and 95% confidence intervals are shown in Table 2. By far the highest cumulative mortality was due to gastric cancer, followed by the combined group of non-cancer causes of death excluding heart diseases. Cumulative mortality due to diseases of heart was marginally higher than that caused by other subsequent cancers in all resected patients, but was slightly lower in those also receiving chemotherapy. Compared to all resected patients, cumulative mortality in the longer term in those further treated with chemotherapy was more frequently due to gastric cancer, but less often the other causes. Interestingly, cumulative mortality from gastric cancer within 1 year was lower in patients undergoing both resection and chemotherapy. The 8-year cumulative mortalities from heart diseases and gastric cancer were 4.4% and 45.2%, respectively in overall resected patients, and 2.0% and 54.6%, respectively in those also receiving chemotherapy (Table 2).
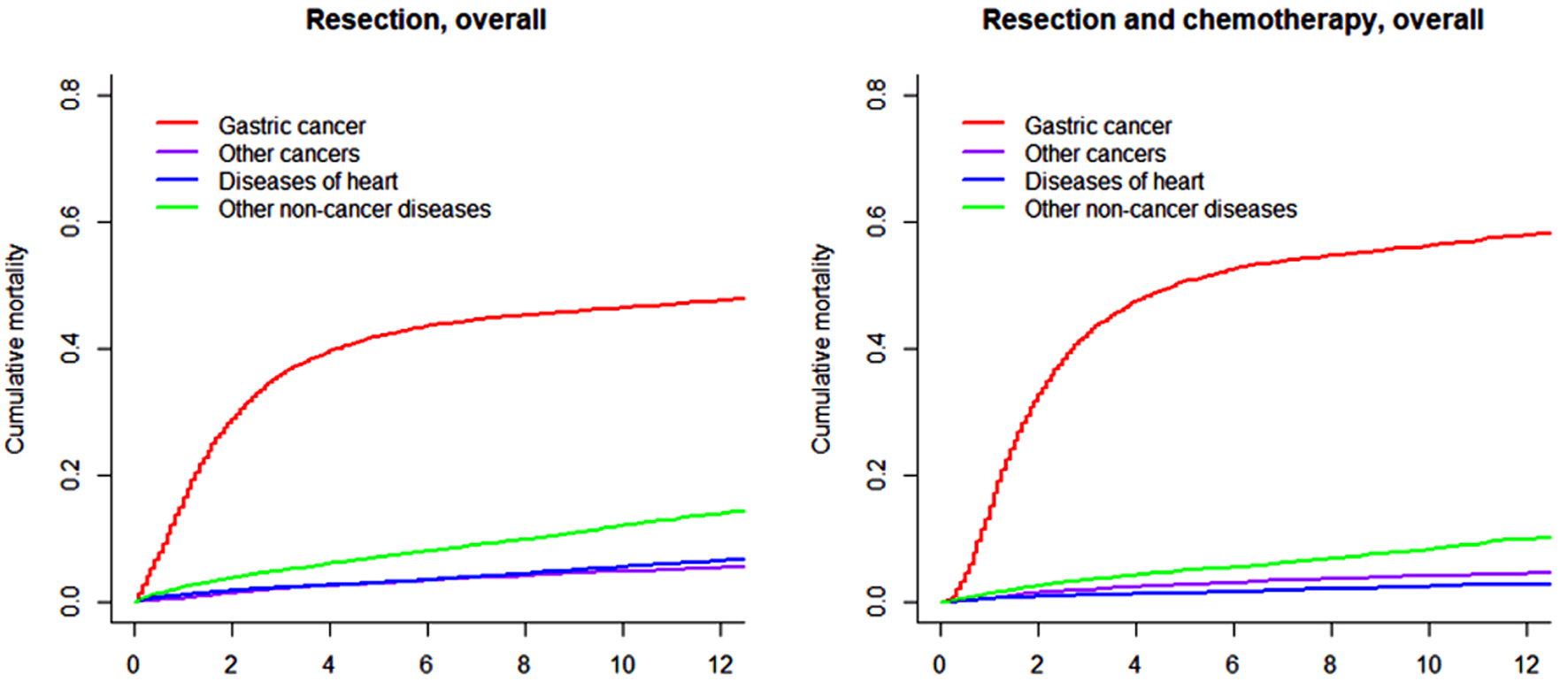 Click for large image | Figure 1. Cumulative incidence function curves illustrating mortality from gastric cancer, other cancers, diseases of heart, and other non-cancer diseases in patients with gastric adenocarcinoma undergoing resection (and chemotherapy). |
