| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 5, October 2022, pages 272-288
Vincosamide Has a Function for Inhibiting Malignant Behaviors of Hepatocellular Carcinoma Cells
Ming Yue Zhua, e, Zhi Sun Gongb, e, Hai Peng Fenga, e, Qiu Yue Zhanga, Kun Liua, Bo Lina, Min Ni Zhanga, Hai Feng Linc, f, Meng Sen Lia, c, d, f
aHainan Provincial Key Laboratory of Carcinogenesis and Intervention, Hainan Medical College, Haikou 571199, Hainan Province, China
bDepartment of Radiotherapy, Second Affiliated Hospital, Hainan Medical College, Haikou, China
cDepartment of Medical Oncology, Second Affiliated Hospital, Hainan Medical College, Haikou, China
dInstitution of Tumor, Hainan Medical College, Hiakou 570102, Hainan Province, China
eThese authors contributed equally to this work and are co-first authors.
fCorresponding Author: Meng Sen Li, Hainan Provincial Key Laboratory of Carcinogenesis and Intervention, Hainan Medical College, Haikou 571199, Hainan Province, China; Hai Feng Lin, Department of Medical Oncology, Second Affiliated Hospital, Hainan Medical College, Haikou 570216, Hainan Province, China
Manuscript submitted July 3, 2022, accepted August 13, 2022, published online October 22, 2022
Short title: Vincosamide Inhibits Malignant Behaviors of HCC Cells
doi: https://doi.org/10.14740/wjon1514
| Abstract | ▴Top |
Background: Vincosamide (Vinco) was first identified in the methanolic extract of the leaves of Psychotria leiocarpa, and Vinco has important anti-inflammatory effects and activity against cholinesterase, Vinco also has a trait to anti-tumor. However, whether Vinco can inhibit the malignant behaviors of hepatocellular carcinoma (HCC) cells is still unclear. In the present study, we explored the role of Vinco in suppressing the malignant behaviors of HCC cells.
Methods: MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide), trypan blue exclusion assay, the Cell Counting Kit (CCK)-8 and flow cytometric analysis were applied to detect the proliferation and apoptosis of HCC cells; electron microscopy was performed to observe the change of cellular mitochondrial morphology; scratch repair and Transwell assays were used to analyze the migration and invasion of HCC cells; expression and localization of proteins were detected by laser confocal microscopy and Western blotting; the growth of the cancer cells in vivo was assessed in a mouse tumorous model.
Results: At a dose of 10 - 80 µg/mL, Vinco inhibited the proliferation, migration, invasion and promoted apoptosis of HCC cells in a dose-dependent manner but had low cytotoxicity effect on normal liver cells. Additionally, 80 µg/mL of Vinco could significantly disrupt the morphology of mitochondria, suppress the migration and invasion of HCC cells. The growth of HCC cells in the animal tumorous model was significantly inhibited after treatment with Vinco (10 mg/kg/day) for 3 days. The results of the present study indicated that Vinco (10 - 80 µg/mL) played a role in activating caspase-3, promoting the expression of phosphate and tension homology deleted on chromosome 10 (PTEN), and inhibiting the phosphorylation of AKT (Ser473) and mTOR (Thr2448); Vinco also has a trait for suppressing the expression of CXCR4, Src, MMP9, EpCAM, Ras, Oct4 and cancer stem cell “stemness markers” CD133 and CD44 in HCC cells.
Conclusions: Vinco has a role in inhibiting the malignant behaviors of HCC cells; the role molecular mechanism of Vinco may be involved in restraining expression of the growth-, metastasis-related factors, such as Src, Ras, MMP9, EpCAM, CXCR4; activating the activity of caspase-3 and blocking PI3K/AKT signaling pathway. Thus, Vinco should be considered as a new chemotherapy agent for HCC patients.
Keywords: Vincosamide; Chemotherapy; Hepatocellular carcinoma; Malignant behaviors; Cell signal pathway
| Introduction | ▴Top |
The genus Psychotria (Rubiaceae) contains more than 2,000 species that mostly grow in tropical and subtropical regions [1]. Vincosamide (Vinco) was first identified in the methanolic extract from the leaves of Psychotria leiocarpa. Palicourea species (including Psychotria leiocarpa) have been used in folk medicine for the treatment of some disorders, including inflammatory disorders and cancer [2-4]. Previously, documents showed that alkaloid extracts of Psychotria forsteriana (include Vinco) has a cytotoxicity on rat hepatoma cell lines [5]. Some evidence indicated that Vinco was able to inhibit the growth of glioma cell lines [2], protect against inflammatory diseases and respiratory disturbances, and exert anti-hallucinogenic effects [6]. Monoterpene indolic alkaloid (N-β-glucopyranosyl vincosamide) is the main component of extracts from the leaves of Palicourea species collected from Morro Santana in Porto Alegre, Brazil [7]. It has been reported that Vinco has pharmacological effects, such as anti-oxidant effects and anti-mycobacterial effects, for example, against the growth of Mycobacterium bovis [8] and has an analgesic activity [9]. Additionally, it exhibits anti-inflammatory effects and dose-independent analgesic activity, and its effects are not reversible by naloxone. Vinco also plays a role in antagonizing the myocardial cells toxicity that induced by doxorubicin [10] and inhibiting the growth of glioma cell lines [2] and hepatoma cells [5]. These findings suggested that Vinco not only has anti-inflammatory and anti-oxidant effects and inhibits the growth of cancer cells, but also has low cytotoxicity in normal cells. Although Vinco has been reported to possess these pharmacological effects, the role of Vinco in hepatocellular carcinoma (HCC) cells is still unclear, and the bio-target of Vinco in suppressing the malignant behaviors of cancer cells has not been determined.
Considering that species of the genus Psychotria have been used as anti-inflammatory agents, anti-microbial drugs and cancer treatments, but we still lack scientific evidence of potential therapeutic applications of Vinco, so research on the potential therapeutic effect of Vinco was performed in this study. The goal of this study was to explore the effect of Vinco on the malignant phenotype of HCC cells in vitro and its anti-cancer role in a mouse model in vivo.
| Materials and Methods | ▴Top |
Cell culture
The human normal liver cell line L-02 and the human HCC cell lines HLE, Bel 7402 and PLC/PRF/5 were selected for the present study. These cells were gifts from the Department of Cell Biology and Department of Biochemistry and Molecular Biology, Peking University Health Science Centre (Beijing, China). The cells were cultured in RPMI 1640 medium supplemented with 10% heat inactivated fetal calf serum (FCS) and were incubated at 37 °C in a humidified atmosphere containing 5% CO2 as previously described [11, 12].
MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide) assay
L-02, HLE, Bel 7402 and PLC/PRF/5 cells were digested with trypsin and diluted in RPMI 1640 medium containing 10% FCS to form a suspension of 2.5 × 104cells/mL, and 200 µL/well was subcultured in 96-well plates. After incubation for 48 h in the plates, the cells were treated with different concentrations of Vinco (5, 10, 20, 40, or 80 µg/mL) (purchased from Chengdu Greenpurify Biology Medicine Company, Chengdu, China. Purity 99.7%) for 48 h. Then, MTT solution (5 mg/mL) was added to the cells in each well, and the cells were cultured for another 4 h. The culture medium containing MTT was discarded, and 200 µL of dimethylsulfoxide was added to each well. The plates were oscillated for 10 min. The absorbance values of the experimental group were measured by a microplate reader (Bio-Rad) at a wavelength of 490 nm. The growth ratio was calculated by the following formula: growth ratio = (treated A490/control A490) × 100%. The procedure and calculations were based on a previous description [11].
Cell Counting Kit (CCK)-8 assay
The CCK-8 (CK04, Dojindo, Tokyo, Honshu, Japan) was used to assess the effect of Vinco on the cell proliferation. L-02, HLE, Bel 7402 and PLC/PRF/5 cells were cultured in RPMI 1640 medium supplemented with 10% FCS at 37 °C in a humidified atmosphere of 5% CO2 for 48 h, and the cells were seeded at a density of 2.5 × 104 cells/well in a 96-well microplate. Then the cells were treated with Vinco (10 µg/mL and 80 µg/mL) for 24 h, 48 h and 72 h, respectively; HLE cells were transfected with alpha fetoprotein (AFP) expressed vectors, and Bel 7402, PLC/PRF/5 cells were transfected with siRNA-AFP vectors for 24 h as previously described [13, 14]; then these cells were treated with Vinco (80 µg/mL) for 48 h, and a total of 10 µL of CCK-8 solution was added to each well. The detected operation was done according to the procedure of the kit. The absorbance at 450 nm was measured using a Universal Microplate Reader (ELx800). The percentage of cell proliferation (viability) was calculated as the following formula: Proliferation ratio = (untreated A450 - treated A450)/(untreated A450) × 100%.
Trypan blue exclusion dye method to analyze cell viability and metabolic activity
To evaluate cell viability, L-02, HLE, Bel 7402 and PLC/PRF/5 cells were seeded at a density of 2.5 × 104 cells/well in six-well plates, and the cells were cultured in RPMI 1640 medium supplemented with 10% FCS at 37 °C in a humidified atmosphere of 5% CO2 for 48 h. Following treatment with different concentrations of Vinco (10 µg/mL or 80 µg/mL) for 48 h, cell viability was determined by the trypan blue exclusion dye assay using a Trypan Blue Staining Cell Viability Assay Kit (Beyotime Biotech Corp, Haimen, Jiangshu, China). Cells restricting trypan blue entry were considered viable. Five different visual fields were used to analyze the quantity of cell viability ratio; the cell viability ratio was calculated according to the following formula: cell viability ratio = (total cells - trypan blue stained cells)/total cells × 100%. The procedure and calculations were based on as previously described [15].
Microscopy and 4’,6-diamidino-2-phenylindole dihydrochloride (DAPI) nuclear staining to observe cell morphology
To observe the alterations of cellular morphology that induced by Vinco, L-02, HLE, Bel 7402, and PLC/PRF/5 cells were plated at a density of 2.0 × 104 cells/mL in 24-well plates. The cells were cultured in RPMI 1640 medium supplemented with 10% FCS at 37 °C in a humidified atmosphere of 5% CO2 for 48 h, and then treated with 10 µg/mL or 80 µg/mL Vinco. After treatment for 48 h, cellular morphology was observed under a light microscope, and the cells were stained with DAPI solution. The cells were imaged using a fluorescence microscope at × 100 magnification. In this study, nuclear pyknosis and fragmentation were used as evidence of apoptosis, and these criteria were evaluated by fluorescence microscopy as previously described [13, 14]. Five different visual fields were used to analyze the quantity of nuclear pyknosis with the formula: nuclear pyknosis (%) = numbers of nuclear pyknosis/total nucleus × 100%.
Flow cytometry method to analyses HCC cell apoptosis
L-02 cells, HLE cells, Bel 7402 cells, and PLC/PRF/5 cells were cultured in RPMI 1640 medium supplemented with 10% FCS at 37 °C in a humidified atmosphere with 5% CO2. Then the cells were treated with Vinco (10 µg/mL or 80 µg/mL, respectively) for 48 h. The extent of apoptosis of these cells was analyzed by flow cytometry. The detailed procedure was described in a previous study [13, 16, 17].