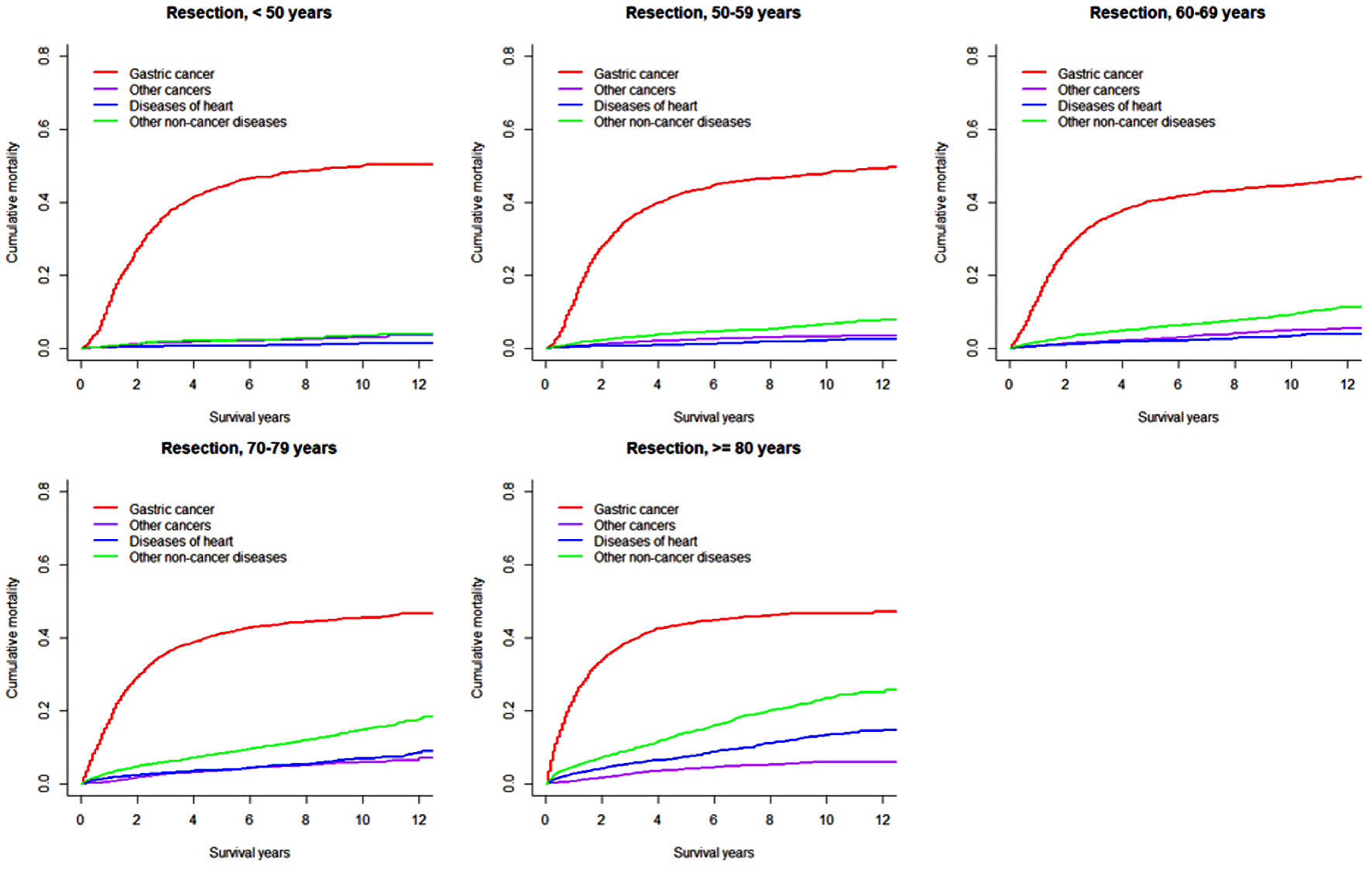 Click for large image | Figure 2. Cumulative incidence function curves illustrating mortality from gastric cancer, other cancers, diseases of heart, and other non-cancer diseases in patients with gastric adenocarcinoma undergoing resection, by age at diagnosis. |
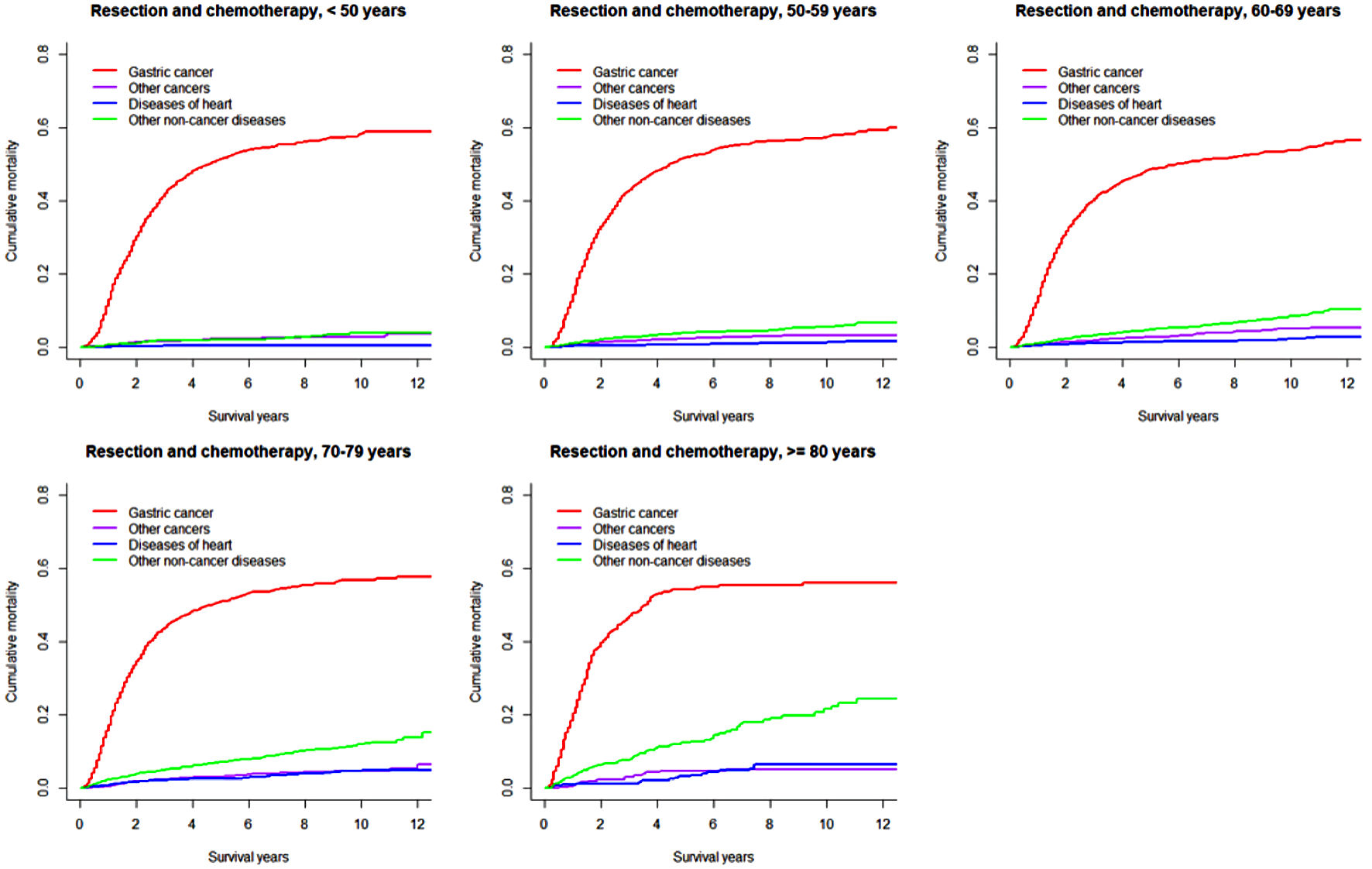 Click for large image | Figure 3. Cumulative incidence function curves illustrating mortality from gastric cancer, other cancers, diseases of heart, and other non-cancer diseases in patients with gastric adenocarcinoma undergoing resection and chemotherapy, by age at diagnosis. |
 Click to view | Table 2. Cumulative Incidences of Mortality (%) and 95% Confidence Intervals due to Diseases of Heart and Gastric Cancer in Patients With Non-Metastatic Gastric Adenocarcinoma Undergoing Resection (and Chemotherapy), Overall and Stratified by Patient Age |
In both overall resected patients and those further receiving chemotherapy, the cumulative gastric cancer-specific mortality was similar in the long term across all age groups, but increased more quickly with older age. Cumulative mortality due to other subsequent cancers was higher in patients ≥ 70 years. Cumulative mortalities due to heart and other non-cancer diseases mostly steadily increased with older age and longer length of follow-up, except that early rapid increases were observed in patients ≥ 70 years. Notably, heart-specific cumulative mortality was lower than mortality due to other subsequent cancers in patients < 70 years, while the difference disappeared or the comparison pattern was reversed in those ≥ 70 years. In patients receiving both resection and chemotherapy compared to all resected patients, cumulative mortalities from gastric cancer were higher after 1 year (within 1 year moralities were lower or similar), while mortalities from other subsequent cancers were mostly similar; cumulative mortalities from heart-specific and other non-cancer diseases were lower especially in patients ≥ 70 years. The 8-year cumulative mortalities from heart diseases in overall resected patients were as high as 11.1% in patients ≥ 80 years compared to 1.4% in those < 60 years (Table 2).
Subdistribution and cause-specific hazard ratios
The associations of individual prognostic factors with heart- and gastric cancer-specific mortalities within patients undergoing resection and those further receiving chemotherapy are shown in Tables 3 and 4, respectively. The associations with gastric cancer-specific mortality were described here (Supplementary Material 5, www.wjon.org).
 Click to view | Table 3. Fine-Gray Subdistribution and Cause-Specific Hazard Ratios and 95% Confidence Intervals for Heart- and Gastric Cancer-Associated Mortalities Among Patients With Non-Metastatic Gastric Adenocarcinoma Undergoing Resectiona |
 Click to view | Table 4. Fine-Gray Subdistribution and Cause-Specific Hazard Ratios and 95% Confidence Intervals for Heart- and Gastric Cancer-Associated Mortalities Among Patients With Non-Metastatic Gastric Adenocarcinoma Undergoing Resection and Chemotherapya |
Among all patients undergoing resection, those diagnosed in 2004 - 2009 had higher risks of heart-specific mortalities relative to those diagnosed in later period (HRSD = 1.66; HRCS = 1.24). While the subdistribution model did not reveal a significant association between heart-specific mortality and sex, the cause-specific model showed that females had lower heart-specific mortalities (HRCS = 0.83). Heart-specific mortality most strongly increased with older age (e.g., for patients aged 60 - 69 years relative to those ≥ 80 years: HRSD = 3.88; HRCS = 6.06). Compared to white people, black patients had higher heart-associated mortalities (HRSD = 1.31; HRCS = 1.41), while Asians/Pacific Islanders were at lower risks of such mortalities (HRSD = 0.81; HRCS = 0.73). Regarding the associations with tumor location, local invasion, and positive lymph node count, only the subdistribution model revealed statistical significance. Patients with cancers located at gastric antrum/pylorus had higher heart-specific mortalities (HRSD = 1.39), those with cancers invading serosa had lower heart-specific mortalities (HRSD = 0.76), and heart-specific mortalities decreased with more positive lymph nodes (e.g., for ≥ 16 vs. 0 positive nodes: HRSD = 0.52).
Compared to overall resected patients, the association with period of diagnosis was only significant when using the subdistribution model (HRSD = 1.40), and the association with age was less pronounced (e.g., for patients aged 60 - 69 years relative to those ≥ 80 years: HRSD = 2.72; HRCS = 3.76). Relative to white patients, black patients were at higher risks for heart-specific mortality with stronger association strength (HRSD = 1.63; HRCS = 1.74).
In sensitivity analyses of patients diagnosed before 2012 with follow-up time restricted to 5 years, results remained similar (Supplementary Materials 6, 7, www.wjon.org).
| Discussion | ▴Top |
By analyzing > 21,000 patients with nmGaC resected in the early 21st century, our population-based cohort study comprehensively depicted the competing causes of mortalities from heart diseases, other non-cancer diseases, gastric cancer, and other subsequent cancers, both overall and in age-stratified subgroups, and provided the long-term heart- and gastric cancer-specific mortalities using the CIF for clinical counseling and decision-making, which take into account all competing risks and which are more valid than the Kaplan-Meier estimates [24]. We found that while the gastric cancer-associated mortality in patients further receiving chemotherapy was higher than in overall resected patients, the heart-specific mortality did not increase and was even lower especially in patients ≥ 70 years. We further investigated factors associated with mortalities from different causes using both the Fine-Gray subdistribution and cause-specific regression models, and found that age and ethnicity were two major factors associated with heart-specific mortality.
For patients with resectable nmGaC, whose survival has continued to improve in recent decades due to medical advancement, resection remains the most important local treatment modality which can possibly cure disease and guarantee long-term survival [30]. Notably, some patients have local and/or distant disease relapse years after resection, which largely compromises prognosis [31]. To tackle this, systemic chemotherapy has been additionally recommended for resected patients who are in good medical condition to receive other treatment, and has contributed to further enhanced survival [32]; however, the survival improvement appears small, and not every patient with resected gastric cancer benefits from additional systemic treatment [13-16]. Outstandingly, the chemotherapy-induced cardiotoxicity is increasingly causing concern and may compete with the underlying cancer as a major cause of mortality, especially in older patients more often with basic heart diseases as comorbidity [18-22]. A retrospective study reports that cardiotoxicity occurs in 30% of gastric cancer patients managed with fluorouracil-based chemotherapy, where 10% experienced toxicity greater than grade 2 and 2% eventually died [18]. Accordingly, we found that heart diseases were the most common non-cancer cause of death in patients with nmGaC. Nevertheless, commonly only those with good performance status who are believed to well tolerate additional non-surgical treatment are selected for systemic therapy.