Electron microscopy to observe mitochondria
HLE, Bel 7402, and PLC/PRF/5 cells were cultured in RPMI 1640 medium supplemented with 10% FCS at 37 °C in a humidified atmosphere of 5% CO2. The cells were treated with Vinco (80 µg/mL) for 48 h and then harvested, washed with phosphate-buffered saline (PBS) for two times (4 °C, 10 mL, 8 min), and transferred to 1.5-mL EP tubes. The cells were fixed, and monolayer section of cells were prepared. The ultrathin sections were examined under a TECNA 10 transmission electron microscope (Philips, Holland). The morphological characteristics of cellular mitochondria were observed by randomly selecting 10 cells from each group. Mitochondrial damage was evaluated by electron microscopy, and five different visual fields were selected to evaluate the death cells. The method was performed as previously described [18].
Cellular wound repair assay
Cell motility was analyzed by a wound repair assay. HLE, Bel 7402, and PLC/PRF/5 cells (5 × 104 cells/mL) were seeded in 12-well plates and allowed to reach almost total confluence in 24 h. A scratch was made by scraping the middle of the cell monolayer with a sterile micropipette tip. The cells were cultured with RPMI 1640 medium containing 10% FCS and Vinco (10 µg/mL). Then, images of the cells that migrated into the wound area were captured at 0, 24, and 48 h by an inverted microscope (× 100), and their distances traveled were recorded. Cell repair motility was evaluated using the following formula: cell repair ratio (%) = (distance at 0 h - distance at X h)/distance at 0 h × 100%, with X representing the time points of observation. The procedure was performed as previously described [16].
Cell invasion assay
The cell invasion assay was carried out on HLE, Bel 7402, and PLC/PRF/5 cells according to the manufacturer’s protocols. Cell invasion was measured by observing cells cultured on inserts in Transwell chambers (Transwell chamber; 8-mm pore size; Costar, High Wycombe, UK) covered with Matrigel (BD Falcon, NJ, USA). The cells were plated in 12-well culture plates with separate upper and lower chambers. The cells were added to the upper chambers (5 × 104 cells/mL), cultured with serum-free RPMI 1640 medium and treated with Vinco (10 µg/mL and 80 µg/mL), whereas the lower chamber was filled with complete medium (containing 20% FCS). After 48 h of incubation, the cells in the upper chamber were carefully removed with a cotton swab, and those that had invaded through the membrane to the lower surface were fixed with 90% methanol and stained with 0.1% crystal violet. The number of cells that had invaded through the membrane was quantified by counting the cells in five different visual fields under a microscope (Olympus) with a × 20 objective. Three independent assays were performed.
Laser confocal microscopy
HLE, Bel 7402, and PLC/PRF/5 cells were stained as described previously [19]. To observe the migration of caspase-3 molecules, cells were treated with Vinco (10 µg/mL and 80 µg/mL); to evaluate the expression and location of Src, Oct4, and phosphate and tension homology deleted on chromosome 10 (PTEN); cells were treated with Vinco (80 µg/mL). Laser confocal microscopy was performed to observe the expression and location of the target proteins. Briefly, cells were fixed in 4% paraformaldehyde and incubated with mouse anti-human caspase-3, anti-Src antibodies, rabbit anti-human Oct4, anti-PTEN antibodies (Abcam Trading (Shanghai) Company, Ltd., Shanghai, China) for 24 h. Alex488-, Alex647-, or fluorescein isothiocyanate (FITC)-conjugated secondary anti-mouse immunoglobulin G (IgG) was added, and the cells were incubated for 2 h. Afterwards, 100 µL of DAPI (1 µg/mL) was added for 30 min. The cells were visualized with a Leica TCS-NT SP2 laser confocal microscope (Leica Camera, Wetzlar, Germany).
Western blotting analysis
To estimate the influence of Vinco on the expression of proteins related to metastasis, apoptosis, cancer stem cell (CSC)-related markers, and the PI3K/AKT signaling pathway, HLE, Bel 7402, and PLC/PRF/5 cells were treated with Vinco (10 µg/mL or 80 µg/mL) for 24 h. The expression of metastasis-related proteins, such as MMP9, CXCR4, and EpCAM; apoptosis-related proteins, such as activated caspase-3, PARP-1, PTEN, Bax, and Bcl-2; CSC-related markers, such as CD133 and CD44; and factors regulated by the PI3K/AKT signaling pathway, such as pAKT (Ser473), and pmTOR (Thr2448) in these cells was analyzed by Western blotting was done as previously described [16].
Immunohistochemical analysis
The protein expression of c-myc, Ras, activated caspase-3 and PARP-1 in tumor-bearing mice were evaluated by immunohistochemical analysis. Briefly, tumorous tissue was removed from tumor-bearing mice and cut into 5-mm-thick paraffin sections. The sections were deparaffinized and rehydrated according to standard protocols. The sections were then incubated with primary antibody (1:100 dilution; Abcam Trading Company, Ltd. Shanghai, China) at 4 °C overnight. Nonimmune (IgG) was used as a negative control, and antigenic sites were identified using an SP9000 Polymer Detection System and a 3,3’-diaminobenzidine kit (ZSGB-BIO, Beijing, China). The standard protocols were performed in accordance with approved guidelines [19, 20].
Analysis of caspase-3 activity
HLE, Bel 7402 and PLC/PRF/5 cells were treated with tumor necrosis factor-related apoptosis-induced ligand (TRAIL) (2 µmol/L) or Vinco (80 µg/mL) and a caspase-3 inhibitor (Z-DEVD-FMK, 1.0 µmol/L) (Selleck Chemicals Company, USA) for 24 h. Caspase-3 activity was measured with a commercial kit (APOP-CYTO Caspase-3 Colorimetric Assay Kit; Medical and Biological Laboratories, Japan) according to the manufacturer’s protocol as described in a previous study [21].
Animal experiments
Male pathogen-free athymic nude mice (BALB/C, male, 6 weeks, 20 - 25g) were purchased from the Guangzhou Animal Research Center (Guangzhou, China), the mice were fed in no specific pathogen environment, and sterile feed. The animals were maintained in a facility approved by the Ethical Committee of Hainan Medical College. The animal experiments were approved by the Institutional Animal Care and Use Committee at the Hainan Medical College, Haikou, Hainan Province, China. All experimental procedures follow the relevant requirements of animal protection, animal welfare, and ethical principles. To analyze tumorigenicity, Bel 7402 and PLC/PRF/5 cells (1 × 106) in 0.1 mL of Hank’s balanced salt solution were subcutaneously injected into the right scapular region of nude mice (10 per group). The mice were intraperitoneally injected with Vinco (10 mg/kg/day) every day. Tumor-bearing mice were killed every 3 days after inoculation (during the fed, the lethality rate is 30%), tumorous tissues were removed, the length (L) and weight (W) of the tumors were measured, and the volume (V) was calculated by the following formula as described previously [22, 23], V = π/6 × L × W2 (π = 3.14). On day 21, tumorous tissues, and the expression of activated caspase-3, PARP-1, PTEN, pAKT (Ser473), C-myc, Ras, CD133 and CD44 were detected by immunohistochemical analysis or Western blotting.
Statistical analysis
The data are presented as the mean ± standard deviation (SD). Statistical analysis was performed using Student’s t-test (for two experimental groups). Significance was set at P < 0.05. Statistical significance was determined using Student’s t-test, Kruskal-Wallis test and the F test (SPSS 11.5 software for Windows, SPSS Inc., Chicago, IL, US).
| Results | ▴Top |
Vinco inhibited the proliferation of HCC cells and promoted their apoptosis
First, the molecular structure of Vinco was displayed in Figure 1a. In the MTT assay, L-02, HLE, Bel 7402, and PLC/PRF/5 cells were treated with different concentrations of Vinco (5 - 80 µg/mL) for 48 h. At a concentration of 10 µg/mL, Vinco inhibited the growth of HLE cells but had weak effect on the growth of Bel 7402 and PLC/PRF/5 cells. When the concentration of Vinco was greater than 40 µg/mL, the proliferation of HLE, Bel 7402, and PLC/PRF/5 cells was significantly suppressed. The results also revealed that Vinco had little effect on normal human liver L-02 cells (Fig. 1b). CCK-8 detected results also indicated that concentration of 10 µg/mL of Vinco was able to inhibit the proliferation of HLE cells while treated for 48 h and 72 h, but weak effect on the proliferation of L-02, Bel7402 and PLC/PFR/5 cells (Fig.1c). The high concentration of Vinco (80 µg/mL) significantly inhibited the proliferation of L-02, HLE, Bel7402, and PLC/PFR/5 cells while treated for 24 h, 48 h and 72 h, but the effect higher in HLE, Bel7402 and PLC/PFR/5 cells than in L-02 cell (Fig. 1d). To further evaluate the role of Vinco in viability and apoptosis of HCC cells, L-02, HLE, Bel 7402, and PLC/PRF/5 cells were treated with different concentrations of Vinco (10 µg/mL or 80 µg/mL) for 48 h. We applied trypan blue exclusion dye method to analyze cell viability and metabolic activity and observed the changes in cellular morphology by microscopy. The results showed that the number of dead cells in the Vinco-treated groups were significantly increased compared to the untreated groups (Fig. 1e), and cell morphological changes occurred in HLE, Bel 7402, and PLC/PRF/5 cells while treated with Vinco (Supplementary Material 1, www.wjon.org), while HLE cells were transfected with AFP-expressed vectors, and Bel 7402, or PLC/PRF/5 cells were transfected with siRNA-AFP vectors for 24 h followed treated with Vinco (80 µg/mL) for 48 h. CCK-8 was applied to detect the proliferation of these cells. The results displayed that the expression of AFP in HLE cells, the role of Vinco in HLE was suppressed; and silenced expression of AFP in Bel 7402 or PLC/PRF/5 cells, the role of Vinco was stimulated (Supplementary Material 2, www.wjon.org). DAPI staining results showed that nuclear morphological changes were observed in HLE, Bel 7402, and PLC/PRF/5 cells (Fig.1f); the degree of cellular nuclear condensation and pyknosis was significantly increased compared to that in untreated cells, and morphological characteristics of apoptosis, including nuclear shrinkage, were apparent. However, few changes were observed in the untreated group and in normal liver cell (L-02) (Fig. 1f). Flow cytometry analysis indicated that Vinco was able to promote apoptosis of these HCC cells, but little effect on human normal liver cell line, L-02 cells (Fig. 2). These results indicate that Vinco played a role in inhibiting the growth, metabolism and promoting apoptosis of HCC cells.