We found that compared to overall resected patients, those further receiving chemotherapy were more often male and averagely 4-year younger, and patients ≥ 70 years comprised < 20%; however, they more often had gastric cardia cancers and cancers with more advanced stage, poorer differentiation, and larger size. These differences in baseline characteristics suggest the careful selection of patients to receive further chemotherapy, and may well explain the observations that heart-specific mortality did not increase and even decreased in the further chemotherapy-treated patients, who instead died more often from gastric cancer in the long term. The decrease in heart-specific mortality was more pronounced in patients ≥ 70 years. Notably, gastric cancer-specific mortality within 1 year among patients receiving both resection and chemotherapy did not surpass that within overall resected patients, possibly suggesting the good short-term disease control of systemic therapy.
Together with most cancers, cardiovascular diseases and corresponding adverse events become more frequent when one gets older. With older age, heart-specific mortality sequentially increased (e.g., in overall resected patients, the 8-year mortality in patients ≥ 80 years was about eight times that in those < 60 years), and in those ≥ 70 years, it rapidly increased within the first few months. This highlights the importance of careful monitoring of cardiovascular events and timely implementation of corresponding prevention and management measures in older patients. Older patients are more prone to dying early from the underlying cancer, but have similar survival in the longer term with younger patients. This may indicate the heterogeneity of cancers occurring among patients of different age groups. To estimate the crude cumulative mortalities from different causes, we used the CIF rather than the Kaplan-Meier survival function, which resulted in overestimation of mortalities in the presence of competing risks [24, 33]. The overestimation may be due to the increased risk of death attributable to competing risks [34].
We further investigated the risk factors for mortifies from heart diseases and gastric cancer using both the subdistribution and cause-specific models [35]. Patients diagnosed in more recent period had lower heart-specific mortality, possibly thanks to the advancement in postsurgical care and chemotherapeutic strategies. As expected, older age was the strongest risk factor for heart-specific mortality, while the association was weaker in patients receiving both resection and chemotherapy compared to overall resected patients; this may also be due to meticulous patient selection. The discrepancies in heart-related mortality by ethnicity is in line with literature [36] and highlights the importance of individualized risk assessment and care and of special attention to black patients, who may have even greater heart-associated mortalities when chemotherapy was further added.
While both the subdistribution and cause-specific hazard function models account for competing risks, the interpretations are different. The former denotes the instantaneous rate of mortality (incidence) from the underlying cause in subjects who have not yet experienced any event (i.e., who are still alive) or who have died of causes other than the underlying one of interest (e.g., those having died of cardiovascular diseases when investigating cancer-specific mortality), while the latter denotes the instantaneous rate of mortality (rate of occurrence) from the underlying cause in subjects who have not yet experienced any event (i.e., who are still alive) [24]. We found that in overall resected patients, the subdistribution model revealed that those with gastric cardia cancers and those with more advanced cancers had lower heart-specific mortalities, while the cause-specific model did not show any statistical significance. The three strong prognostic factors, tumor location, local invasion, and positive lymph node count, for the cause-specific hazard for gastric cancer-specific death might have led to an apparent decrease in the cumulative incidence for heart-specific death when such factors have no effect on the cause-specific hazard for heart-specific death. This indirect effect of the prognostic factors for gastric cancer-associated death occurred because heart-specific death could not occur in those who died of gastric cancer and hence had a decreased risk for that event [24, 35, 37]. Competing risk analyses using both models are crucial for clinical decision making and personalized management. For example, a patient with high risk of gastric cancer-associated mortality and low risk of heart-specific mortality may be especially suitable for timely and full-course chemotherapy in addition to resection.
Our report has some limitations commonly shared by population-based registry-based studies. First, information on chemotherapy was registered with low sensitivity albeit high specificity in the SEER-18 database [27], and detailed information on chemotherapy (e.g., agents and course) was unavailable. We could not really select patients not receiving chemotherapy out from the total group of patients undergoing resection, i.e., the subgroup of patients undergoing resection alone could not be accurately determined. The time sequence between chemotherapy and resection was unknown in the SEER database, where neoadjuvant chemotherapy could not be differentiated from adjuvant chemotherapy. Accordingly, we did not look into a “non-chemotherapy” group, but analyzed two groups of patients with nmGaC in our study: the total group of all patients who underwent resection, and among them, the subgroup of patients who were known to have also received chemotherapy. The chemotherapy strategies might have changed over the investigation period. To account for this, we included year of diagnosis as a covariate in multivariable analyses and found decreased risks of both heart-specific and gastric cancer-specific mortalities in more recent period. Second, data on comorbidities and health condition were unavailable, and the original proportion of patients with cardiovascular diseases was unknown in those eventually dying of heart diseases or those who did not. Nevertheless, to be eligible for resection and particularly further systemic therapy, one should have been excluded from major cardiovascular comorbidities and been relatively medically fit (e.g., performance status score 0 - 1). Third, some other prognostic factors (e.g., resection margin) were unavailable. Nevertheless, we had included most of the common risk factors for mortalities from various causes in multivariable modeling. Furthermore, the findings were based on US patients, and may not be generalizable to other nations especially Asians, where gastric cancer is far more prevalent. Analyses of datasets from other countries are strongly encouraged. Notably, the patients who receive chemotherapy are most likely assessed to be eligible with healthy hearts, as opposed to patients with older age and those with black ethnicity who are known to more frequently, and females and Asians who are known to less often have heart diseases. Furthermore, when patients have more aggressive cancers (e.g., with invasion of serosa and/or lymph node metastasis), they are more likely to die with cancer rather than any other causes including heart issues. To this end, the findings of this study may be a result of selection bias.
To our knowledge, this is the largest population-based real-world study using individual-level data and comprehensive competing risk analytical methods to explore heart- and cancer-specific mortalities and associated prognostic factors in patients with nmGaC. The careful and strict patient enrollment, use of both the commonly used multivariable competing risk models, comprehensive comparison of findings in patients receiving both resection and chemotherapy to those within overall resected patients, and meticulous subgroup analyses enable this report to provide robust, valid, and useful references for individualized gastric cancer management.
Conclusions
Heart diseases is the most common non-cancer risk factor for mortality in nmGaC. Contrary to gastric cancer-associated mortality, heart-specific mortality among survivors with resected nmGaC receiving chemotherapy is not higher compared to overall resected patients in this observational study, suggesting that chemotherapy may be relatively safely administered to carefully selected patients under strict indications. Age and ethnicity were major factors associated with heart-specific mortality in both overall resected patients and those further receiving chemotherapy. Patients ≥ 70 years should undergo cautious risk assessment before receiving any treatment and be timely and carefully monitored for cardiovascular events during chemotherapy. Overall and stratified cumulative incidences of mortalities from heart diseases and gastric cancer are provided, which can be more clinically useful and relevant than the Kaplan-Meier estimates.
| Supplementary Material | ▴Top |
Suppl 1. Patient selection flow diagram.
Suppl 2. Inclusion and exclusion codes according to International Classification of Diseases for Oncology, Third Edition.
Suppl 3. Non-cancer and cancer causes of mortalities according to SEER Recode among patients with non-metastatic gastric adenocarcinoma undergoing resection with/without chemotherapy.
Suppl 4. Cumulative incidence functions and Kaplan-Meier estimates in patients with gastric adenocarcinoma undergoing resection (and chemotherapy).
Suppl 5. Subdistribution and cause-specific hazard ratios for gastric cancer-specific mortality.
Suppl 6. Fine-Gray subdistribution and cause-specific hazard ratios and 95% confidence intervals for heart- and gastric cancer-associated mortalities among patients with non-metastatic gastric adenocarcinoma undergoing resection, who were diagnosed before 2012 with follow-up time restricted to 5 years.
Suppl 7. Fine-Gray subdistribution and cause-specific hazard ratios and 95% confidence intervals for heart- and gastric cancer-associated mortalities among patients with non-metastatic gastric adenocarcinoma undergoing resection and chemotherapy, who were diagnosed before 2012 with follow-up time restricted to 5 years.
Acknowledgments
We would like to thank very much Dr. Sara Schonfeld from the National Institutes of Health (NIH)/National Cancer Institute (NCI) for her constructive, thoughtful, and insightful comments and suggestions, and great and kind assistance in revising the work and editing the English language, and Dr. Lindsay Morton from the NIH/NCI for her great support. We are very grateful to the members of the Surveillance, Epidemiology, and End Results Program (SEER) team for their kind work in data collection and delivery and to the staff of the Surgical Society of Chinese Medical Association for the great support.
Financial Disclusure
Our study was supported by the Start-up Fund for the Introduction of High Level Talents and the Fund for Medical Center on Aging (GB202103), Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. The supporter played no role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Conception or design: Huang L, Hu WG, Zhu ZG, and Zhang J. Acquisition, analysis, or interpretation of data: Huang L, Shi Y, Zhao YJ, Wang L, Hu WG, Zhu ZG, and Zhang J. Drafting of the manuscript: Huang L. Critical revision of the manuscript for important intellectual content: Huang L, Shi Y, Zhao YJ, Wang L, Hu WG, Zhu ZG, and Zhang J. Statistical analysis: Huang L. Administrative, technical, or material support: Hu WG, Zhu ZG, and Zhang J. All authors have approved the current version of the manuscript for submission and publication.
Data Availability
Data from the Surveillance, Epidemiology, and End Results Program database are available on reasonable request and with permission of the program.