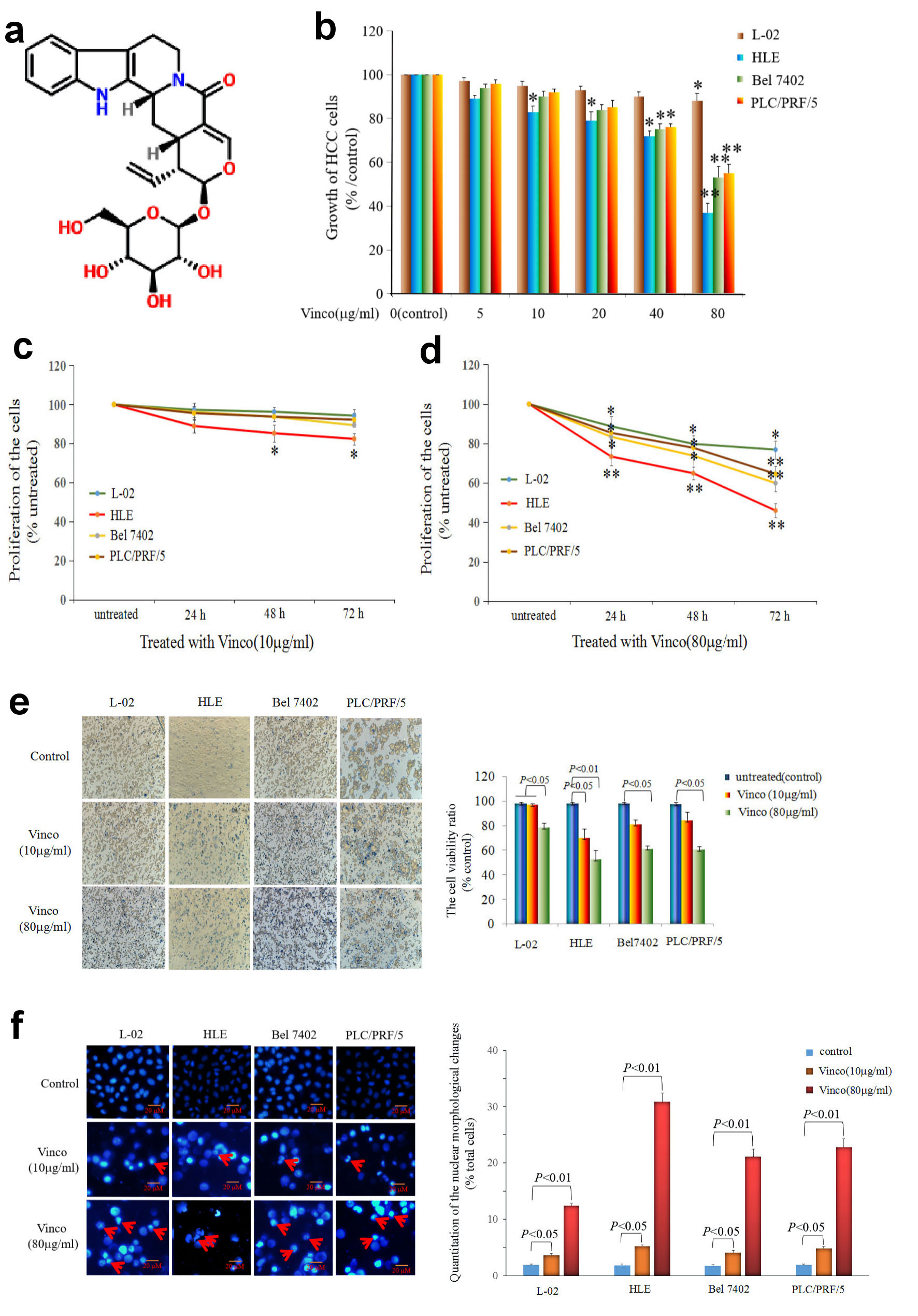 Click for large image | Figure 1. Influence of Vinco on the growth and viability of the normal liver cell line L-02 and the HCC cell lines HLE, Bel 7402, and PLC/PRF/5. (a) Molecular structure of Vinco (element components: C26H30N2O8; molecular weight: 498.532 Da). (b) L-02 cells and the HCC cell lines HLE, Bel 7402, and PLC/PRF/5 were treated with different concentrations (5 µg/mL, 10 µg/mL, 20 µg/mL, 40 µg/mL or 80 µg/mL) of Vinco for 48 h. The MTT assay was applied to detect the growth of the cells. *P < 0.05, and **P < 0.01 vs. the control groups (0 µg/mL) (n = 6). (c, d) L-02, HLE, Bel 7402, and PLC/PRF/5 cells were treated with different concentrations (10 µg/mL or 80 µg/mL) of Vinco for 24 h, 48 h, 72 h, respectively, and CCK-8 assay was applied to detect the proliferation of the cells, *P < 0.05 and **P < 0.01 (n = 6). (e) L-02, HLE, Bel 7402, and PLC/PRF/5 cells were treated with different concentrations (10 µg/mL or 80 µg/mL) of Vinco for 48 h, the trypan blue exclusion dye assay was used to analyze the viability of these cells, the bar graphs on the right show a quantitative of viability cells, P < 0.05 indicating statistical significance. (f) The nuclei of these cells were stained with DAPI and observed by fluorescence microscopy, the bar graphs on the right show a quantitative assessment of the frequency of nuclear morphological changes, P < 0.05 indicating statistical significance. The red arrows indicate cellular nucleus condensation and pyknosis. The images are representative of at least three independent experiments. Vinco: vincosamide; HCC: hepatocellular carcinoma; MTT: 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide; CCK: Cell Counting Kit; DAPI: 4’,6-diamidino-2-phenylindole dihydrochloride. |
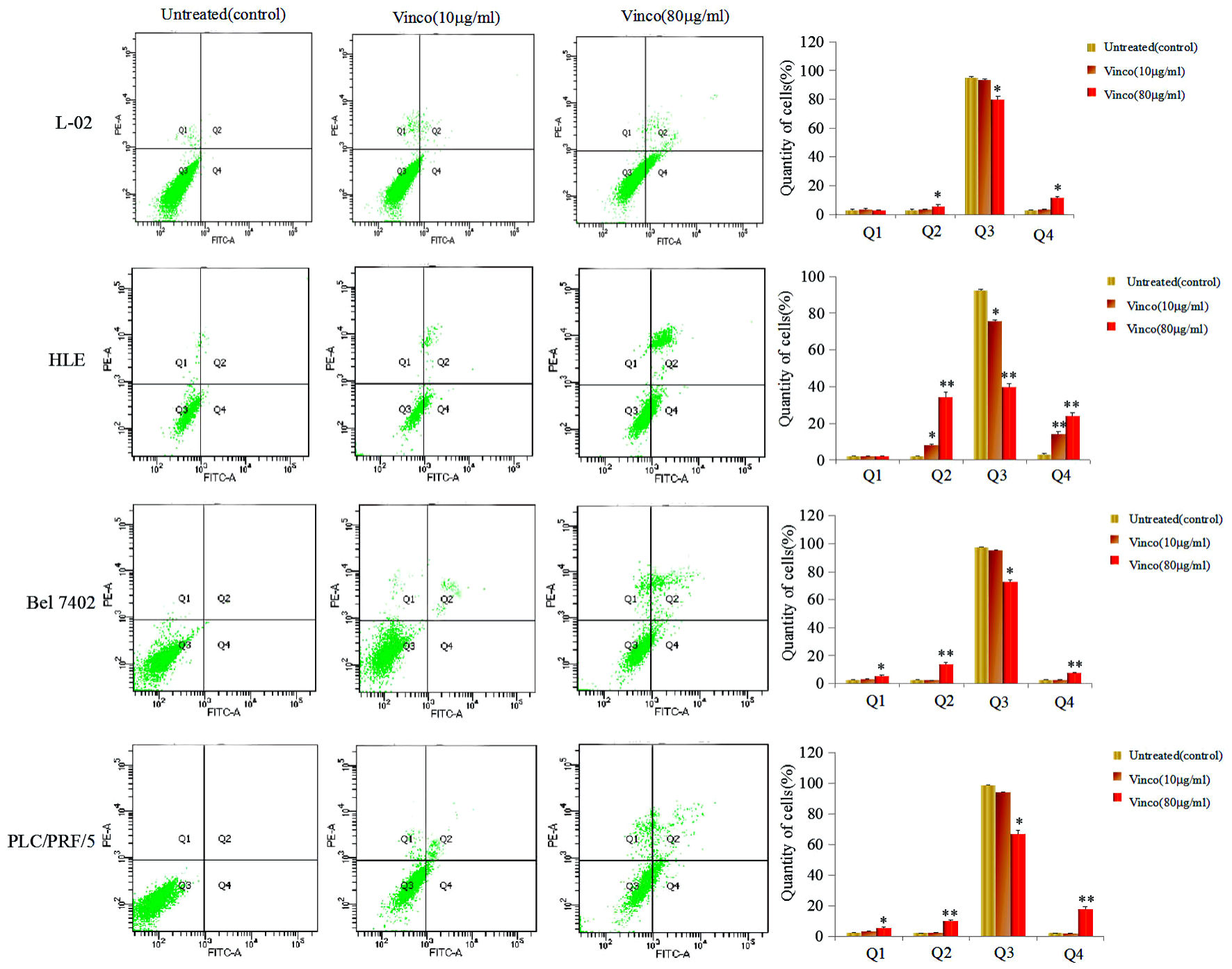 Click for large image | Figure 2. Effects of Vinco in the apoptosis of human normal liver cell line and HCC cells lines. L-02, HLE, Bel 7402, and PLC/PRF/5 were treated with 10 µg/mL or 80 µg/mL Vinco for 48 h. Apoptosis of these HCC cells were analyzed by flow cytometry. The right column picture depicts the statistical analysis of the apoptosis ratio. *P < 0.05 and **P < 0.01 vs. untreated (control). The images were representative of at least three independent experiments. Vinco: vincosamide; HCC: hepatocellular carcinoma |
Vinco disrupted the morphology and function of mitochondria in HCC cells
To reveal the role of Vinco in suppressing the growth of HCC cells and stimulating their apoptosis, we used electron microscopy to observe morphological and structural changes of mitochondria in HCC cells. HLE, Bel 7402, and PLC/PRF/5 cells were treated with Vinco (80 µg/mL) for 48 h. The results indicated that mitochondrial swelling and ridge breakage occurred in apoptotic HCC cells, but the morphology of mitochondria in the untreated groups remained normal (Fig. 3). These results demonstrated that Vinco was able to disrupt the morphology and function of mitochondria in HCC cells.
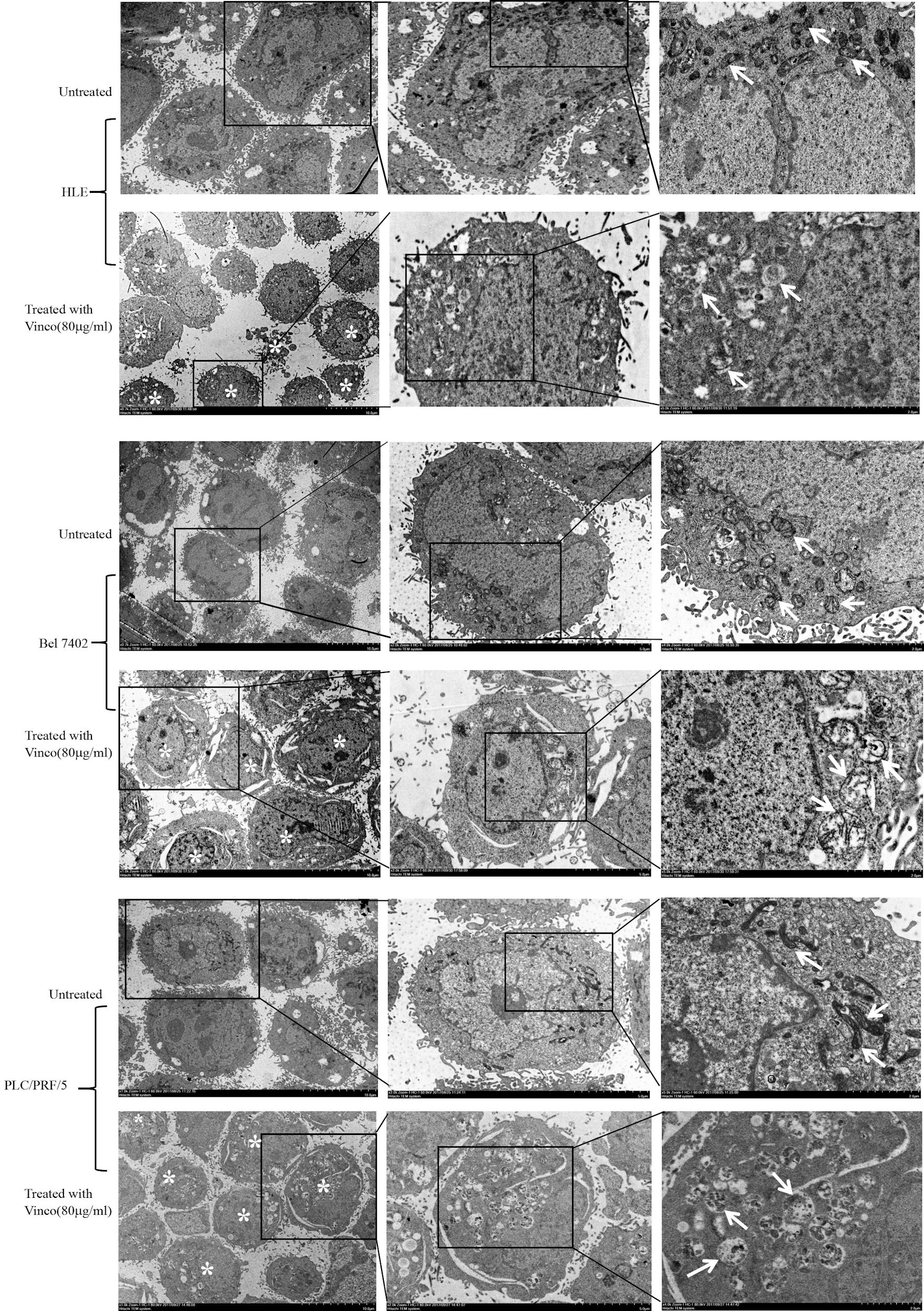 Click for large image | Figure 3. Effects of Vinco on mitochondrial morphology in HLE, Bel 7402, and PLC/PRF/5 cells. HCC cells were treated with Vinco (80 µg/m>) for 12 h, and changes in the morphology of mitochondria in the cells were observed by electron microscopy. *Indicates death cells, and the white arrows indicate mitochondria. The images are representative of three independent experiments. Vinco: vincosamide; HCC: hepatocellular carcinoma. |
Vinco inhibited the migration and invasion of HCC cells
To observe the effect of Vinco on the migration and invasion of HCC cells, cellular wound repair and invasion assays were performed. In the cellular wound repair assay, HLE, Bel 7402, and PLC/PRF/5 cells were treated with Vinco (10 µg/mL) for 24 h or 48 h. The scratch repair assay results revealed that repair migration of Vinco-treated HLE cells was significantly decreased compared to that of untreated HLE cells. Few changes were observed in Bel 7402 and PLC/PRF/5 cells while treated with Vinco (10 µg/mL) for 24 h, but when these cells were treated with Vinco (10 µg/mL) for 48 h, their repair migration was significantly decreased compared to that of untreated (control) cells (Fig. 4a). The invasion assay indicated that the pore transfer capacity of HLE, Bel 7402, and PLC/PRF/5 cells which were treated with Vinco (10 µg/mL or 80 µg/mL) for 48 h were significantly decreased compared to that of untreated cells (control); the results also showed that the pore transfer capacity of HCC cells which were treated with a high concentration of Vinco (80 µg/mL), was significantly decreased compared to that of HCC cells which were treated with a low concentration of Vinco (10 µg/mL) (Fig. 4b). These results reveal that Vinco could inhibit the migration and invasion of HCC cells in a dose-dependent manner.
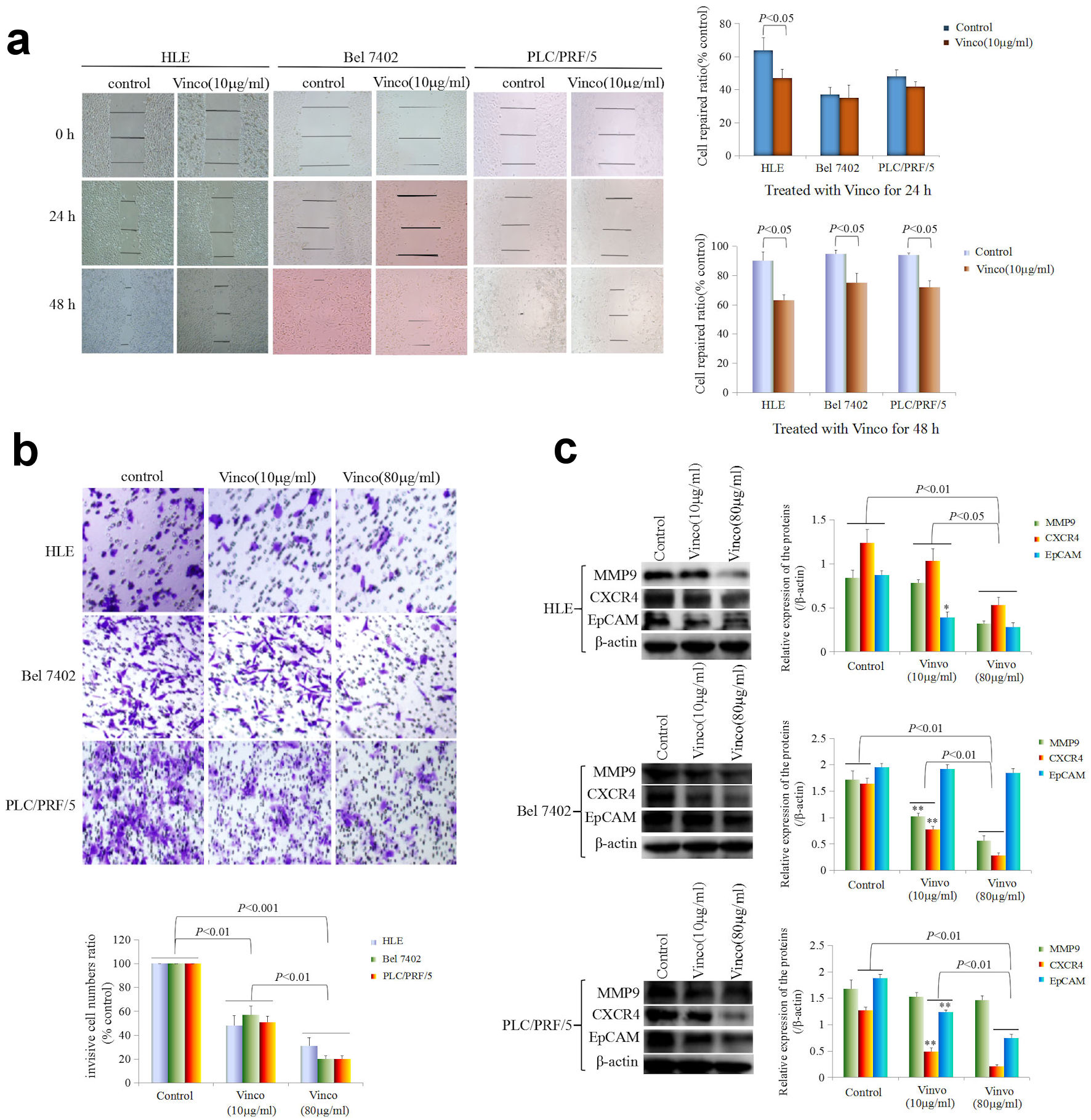 Click for large image | Figure 4. Influence of Vinco on scratch repair and migration, and the expression of metastasis-related proteins in HCC cells. (a) HLE, Bel 7402, and PLC/PRF/5 cells were treated with Vinco (10 µg/mL) for 24 h or 48 h. Scratch repair was observed by microscopy. The bar graph on the right shows the repair ratio of the cells, with P < 0.05 indicating statistical significance. (b) HLE, Bel 7402, and PLC/PRF/5 cells were treated with Vinco (10 µg/mL or 80 µg/mL) for 48 h. Migratory cells were stained with 0.1% crystal violet and observed by microscopy. The lower bar graph shows the number of migratory cells; with P < 0.01 indicates statistical significance. (c) HLE, Bel 7402, and PLC/PRF/5 cells were treated with Vinco (10 µg/mL or 80 µg/mL) for 48 h, and the expression of the metastasis-related proteins MMP9, CXCR4 and EpCAM were detected by Western blotting. The bar graphs on the right show the relative expressed quantity of these proteins, with P < 0.05 indicating statistical significance. The images are representative of three independent experiments. Vinco: vincosamide; HCC: hepatocellular carcinoma. |
Furthermore, we performed Western blotting to explore the effect of Vinco on the expression of migration- and invasion-related proteins. The results indicated that the expression of MMP9, CXCR4, and EpCAM was significantly reduced in HLE cells while treated with Vinco (10 µg/mL or 80 µg/mL) for 48 h, compared to that in untreated cells (control) (Fig. 4c). The expression of these proteins was also significantly lower in HLE cells while treated with a high concentration of Vinco (80 µg/mL) than in those treated with a low concentration of Vinco (10 µg/mL). Bel 7402 cells were treated with Vinco (10 µg/mL or 80 µg/mL) for 48 h, the expression of MMP9 and CXCR4 was significantly reduced compared to untreated cells (control) (Fig. 4c), and the expression of these proteins was also significantly lower in Bel 7402 cells while treated with a high concentration of Vinco (80 µg/mL) than that were treated with a low concentration of Vinco (10 µg/mL). However, there was weak change in the expression of EpCAM in Bel 7402 cells. PLC/PRF/5 cells were treated with Vinco (10 µg/mL or 80 µg/mL) for 48 h, the expression of CXCR4 and EpCAM were significantly reduced compared to untreated cells (control) (Fig. 4c), and the expression of these proteins were also significantly lower in PLC/PRF/5 cells while treated with a high concentration of Vinco (80 µg/mL) than that were treated with a low concentration of Vinco (10 µg/mL). However, there was few changes in the expression of MMP9 in PLC/PRF/5 cells (Fig. 4c). These results demonstrated that Vinco has a function for inhibiting the expression of migration- and invasion-related proteins.