Abbreviations
nmGaC: non-metastatic gastric adenocarcinoma; CIF: cumulative incidence function; HRCS: cause-specific hazard ratio; HRSD: subdistribution hazard ratio; CI: confidence interval
| References | ▴Top |
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
doi pubmed - Luciani LG, Cestari R, Tallarigo C. Incidental renal cell carcinoma-age and stage characterization and clinical implications: study of 1092 patients (1982-1997). Urology. 2000;56(1):58-62.
doi - Jahanafrooz Z, Mosafer J, Akbari M, Hashemzaei M, Mokhtarzadeh A, Baradaran B. Colon cancer therapy by focusing on colon cancer stem cells and their tumor microenvironment. J Cell Physiol. 2020;235(5):4153-4166.
doi pubmed - Zardavas D, Pugliano L, Piccart M. Personalized therapy for breast cancer: a dream or a reality? Future Oncol. 2013;9(8):1105-1119.
doi pubmed - Yu YZ, Lv DJ, Wang C, Song XL, Xie T, Wang T, Li ZM, et al. Hsa_circ_0003258 promotes prostate cancer metastasis by complexing with IGF2BP3 and sponging miR-653-5p. Mol Cancer. 2022;21(1):12.
doi pubmed - Matsubara T, Kokabu S, Nakatomi C, Kinbara M, Maeda T, Yoshizawa M, Yasuda H, et al. The actin-binding protein PPP1r18 regulates maturation, actin organization, and bone resorption activity of osteoclasts. Mol Cell Biol. 2018;38(4):e00425-17.
doi pubmed - Yasuda K, Matsubara T, Shirakawa T, Kawamoto T, Kokabu S. Protein phosphatase 1 regulatory subunit 18 suppresses the transcriptional activity of NFATc1 via regulation of c-fos. Bone Rep. 2021;15:101114.
doi pubmed - Ha YJ, Tak KH, Kim CW, Roh SA, Choi EK, Cho DH, Kim JH, et al. PSMB8 as a candidate marker of responsiveness to preoperative radiation therapy in rectal cancer patients. Int J Radiat Oncol Biol Phys. 2017;98(5):1164-1173.
doi pubmed - Wang R, Li S, Wen W, Zhang J. Multi-Omics Analysis of the Effects of Smoking on Human Tumors. Front Mol Biosci. 2021;8:704910.
doi pubmed - Su YA, Yang J, Tao L, Nguyen H, He P. Undetectable and decreased expression of KIAA1949 (Phostensin) encoded on chromosome 6p21.33 in human breast cancers revealed by transcriptome analysis. J Cancer. 2010;1:38-50.
doi pubmed - Bonneville R, Krook MA, Kautto EA, Miya J, Wing MR, Chen HZ, Reeser JW, et al. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis Oncol. 2017;2017:1-15.
doi pubmed - Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899-905.
doi pubmed - Attalla K, DiNatale RG, Rappold PM, Fong CJ, Sanchez-Vega F, Silagy AW, Weng S, et al. Prevalence and landscape of actionable genomic alterations in renal cell carcinoma. Clin Cancer Res. 2021;27(20):5595-5606.
doi pubmed - Peng D, Wei C, Zhang X, Li S, Liang H, Zheng X, Jiang S, et al. Pan-cancer analysis combined with experiments predicts CTHRC1 as a therapeutic target for human cancers. Cancer Cell Int. 2021;21(1):566.
doi pubmed - Liu J, Wang Y, Yin J, Yang Y, Geng R, Zhong Z, Ni S, et al. Pan-cancer analysis revealed SRSF9 as a new biomarker for prognosis and immunotherapy. J Oncol. 2022;2022:3477148.
doi pubmed - Ma L, Jin G, Yao K, Yang Y, Chang R, Wang W, Liu J, et al. Safety and Efficacy of Anti-PD-1/PD-L1 inhibitors compared with docetaxel for NSCLC: a systematic review and meta-analysis. Front Pharmacol. 2021;12:699892.
doi pubmed - Hung JH, Yang TH, Hu Z, Weng Z, DeLisi C. Gene set enrichment analysis: performance evaluation and usage guidelines. Brief Bioinform. 2012;13(3):281-291.
doi pubmed - Zhang SS, Zhu L, Peng Y, Zhang L, Chao FL, Jiang L, Xiao Q, et al. Long-term running exercise improves cognitive function and promotes microglial glucose metabolism and morphological plasticity in the hippocampus of APP/PS1 mice. J Neuroinflammation. 2022;19(1):34.
doi pubmed - Zhu Z, Cao C, Zhang D, Zhang Z, Liu L, Wu D, Sun J. UBE2T-mediated Akt ubiquitination and Akt/beta-catenin activation promotes hepatocellular carcinoma development by increasing pyrimidine metabolism. Cell Death Dis. 2022;13(2):154.