Vinco activated caspase-3 activity in HCC cells
Activated caspase-3 activity is a pivotal factor for promoting apoptosis of cell. In the present investigation, we applied laser confocal microscopy to observe the expression, location, and migration of caspase-3. The results showed that HLE, Bel 7402, and PLC/PRF/5 cells expressed caspase-3 (green fluorescence), and the caspase-3 protein was located in the cytoplasm in these cells (Fig. 5a). The results also showed that a small number of caspase-3 molecules migrated from the cytoplasm to nucleus when the cells were treated with a low concentration of Vinco (10 µg/mL) for 48 h. However, a large number of caspase-3 molecules were assembled in the nucleus (green fluorescence) when the cells were treated with a high concentration of Vinco (80 µg/mL) for 48h (Fig. 5a). To explore the effect of Vinco on the expression of apoptosis-related proteins, Western blotting was used to detect the expression of Bax, Bcl-2, activated caspase-3, and PARP-1. The results indicated that the expression of Bax was significantly stimulated, but the expression of Bcl-2 was significantly inhibited, and the expression of activated caspase-3 and PARP-1 was significantly promoted in HLE, Bel 7402, and PLC/PRF/5 cells while treated with Vinco (10 µg/mL or 80 µg/mL) for 48h (Fig. 5b). The Bax/Bcl-2 ratio was also elevated in these cells while treated with Vinco (10 µg/mL or 80 µg/mL), but the elevation in the ratio was more significant in the cells while treated with a high concentration of Vinco (80 µg/mL) than that treated with a low concentration of Vinco (10 µg/mL); the elevation of Bax/Bcl-2 ratio implies the promotion of apoptosis (Fig. 5c). Furthermore, we analyzed the effect of Vinco on the activity of caspase-3. HLE, Bel 7402, and PLC/PRF/5 cells were treated with TRAIL, which promotes TRAIL receptor-mediated caspase-3 activity, as a positive control, and Z-DEVD-FMK, which inhibits caspase-3 activity for 48 h. The results indicated that Vinco (80 µg/mL) was able to activate caspase-3 activity similarly to TRAIL (2 µmol/L), and the inhibitor (Z-DEVD-FMK) blocked the effect of Vinco. Vinco synergized with TRAIL to stimulate caspase-3 activity (Fig. 5d). These results proved that Vinco has the capacity to activate the activity of caspase-3 in HCC cells.
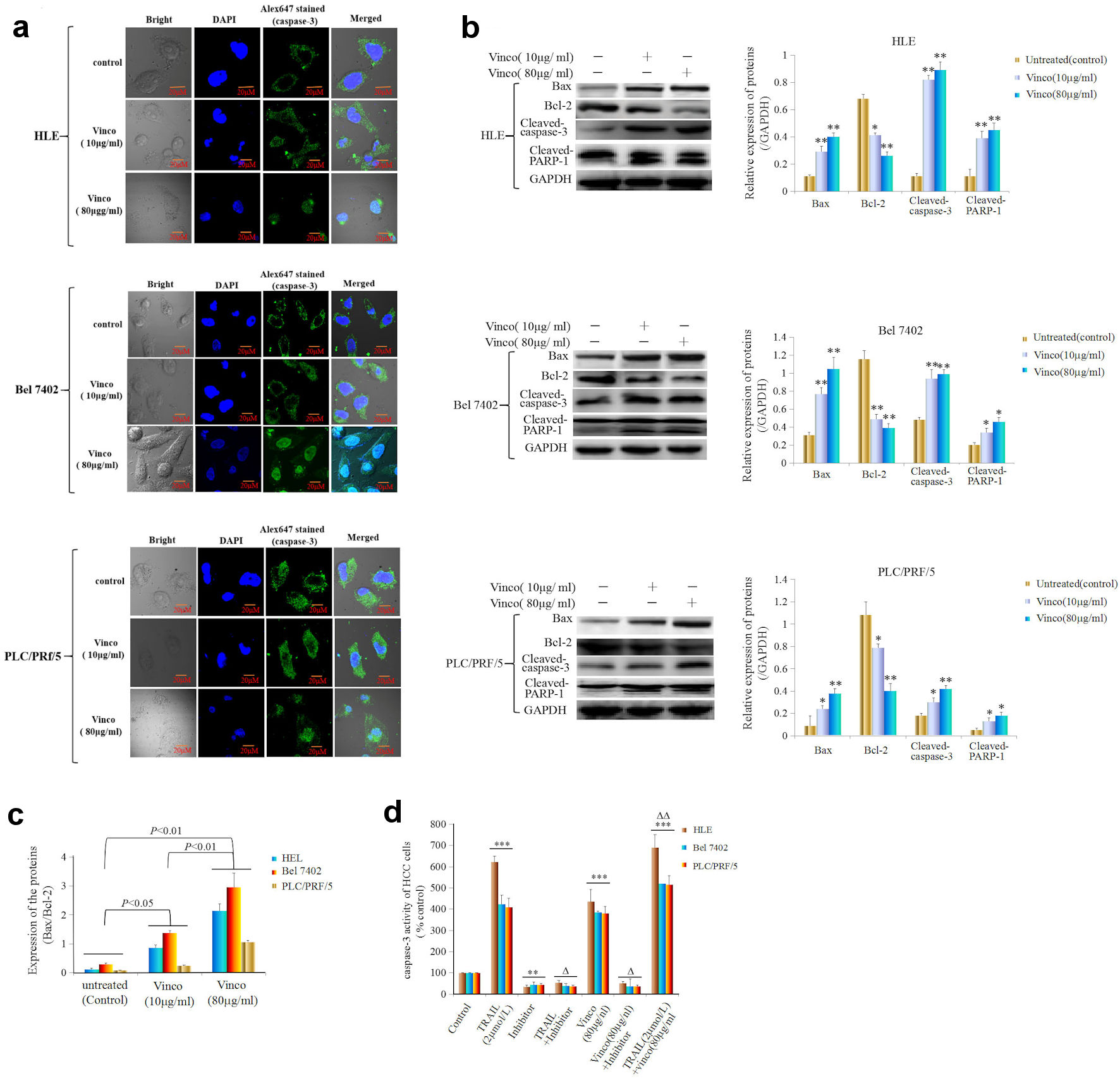 Click for large image | Figure 5. Effects of Vinco on nuclear migration and activity of caspase-3, and the expression of apoptosis-related proteins in HCC cells. HLE, Bel 7402, and PLC/PRF/5 cells were treated with Vinco (10 µg/mL or 80 µg/mL) for 48 h, and the nuclear migration of caspase-3 molecules in these cells was observed by laser confocal microscopy (a). The expression of the apoptosis-related proteins Bax, Bcl-2, cleaved caspase-3 and PARP-1 was detected by Western blotting. The bar graphs on the right show the relative expression of these proteins. *P < 0.05, **P < 0.01 vs. the untreated group (control) (b). The quantitative expression ratio of Bax/Bcl-2 (c), with P < 0.05 indicating statistical significance. The images are representative of at least three independent experiments. (d) HLE, Bel 7402, and PLC/PRF/5 cells were treated with TRAIL (2 µmol/L) or Vinco (80 µg/mL) and a caspase-3 inhibitor (Z-DEVD-FMK) for 48 h. The activity of caspase-3 in these cells was measured using an enzymatic reaction kit. **P < 0.01, ***P < 0.001 vs. control group, ΔP < 0.01 vs. the control group, TRAIL (2 µmol/L)-treated group or Vinco (80 µg/mL)-treated group; ΔΔP < 0.05 vs. the TRAIL (2 µmol/L)- and Vinco (80 µg/mL)-treated group (n = 6). The images are representative of at least three independent experiments. Vinco: vincosamide; HCC: hepatocellular carcinoma; TRAIL: tumor necrosis factor-related apoptosis-induced ligand. |
Vinco blocked PI3K/AKT signaling pathway, inhibited the expression of oncogenes and CSC stemness markers
In the present study, we explored the effect of Vinco on the PI3K/AKT signaling pathway and the expression of oncogenes. HLE, Bel 7402, and PLC/PRF/5 cells were treated with Vinco (80 µg/mL) for 48 h, and the expression and location of PTEN, Src and Oct4 were observed by laser confocal microscopy. The results indicated that Vinco promoted the expression of PTEN; and PTEN was located in the cytoplasm (Fig. 6a). The expression of Src and Oct4 was significantly inhibited; Src was located in the cytoplasm and Oct4 was located in the nucleus (Fig. 7a, b). We also used Western blotting method to detect the expression of PTEN and other proteins which related to the PI3K/AKT signaling pathway. The results revealed that the expression of PTEN was significantly stimulated and the expression of pAKT (Ser473), pmTOR (Thr2448) was significantly inhibited when these cells were treated with Vinco (10 µg/mL or 80 µg/mL) for 48 h (Fig. 6b). Additionally, a high concentration of Vinco (80 µg/mL) has a greater effect on suppressing the expression of pAKT (Ser473) and pmTOR (Thr2448) in HCC cells (Fig. 6b). Vinco not only inhibited the expression of pAKT (Ser473), Src and Oct4, but also synergized with a PI3K inhibitor (Ly294002) to inhibit the expression of pAKT (Ser473), Src, Oct4 (Fig. 7c); and CSC stemness markers CD133 and CD44 (Supplementary Material 3, www.wjon.org). These results proved that Vinco was able to block the activation of the PI3K/AKT signaling pathway and inhibit the expression of oncogenes and CSC stemness markers in vitro.
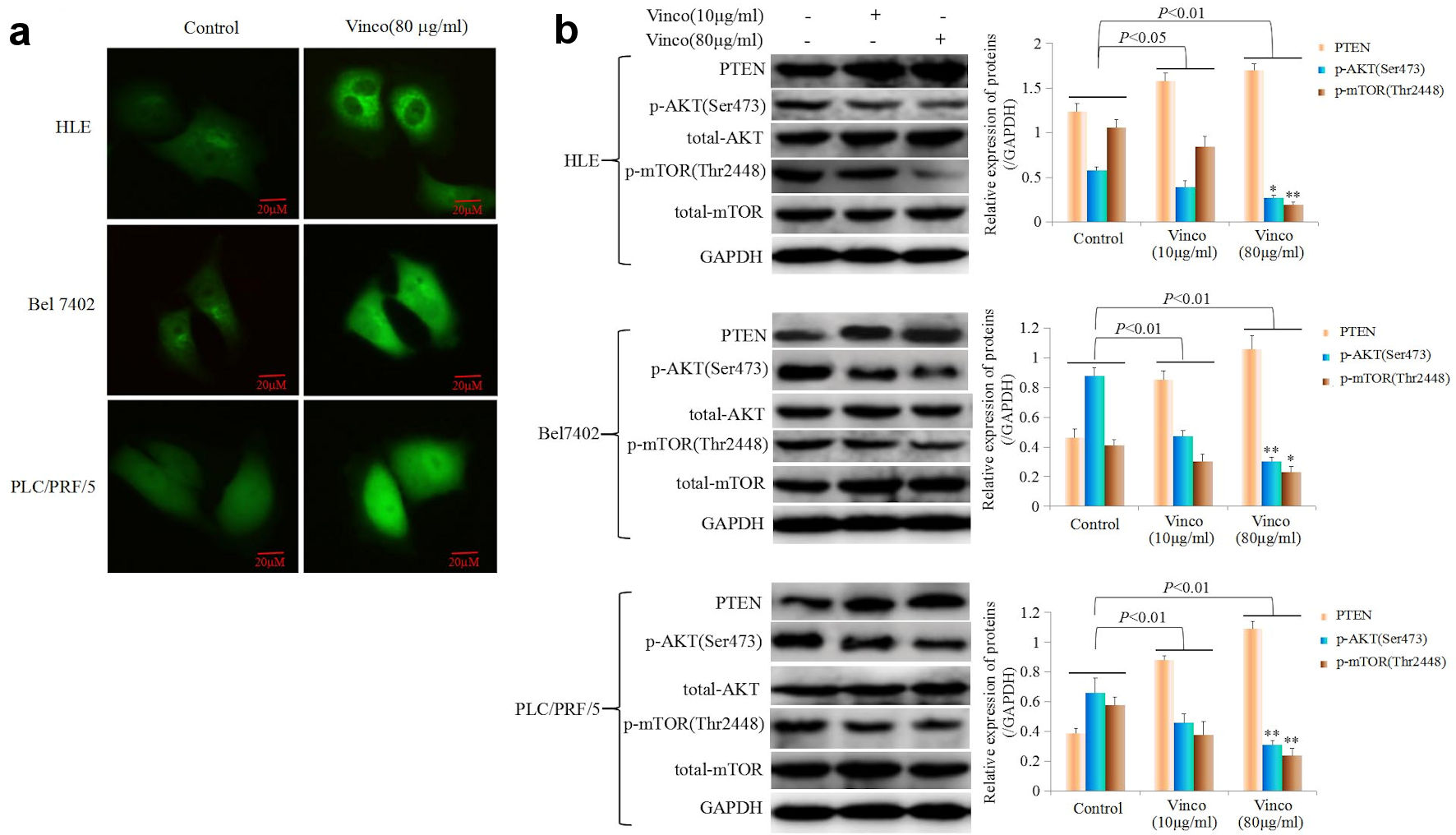 Click for large image | Figure 6. The influence of Vinco on the expression of PTEN, and the PI3K/AKT signaling pathway is regulated by PTEN express in HCC cells. (a) HLE, Bel 7402 and PLC/PRF/5 cells were treated with Vinco (80 µg/mL) for 48 h, and the expression and localization of PTEN in these cells were observed by laser confocal microscopy. (b) HLE, Bel 7402 and PLC/PRF/5 cells were treated with Vinco (10 µg/mL or 80 µg/mL) for 48 h, and the expression of PTEN, pAKT (Ser473), pmTOR (Thr2448) was detected by Western blotting. The bar graphs on the right show relative expressed quantity of these proteins, with P < 0.05 indicating statistical significance; *P < 0.05 and **P < 0.01 vs. control and Vinco (10 µg/mL)-treated groups. The images are representative of at least three independent experiments. Vinco: vincosamide; HCC: hepatocellular carcinoma; PTEN: phosphate and tension homology deleted on chromosome 10. |
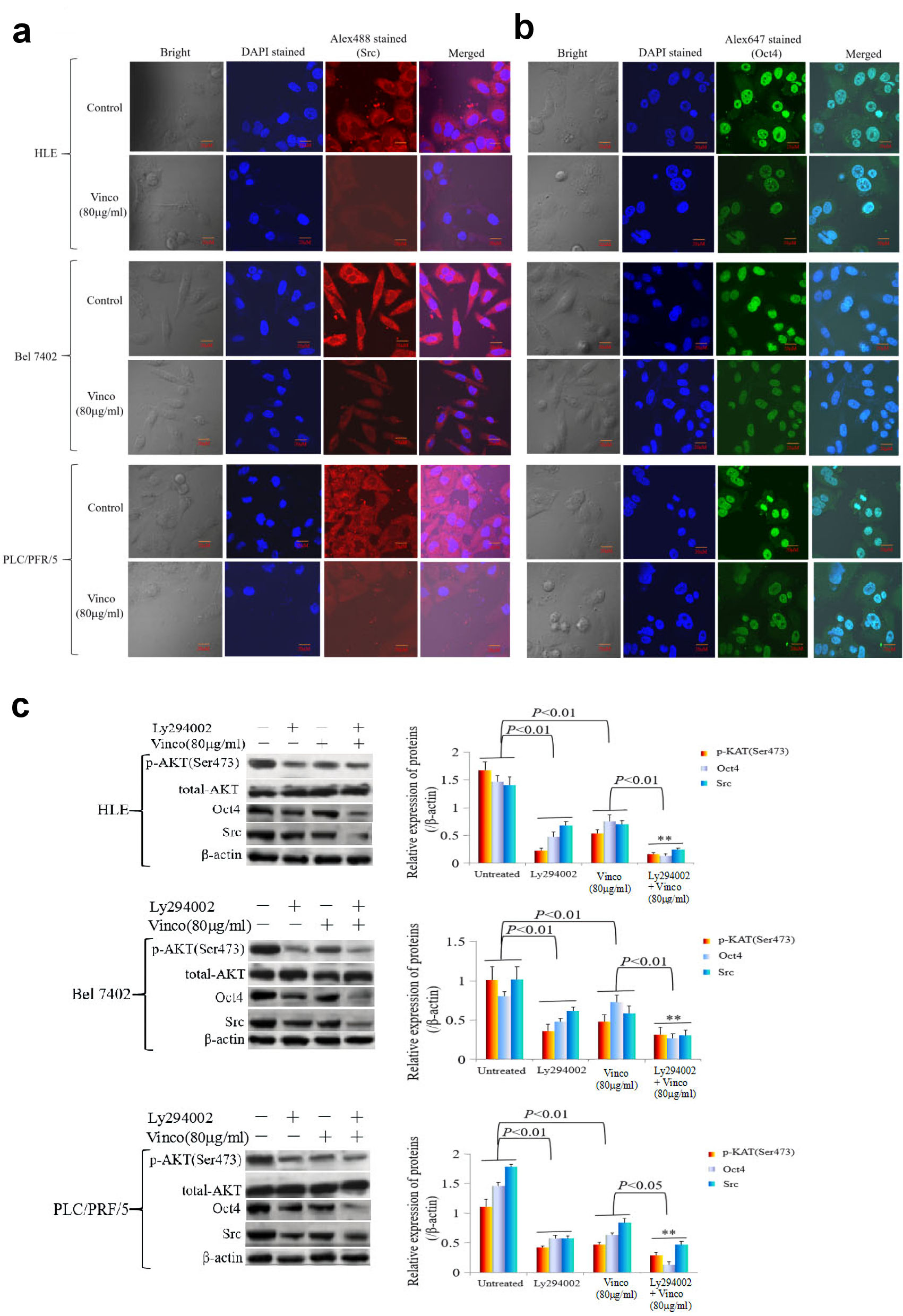 Click for large image | Figure 7. The influence of Vinco on the expression of the oncogene Src, the reprogramming gene Oct4, and the proteins related to the regulation by PI3K/AKT signaling pathway in HCC cells. HLE, Bel 7402 and PLC/PRF/5 cells were treated with Vinco (80 µg/mL) for 48 h, and the expression and localization of Src (a) and Oct4 (b) in these cells were observed by laser confocal microscopy. (c, d) HLE, Bel 7402 and PLC/PRF/5 cells were treated with Vinco (80 µg/mL) or the PI3K inhibitor Ly294002 (2 µmol/L) or co-treated with Vinco (80 µg/mL) and Ly294002 (2 µmol/L) for 48 h, and the expression of pAKT (Ser473), Src and Oct4 were detected by Western blotting. The bar graphs on the right showed relative expressed quantity of these proteins, with P < 0.05 indicating statistical significance; **P < 0.01 vs. Ly294002- treated groups. The images are representative of at least three independent experiments. Vinco: vincosamide; HCC: hepatocellular carcinoma; DAPI: 4’,6-diamidino-2-phenylindole dihydrochloride. |
Vinco inhibited cancer growth, activated caspase-3 activity, and blocked the PI3K/AKT signaling pathway in vivo
In the present study, the therapeutic effect of Vinco on a tumorous model was observed by injecting Bel 7402 and PLC/PRF/5 cells into the right scapular region of male nude mice. The mice were intraperitoneally injected with Vinco (10 mg/kg/day) every day. Tumor-bearing mice were killed every 3 days after inoculation, and the tumorous volume was calculated. The results showed that the tumorous volume of Vinco-treated mice was significantly reduced compared to that of untreated mice (control) at the same time point after 9 days (Fig. 8a, b). Furthermore, immunohistochemical analysis was applied to detect the expression of C-myc, Ras, activated caspase-3, and PARP-1 in tumorous tissues at 21 days. The results indicated that the expression of C-myc and Ras was significantly decreased in tissues extracted from Vinco-treated mice compared to the tissues extracted from untreated mice; but the expression of activated caspase-3 and PARP-1 was significantly elevated in tissues extracted from Vinco-treated mice compared to the tissues extracted from untreated mice (Fig. 8c, d). Western blotting results revealed that the expression of activated caspase-3, PARP-1, and PTEN were significantly elevated in tissues extracted from Vinco-treated mice compared to the tissues extracted from untreated mice, but the expression of pAKT (Ser473), C-myc, Ras (Fig. 8e, f), and CSC stemness markers CD133 and CD 44 were significantly decreased (Supplementary Material 4, www.wjon.org). These results further demonstrated that Vinco inhibited the growth of liver cancer cells, activated caspase-3 activity, and blocked transduction of PI3K/AKT signaling pathway to suppress the genesis of CSC. The role mechanism of Vinco in inhibiting the malignant behaviors of HCC cells was shown in Figure 8g.