doi pubmed - Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X, Li Z, et al. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018;24(10):1550-1558.
doi pubmed - Hopkins AM, Kichenadasse G, Karapetis CS, Rowland A, Sorich MJ. Concomitant antibiotic use and survival in urothelial carcinoma treated with atezolizumab. Eur Urol. 2020;78(4):540-543.
doi pubmed - Wang A, Bao Y, Wu Z, Zhao T, Wang D, Shi J, Liu B, et al. Long noncoding RNA EGFR-AS1 promotes cell growth and metastasis via affecting HuR mediated mRNA stability of EGFR in renal cancer. Cell Death Dis. 2019;10(3):154.
doi pubmed - Wang Y, Tian Y, Liu S, Wang Z, Xing Q. Prognostic value and immunological role of AXL gene in clear cell renal cell carcinoma associated with identifying LncRNA/RBP/AXL mRNA networks. Cancer Cell Int. 2021;21(1):625.
doi pubmed - Wang W, Hu W, Wang Y, An Y, Song L, Shang P, Yue Z. Long non-coding RNA UCA1 promotes malignant phenotypes of renal cancer cells by modulating the miR-182-5p/DLL4 axis as a ceRNA. Mol Cancer. 2020;19(1):18.
doi pubmed - Inamura K. Renal Cell Tumors: Understanding Their Molecular Pathological Epidemiology and the 2016 WHO Classification. Int J Mol Sci. 2017;18(10):2195.
doi pubmed - Jonasch E, Walker CL, Rathmell WK. Clear cell renal cell carcinoma ontogeny and mechanisms of lethality. Nat Rev Nephrol. 2021;17(4):245-261.
doi pubmed - Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913-924.
doi pubmed - Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1(6):489-495.
doi pubmed - Scholtes MP, Alberts AR, Ifle IG, Verhagen P, van der Veldt AAM, Zuiverloon TCM. Biomarker-oriented therapy in bladder and renal cancer. Int J Mol Sci. 2021;22(6):2832.
doi pubmed - Tian ZH, Yuan C, Yang K, Gao XL. Systematic identification of key genes and pathways in clear cell renal cell carcinoma on bioinformatics analysis. Ann Transl Med. 2019;7(5):89.
doi pubmed - Bai D, Chen S, Feng H, Yin A, Lu J, Ma Y, Sugiyama H. Integrated analysis of immune-related gene subtype and immune index for immunotherapy in clear cell renal cell carcinoma. Pathol Res Pract. 2021;225:153557.
doi pubmed - Du T, Gao J, Li P, Wang Y, Qi Q, Liu X, Li J, et al. Pyroptosis, metabolism, and tumor immune microenvironment. Clin Transl Med. 2021;11(8):e492.
doi - Zhang H, Luo YB, Wu W, Zhang L, Wang Z, Dai Z, Feng S, et al. The molecular feature of macrophages in tumor immune microenvironment of glioma patients. Comput Struct Biotechnol J. 2021;19:4603-4618.
doi pubmed - Upadhaya S, Neftelinov ST, Hodge J, Campbell J. Challenges and opportunities in the PD1/PDL1 inhibitor clinical trial landscape. Nat Rev Drug Discov. 2022.
doi pubmed - Yamaguchi H, Hsu JM, Yang WH, Hung MC. Mechanisms regulating PD-L1 expression in cancers and associated opportunities for novel small-molecule therapeutics. Nat Rev Clin Oncol. 2022.
doi pubmed - Lv Q, Wang G, Zhang Y, Shen A, Tang J, Sun Y, Ma C, et al. CircAGAP1 promotes tumor progression by sponging miR-15-5p in clear cell renal cell carcinoma. J Exp Clin Cancer Res. 2021;40(1):76.
doi pubmed - Qi Y, Ma Y, Peng Z, Wang L, Li L, Tang Y, He J, et al. Long noncoding RNA PENG upregulates PDZK1 expression by sponging miR-15b to suppress clear cell renal cell carcinoma cell proliferation. Oncogene. 2020;39(22):4404-4420.
doi pubmed - Lv Q, Dong F, Zhou Y, Cai Z, Wang G. RNA-binding protein SORBS2 suppresses clear cell renal cell carcinoma metastasis by enhancing MTUS1 mRNA stability. Cell Death Dis. 2020;11(12):1056.
doi pubmed - Zou Z, Ma T, He X, Zhou J, Ma H, Xie M, Liu Y, et al. Long intergenic non-coding RNA 00324 promotes gastric cancer cell proliferation via binding with HuR and stabilizing FAM83B expression. Cell Death Dis. 2018;9(7):717.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