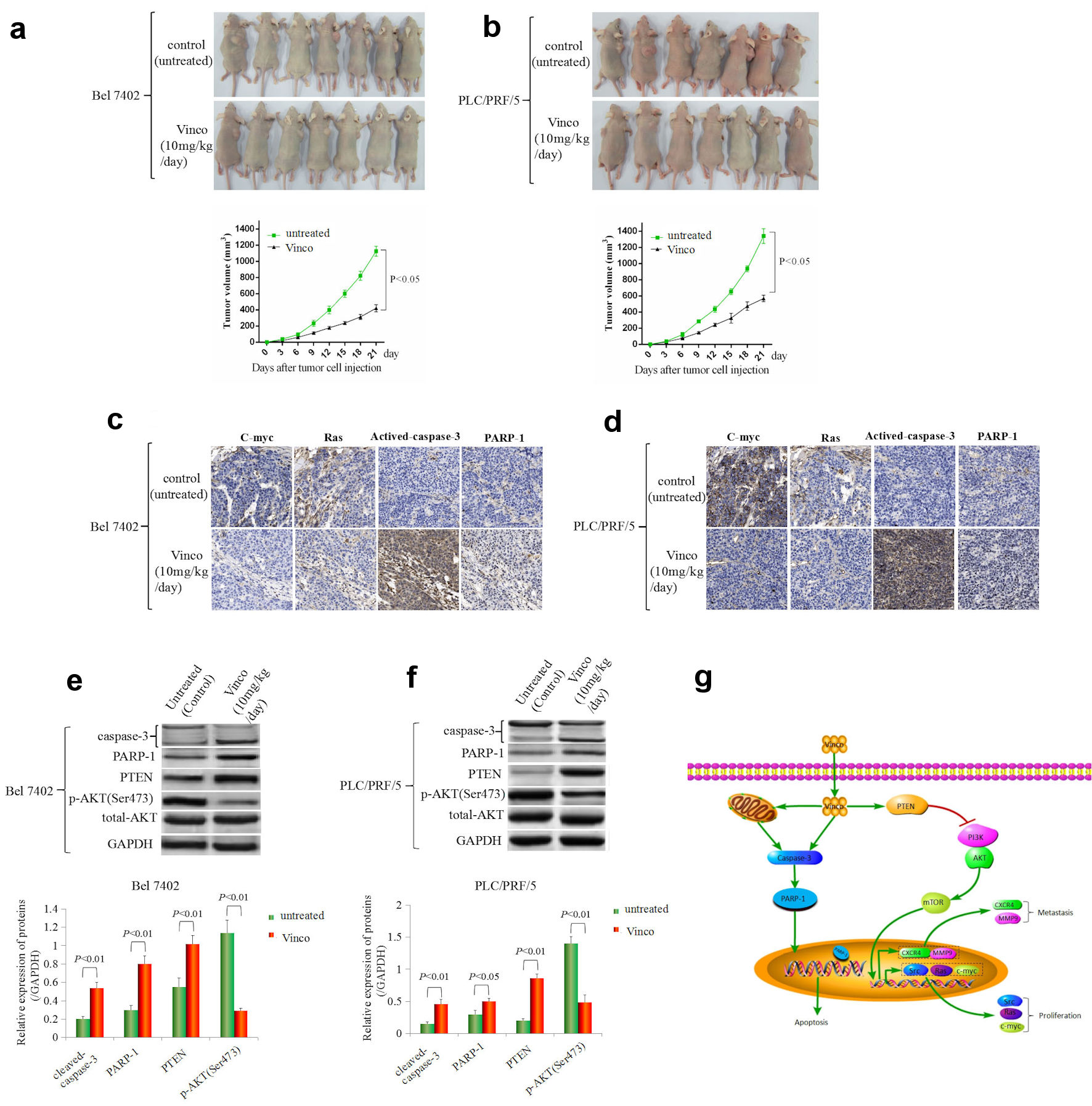 Click for large image | Figure 8. The effect of Vinco on HCC cells growth, the expression of the growth-related factors C-myc, Ras, PTEN, pAKT (Ser473) and the activity of caspase-3 in vivo. Bel 7402 (a) and PLC/PRF/5 (b) cells (1 × 106) in 0.1 mL of Hank balanced salt solution were subcutaneously injected into the right scapular region of male nude mice (n = 7). The mice were intraperitoneally injected with Vinco (10 mg/kg/day) every day. Tumor-bearing mice were killed every 3 days after inoculation, the tumors were removed, and the measured length (L), weight (W) of the tumors were measured, and the volume (V) was calculated by the following formula: V = π/6 × L × W2 (π = 3.14). The lower bar graphs show tumorous volume. (c, d) The expression of c-myc, Ras, cleaved caspase-3 and PARP-1 in tumorous tissues (extracted from tumor-bearing mice on day 21) were detected by immunohistochemistry. (e, f) The expression of cleaved caspase-3, PARP-1, PTEN, pAKT (Ser473) in tumorous tissues were detected by Western blotting. The bar graphs on the right show relative expressed quantity of these proteins. with P < 0.05 indicating statistical significance. The images are representative of at least three independent experiments. (g) Schematic of the mechanism underlying the role of Vinco in inhibiting the malignant behaviors of HCC cells. Vinco: vincosamide; HCC: hepatocellular carcinoma; PTEN: phosphate and tension homology deleted on chromosome 10. |
| Discussion | ▴Top |
Monoterpene indolic alkaloid (N-β-glucopyranosyl vincosamide) is the main component of extracts from the leaves of Psychotria leiocarpa, which is found in tropical and subtropical regions. Many studies have shown that Vinco has anti-inflammatory activities in vivo and anti-proliferative effects in vitro [1, 7, 24-26]; and Vinco also has cytotoxic, anthelmintic, analgesic, anti-bactericidal activities [2, 27-29] and with potential therapeutic effects on type 2 diabetes [30]. Evidence indicated Vinco as protective agent in cardiac damage and vascular dysfunction [31]. Some investigations over the past three decades have indicated that Vinco inhibited the proliferation of cancer cells and promoted their apoptosis [3, 5], and that it antagonized cardiotoxicity that induced by doxorubicin in rats in vitro and in vivo [10, 31]. These evidences indicated that Vinco played a complex role in regulating anti-inflammatory and anti-bacterial activities and antagonizing cardiotoxicity; it also has a capacity to inhibit the growth of cancer cells.
Although evidence has revealed that an analog of Vinco induced cytotoxicity in human leukemic and rat hepatoma cell lines [5], inhibited the growth of a glioma cell line (U251) [2] and has anti-oxidant activity to protect against oxidative stress generated upon wounding [7]; the effect of Vinco on the malignant behaviors of human HCC cells is still unclear. In the present study, we observed the cytotoxicity of Vinco in human HCC cells and human normal liver cells. The results of the MTT, CCK-8 and trypan blue exclusion and flow cytometry assays demonstrated that Vinco significantly inhibited the growth of HCC cells and promoted their apoptosis at concentrations greater than 40 µg/mL but had weak cytotoxicity in normal liver cells. DAPI staining also showed that Vinco promoted cellular nuclear condensation and pyknosis, which are the classic feature of apoptosome. Additionally, the cellular wound repair and invasion assays indicated that Vinco significantly suppressed the migration and invasion of HCC cells. To our surprise, the low concentration of Vinco (10 µg/mL) inhibited the growth of HLE cells, implying that the sensitivity of different HCC cells to Vinco is diverse, possibly due to their heterogeneity. Previously, we found that HLE cells are more sensitive to agents such as benzyl isothiocyanate and paclitaxel compared to Bel 7402 and PLC/PRF/5 cells, and that is due to relatively lower expression of AFP [13, 14, 32]. We further found that AFP antagonizes apoptosis induced by these agents [13, 32], and it inhibits autophagy and immune response, leading to the carcinogenesis of liver cancer [33-35]. In the present study, the sensitivity of HLE cells to Vinco was inhibited when they were transfected with AFP-expressing vectors, whereas the sensitivity of Bel 7402 or PLC/PRF/5 cells to Vinco was stimulated when they were transfected with siRNA-AFP vectors (Supplementary Material 2, www.wjon.org). These results indicate that expression of AFP in HCC cells is a crucial factor for the resistance of HCC cells to these agents. Overall, these results demonstrate that Vinco has the capacity to inhibit growth and invasion and stimulate apoptosis of HCC cells.
To explore the mechanism underlying the ability of Vinco to suppress the malignant behaviors of HCC cells, we evaluated the changes in the morphology and function of cellular mitochondria. The results indicated that Vinco significantly disrupted mitochondrial morphology. Western blotting analysis displayed that the expression of Bcl-2 was significantly suppressed, but the expression of Bax, cleaved caspase-3, and PARP-1 were significantly stimulated, and the activity of caspase-3 was activated. Vinco also promoted the migration of activated-caspase-3 molecules from the cytoplasm to the nucleus. These results suggested that Vinco was able to impair the function of mitochondria in HCC cells and activate the activity of caspase-3 in vitro. The activated PI3K/AKT signaling pathway is a pivotal factor for promoting the proliferation, drug-resistance, and metastasis of HCC cells [36-39]. Recently, many studies have indicated that activated PI3K/AKT signaling pathway was able to stimulate the expression of malignant phenotype-related genes, such as Src, CXCR4, MMP2/9, and EpCAM [40-43]. PTEN is critical molecule for inhibiting the activation of PI3K/AKT signaling pathway in HCC cells [44-46]. The results of this study indicated that Vinco inhibits the expression of Src, CXCR4, MMP9, EpCAM and CSC stemness markers CD133 and CD44 in vitro (Supplementary Material 3, www.wjon.org), and stimulates the expression of PTEN, leading to the expression of pAKT (Ser473) and pmTOR (Thr2448) were inhibited. These results reveal that Vinco inhibited transduction of PI3K/AKT signaling pathway, which is critical for suppressing the malignant behaviors of HCC cells.
Vinco has the capacity to inhibit the proliferation of HCC cells in vitro, but whether it has the same effect in vivo is still unclear. In the present study, we observed the therapeutic effect of Vinco on an animal tumorous model. Animal tumorous models were administered with Vinco (10 mg/kg/day) every day. Because HLE cells cannot induce production of tumors when transplanted into mice, only Bel 7402 and PLC/PRF cells were selected to inject into the mice. The results showed that the tumorous volume of Vinco-treated mice was significantly reduced compared to untreated mice. The results also showed that the expression of C-myc, Ras, pAKT (Ser473) and CSC stemness markers CD133 and CD44 (Supplementary Material 4, www.wjon.org) significantly decreased in tissues extracted from Vinco-treated mice compared to the tissues extracted from untreated mice; but the expression of activated caspase-3, PARP-1 and PTEN was significantly elevated. These results further prove that Vinco suppressed the growth of liver cancer cells, and it was able to stimulate the activity of caspase-3 and promote the expression of PTEN to block the transduction of PI3K/AKT signaling pathway and inhibit expression of CSC stemness markers CD133 and CD44 in vivo. We have summarized the mechanism underlying the role of Vinco in inhibiting the malignant behaviors of HCC cells (Fig. 8g).
Conclusions
The present study reported for the first time that Vinco has an effect on inhibiting the malignant behaviors (phenotypes) of HCC cells. The results imply that caspase-3 and PTEN molecules may be the pivotal target for the role of Vinco. Vinco may inhibit the malignant behaviors (phenotypes) of HCC by activating caspase-3, by blocking PI3K/AKT signaling, and by inhibiting CSC generation. Vinco is an agent derived from a natural product that is expected to be used for treating HCC.
| Supplementary Material | ▴Top |
Suppl 1. Influence of Vinco on the morphology of the normal liver cell line L-02 and HCC cell lines, HLE, Bel 7402, and PLC/PRF/5.
Suppl 2. The effect of alpha fetoprotein on Vinco inhibiting proliferation of the HCC cell lines HLE, Bel 7402 and PLC/PRF/5.
Suppl 3. The influence of Vinco on the expression of the cancer stem cell (CSC) stemness markers CD133 and CD44 in HCC cells.
Suppl 4. The effect of Vinco on the expression of c-myc, Ras and CSC stemness markers CD133, CD44 in vivo.
Acknowledgments
We thank Dr. Wen Ting Wang for assisting in the cellular migration assays.
Financial Disclosure
This work was supported by the National Natural Science Foundation of China (Nos. 82060514, 81960519); the Natural Science Foundation of Hainan Province (Nos. 820RC634, 2019CXTD406, 2019CR204 and 20168263); Research Project of Take off the Proclamation and Leadership in Hainan Medical College Natural Science Foundation (No. JBGS202106); and Hainan Provincial Association for Science and Technology Program of Youth Science Talent and Academic Innovation (No. QCXM 201922).
Conflict of Interests
All the authors confirm that no conflicts of interest are associated with the content of this article.
Informed Consent
Not applicable.
Author Contributions
ML and MZ designed the experiments. ZG, HF, QZ, MZ, BL and KL performed the experiment. ML and HL supervised the study and analyzed the data. ML wrote the manuscript. All authors read and approved the final manuscript.
Data Availability
All data generated or analyzed during this study are included in this manuscript.
Abbreviations
HCC: hepatocellular carcinoma; Vinco: vincosamide; AFP: alpha fetoprotein; TRAIL: tumor necrosis factor-related apoptosis-induced ligand; PTEN: phosphate and tension homology deleted on chromosome 10; CSC: cancer stem cell
| References | ▴Top |
- Marques de Oliveira A, Lyra Lemos RP, Conserva LM. β-Carboline alkaloids from Psychotria barbiflora DC(Rubiaceae). Biochem Syst Ecol. 2013;50:339-341.
doi - de Moura VM, Ames FQ, Correa JGS, Peixoto MA, Amorim AMA, Pomini AM, de Carvalho JE, et al. Cytotoxicity and anti-inflammatory effects of the extract, fractions and alkaloids from Palicourea minutiflora (Rubiaceae). Nat Prod Res. 2021;35(22):4715-4719.
doi pubmed - Volobuff CRF, Junior PCO, Dos Santos SM, Pereira ZV, Ferreira DC, Cardoso CAL, Ruiz A, et al. Antitumoral and anticholinesterasic activities of the seven species from rubiaceae. Curr Pharm Biotechnol. 2019;20(4):302-308.
doi pubmed - Formagio ASN, Volobuff CRF, Kassuya CAL, Cardoso CAL, do Carmo Vieira M, Pereira ZV, Bagatin MC, et al. Psychotria leiocarpa extract and vincosamide reduce chemically-induced inflammation in mice and inhibit the acetylcholinesterase activity. Inflammation. 2019;42(5):1561-1574.
doi pubmed - Adjibade Y, Kuballa B, Cabalion P, Jung ML, Beck JP, Anton R. Cytotoxicity on human leukemic and rat hepatoma cell lines of alkaloid extracts of Psychotria forsteriana. Planta Med. 1989;55(6):567-568.
doi pubmed - Caballero-George C, Vanderheyden PM, Solis PN, Pieters L, Shahat AA, Gupta MP, Vauquelin G, et al. Biological screening of selected medicinal Panamanian plants by radioligand-binding techniques. Phytomedicine. 2001;8(1):59-70.
doi pubmed - Matsuura HN, Fett-Neto AG. The major indole alkaloid N,beta-D-glucopyranosyl vincosamide from leaves of Psychotria leiocarpa Cham. & Schltdl. is not an antifeedant but shows broad antioxidant activity. Nat Prod Res. 2013;27(4-5):402-411.
doi pubmed - Moraes TM, de Araujo MH, Bernardes NR, de Oliveira DB, Lasunskaia EB, Muzitano MF, Da Cunha M. Antimycobacterial activity and alkaloid prospection of Psychotria species (Rubiaceae) from the Brazilian Atlantic Rainforest. Planta Med. 2011;77(9):964-970.
doi pubmed - McKenna DJ, Towers GH, Abbott F. Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and beta-carboline constituents of ayahuasca. J Ethnopharmacol. 1984;10(2):195-223.
doi - Cheraghi M, Namdari M, Daraee H, Negahdari B. Cardioprotective effect of magnetic hydrogel nanocomposite loaded N,alpha-L-rhamnopyranosyl vincosamide isolated from Moringa oleifera leaves against doxorubicin-induced cardiac toxicity in rats: in vitro and in vivo studies. J Microencapsul. 2017;34(4):335-341.
doi pubmed - Li MS, Li PF, Yang FY, He SP, Du GG, Li G. The intracellular mechanism of alpha-fetoprotein promoting the proliferation of NIH 3T3 cells. Cell Res. 2002;12(2):151-156.
doi pubmed - Zhu M, Li W, Lu Y, Dong X, Lin B, Chen Y, Zhang X, et al. HBx drives alpha fetoprotein expression to promote initiation of liver cancer stem cells through activating PI3K/AKT signal pathway. Int J Cancer. 2017;140(6):1346-1355.
doi pubmed - Zhu M, Li W, Guo J, Lu Y, Dong X, Lin B, Chen Y, et al. Alpha fetoprotein antagonises benzyl isothiocyanate inhibition of the malignant behaviors of hepatocellular carcinoma cells. Oncotarget. 2016;7(46):75749-75762.
doi pubmed - Zhu M, Li W, Dong X, Chen Y, Lu Y, Lin B, Guo J, et al. Benzyl-isothiocyanate Induces Apoptosis and Inhibits Migration and Invasion of Hepatocellular Carcinoma Cells in vitro. J Cancer. 2017;8(2):240-248.
doi pubmed - Zhu M, Lu Y, Li W, Guo J, Dong X, Lin B, Chen Y, et al. Hepatitis B virus X protein driven alpha fetoprotein expression to promote malignant behaviors of normal liver cells and hepatoma cells. J Cancer. 2016;7(8):935-946.
doi pubmed - Feng H, Zhu M, Zhang R, Wang Q, Li W, Dong X, Chen Y, et al. GATA5 inhibits hepatocellular carcinoma cells malignant behaviours by blocking expression of reprogramming genes. J Cell Mol Med. 2019;23(4):2536-2548.
doi pubmed - Li M, Li H, Li C, Guo L, Liu H, Zhou S, Liu X, et al. Cytoplasmic alpha-fetoprotein functions as a co-repressor in RA-RAR signaling to promote the growth of human hepatoma Bel 7402 cells. Cancer Lett. 2009;285(2):190-199.
doi pubmed - Wang W, Zhu M, Xu Z, Li W, Dong X, Chen Y, Lin B, et al. Ropivacaine promotes apoptosis of hepatocellular carcinoma cells through damaging mitochondria and activating caspase-3 activity. Biol Res. 2019;52(1):36.
doi pubmed - Li M, Zhu M, Li W, Lu Y, Xie X, Wu Y, Zheng S. Alpha-fetoprotein receptor as an early indicator of HBx-driven hepatocarcinogenesis and its applications in tracing cancer cell metastasis. Cancer Lett. 2013;330(2):170-180.
doi pubmed - Lu Y, Zhu M, Li W, Lin B, Dong X, Chen Y, Xie X, et al. Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. J Cell Mol Med. 2016;20(3):549-558.
doi pubmed - Li M, Li H, Li C, Zhou S, Guo L, Liu H, Jiang W, et al. Alpha fetoprotein is a novel protein-binding partner for caspase-3 and blocks the apoptotic signaling pathway in human hepatoma cells. Int J Cancer. 2009;124(12):2845-2854.
doi pubmed - Euhus DM, Hudd C, LaRegina MC, Johnson FE. Tumor measurement in the nude mouse. J Surg Oncol. 1986;31(4):229-234.
doi pubmed - Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148-154.
doi pubmed - Benevides PJC, Young MCM, Bolzani VDS. Biological activities of constituents from Psychotria spectabilis. Pharm Biol. 2004;42:565-569.
doi - Li D, Chen J, Ye J, Zhai X, Song J, Jiang C, Wang J, et al. Anti-inflammatory effect of the six compounds isolated from Nauclea officinalis Pierrc ex Pitard, and molecular mechanism of strictosamide via suppressing the NF-kappaB and MAPK signaling pathway in LPS-induced RAW 264.7 macrophages. J Ethnopharmacol. 2017;196:66-74.
doi pubmed - Mai SY, Li YH, Zhang XG, Wang YR, Zhang JQ, Jia A. A new indole alkaloid with HUVEC proliferation activities from Nauclea officinalis. Nat Prod Res. 2021;35(18):3049-3055.
doi pubmed - Mahmud Z, Musa M, Ismail N, Lajis NH. Cytotoxic and bacteriocidal activities of Psychotria rostrata. Int J Pharmacogn. 1993;31:142-146.
doi - Aderibigbe SA, Idowu SO, Olaniyi AA, Wright CW, Fatokun AA. Bioactivity and cytotoxicity profiling of vincosamide and strictosamide, anthelmintic epimers from Sarcocephalus latifolius (Smith) Bruce leaf. J Ethnopharmacol. 2021;265:113142.
doi pubmed - Zhou M, Xie Z, Guan W, Zhang J. Comparative analysis of bioactive constituents and pharmacological activities from different parts of Nauclea officinalis. Biomed Chromatogr. 2021;35(12):e5214.
doi - Peng ZC, He J, Pan XG, Zhang J, Wang YM, Ye XS, Xia CY, et al. Secoiridoid dimers and their biogenetic precursors from the fruits of Cornus officinalis with potential therapeutic effects on type 2 diabetes. Bioorg Chem. 2021;117:105399.
doi pubmed - Alia F, Putri M, Anggraeni N, Syamsunarno M. The Potency of Moringa oleifera Lam. as Protective Agent in Cardiac Damage and Vascular Dysfunction. Front Pharmacol. 2021;12:724439.
doi pubmed - Zhu M, Li W, Lu Y, Dong X, Chen Y, Lin B, Xie X, et al. Alpha fetoprotein antagonizes apoptosis induced by paclitaxel in hepatoma cells in vitro. Sci Rep. 2016;6:26472.
doi pubmed - Wang S, Zhu M, Wang Q, Hou Y, Li L, Weng H, Zhao Y, et al. Alpha-fetoprotein inhibits autophagy to promote malignant behaviour in hepatocellular carcinoma cells by activating PI3K/AKT/mTOR signalling. Cell Death Dis. 2018;9(10):1027.
doi pubmed - Zhu M, Li M. Inhibition of autophagy and immune response: Alpha-fetoprotein stimulates initiation of liver cancer. J Cancer Immunol. 2020;2(3):69-73.
doi - Zheng Y, Zhu M, Li M. Effects of alpha-fetoprotein on the occurrence and progression of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(10):2439-2446.
doi pubmed - Tu K, Liu Z, Yao B, Han S, Yang W. MicroRNA-519a promotes tumor growth by targeting PTEN/PI3K/AKT signaling in hepatocellular carcinoma. Int J Oncol. 2016;48(3):965-974.
doi pubmed - Fu X, Liu M, Qu S, Ma J, Zhang Y, Shi T, Wen H, et al. Exosomal microRNA-32-5p induces multidrug resistance in hepatocellular carcinoma via the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37(1):52.
doi pubmed - Li X, Yang Z, Song W, Zhou L, Li Q, Tao K, Zhou J, et al. Overexpression of Bmi-1 contributes to the invasion and metastasis of hepatocellular carcinoma by increasing the expression of matrix metalloproteinase (MMP)2, MMP-9 and vascular endothelial growth factor via the PTEN/PI3K/Akt pathway. Int J Oncol. 2013;43(3):793-802.
doi pubmed - Li W, Liu K, Chen Y, Zhu M, Li M. Role of Alpha-Fetoprotein in Hepatocellular Carcinoma Drug Resistance. Curr Med Chem. 2021;28(6):1126-1142.
doi pubmed - Xia H, Ooi LL, Hui KM. MicroRNA-216a/217-induced epithelial-mesenchymal transition targets PTEN and SMAD7 to promote drug resistance and recurrence of liver cancer. Hepatology. 2013;58(2):629-641.
doi pubmed - O'Brien NA, Browne BC, Chow L, Wang Y, Ginther C, Arboleda J, Duffy MJ, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9(6):1489-1502.
doi pubmed - Zhu M, Guo J, Li W, Lu Y, Fu S, Xie X, Xia H, et al. Hepatitis B virus X protein induces expression of alpha-fetoprotein and activates PI3K/mTOR signaling pathway in liver cells. Oncotarget. 2015;6(14):12196-12208.
doi pubmed - Tian T, Nan KJ, Guo H, Wang WJ, Ruan ZP, Wang SH, Liang X, et al. PTEN inhibits the migration and invasion of HepG2 cells by coordinately decreasing MMP expression via the PI3K/Akt pathway. Oncol Rep. 2010;23(6):1593-1600.
doi pubmed - Chen WT, Zhu G, Pfaffenbach K, Kanel G, Stiles B, Lee AS. GRP78 as a regulator of liver steatosis and cancer progression mediated by loss of the tumor suppressor PTEN. Oncogene. 2014;33(42):4997-5005.
doi pubmed - Zhu L, Sun Y, Zhang S, Wang L. Rap2B knockdown suppresses malignant progression of hepatocellular carcinoma by inactivating the PTEN/PI3K/Akt and ERK1/2 pathways. Mol Cell Biochem. 2020;466(1-2):55-63.
doi pubmed - Luo YD, Fang L, Yu HQ, Zhang J, Lin XT, Liu XY, Wu D, et al. p53 haploinsufficiency and increased mTOR signalling define a subset of aggressive hepatocellular carcinoma. J Hepatol. 2021;74(1):96-108.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


