| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 6, December 2022, pages 359-364
Will Patients With Liver Metastasis From Aggressives Cancers Benefit From Surgical Resection?
Abdallah Al Faraia, Jonathan Garniera, Anais Palena, Jacques Ewalda, Jean-Robert Delperoa, Olivier Turrinib, c
aDepartment of Surgery, Institut Paoli-Calmettes, Marseille, France
bDepartment of Surgery, Aix-Marseille University, Institut Paoli-Calmettes, CRCM, Marseille, France
cCorresponding Author: Olivier Turrini, Department of Surgical Oncology, Institut Paoli-Calmettes, 232 Boulevard Sainte Marguerite, 13009 Marseille, France
Manuscript submitted July 21, 2022, accepted August 8, 2022, published online December 1, 2022
Short title: Metastatic Poor Prognostic Cancers
doi: https://doi.org/10.14740/wjon1516
| Abstract | ▴Top |
Background: We aimed to evaluate the outcomes of resections for liver metastases (LMs) originating from pancreatic ductal adenocarcinoma (PDAC), non-small cell lung cancer (NSCLC), and esophagus/gastric cancers (EGCs), which we label as major killers (MKs; overall survival (OS) under 10%). We hypothesized that LM resection must provide the patient with almost a year of OS postoperatively that is considered beneficial.
Methods: From January 2005 to December 2020, 23 patients underwent resection for isolated LM from MKs. These patients underwent surgery after a multidisciplinary discussion about their performance status, disease evolution during prolonged medical treatment, and the existence or absence of extrahepatic metastases.
Results: LM originated from an PDAC, EGC, or NSCLC in 10 patients (43%), nine patients (39%), and four patients (18%), respectively. The median delay between primary cancer and LM diagnoses was 12 months, and the median delay between LM diagnosis and liver resection was 10 months. Most patients, who had objectively responded to medical treatment (57%), had a solitary (61%) and unilobar (70%) LM. Severe morbidity and 90-day mortality rates were 13% and 4.3%, respectively. Margin-free resection was achieved in 16 patients (70%). After liver resection, the median OS was 24 months without a statistical difference when considering the primary tumor site; 1, 3-, and 5-year OS were 70%, 23%, and 23%, respectively.
Conclusion: Selection based on criteria such as good clinical condition, response to treatment, and long observation period helped identify patients with LM of MKs who seemed to benefit from resection.
Keywords: Pancreatic cancer; Lung cancer; Gastric cancer; Liver metastasis
| Introduction | ▴Top |
Some metastatic cancers show an estimated overall survival (OS) below 10% [1] and can be defined as major killers (MKs): pancreatic ductal adenocarcinoma (PDAC), non-small cell lung cancer (NSCLC), and esophagus/gastric cancers (EGCs). Upfront surgery is not recommended in patients presenting with synchronous metastatic MK; however, resections after induction treatment can be performed, as several studies report an advantage in resecting metastases in selected patients [2-4]. However, no criteria have been reported, and patients are selected for surgery based on mixed factors such as performance status (PS), a delay in the initial surgery, disease evolution during medical treatment, number of metastatic sites, ability to obtain complete (R0) resection with the balance of the expected postoperative morbidity, and serum tumor marker evolution, with a final decision made by a multidisciplinary staff.
At the time of surgery, these selected patients have a favorable medical history, which estimates at least 1 year of survival with continuous medical treatment.
This study aimed to determine whether selected patients will benefit from resection of liver metastasis (LM).
| Materials and Methods | ▴Top |
From January 2005 to December 2020, 23 patients (0.9%) who underwent resection for LM from MKs were identified from our databases (NCT02871336 and NCT03686137). The study design was approved by the appropriate ethics review board. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Patient selection
The multidisciplinary staff proposed a liver resection based on the medical history, the patient’s good PS (0 or 1), an exhaustive imaging workup (body computed tomography (CT) scan associated with diffusion-weighted magnetic resonance imaging (MRI) and positon emission tomography-CT (PET-CT) according to the period of treatment) that did not show metastatic sites other than the liver, the disease stabilization/regression after a prolonged (> 6 months) medical treatment, serum tumor marker stabilization/regression during medical treatment (i.e., carcinoembryonic antigen and/or carbohydrate antigen (CA) 19-9 for PDAC and EGC, squamous cell carcinoma (SCC) for NSCLC), the absence of supplementary metastasis identified during medical treatment, and the surgical team that was estimated to achieve R0 resection without excessive expected postoperative morbidity. Consequently, all these factors were considered, but one could not prevent surgery by itself, and not all were considered for resection. No patients with percutaneous destruction of LM were included in the present series as we wanted to focus on the most “aggressive” treatment (i.e., the surgical approach).
Variables studied and primary endpoints
Various routine variables were evaluated, including American Society of Anesthesiologists (ASA) score; primary tumor etiology; number, size, and location of the LM; type of surgery; morbidity [5]; margin resection status (R0 or R1); and tumor recurrence site assessed every 4 months by clinical examination, serum tumor markers, and thoracoabdominal CT scan.
The primary endpoint of the study was the OS after liver resection.
Statistical analysis
Continuous data were expressed as the mean (± standard deviation) or median (range). Survival duration was measured from the liver resection date until death or the sensor date (June 1, 2020). Survival curves were generated using the Wilcoxon method. Statistical significance was set at P < 0.05.
| Results | ▴Top |
None of the patients were lost during a mean postoperative follow-up period of 28 months. LM originated from an PDAC, EGC, or NSCLC in 10 patients (43%), nine patients (39%), and four patients (18%), respectively (Table 1). The median delay between primary cancer and LM diagnoses was 12 months (range, 0 - 67 months), and the median delay between LM diagnosis and liver resection was 10 months (range, 8 - 61 months). The majority of patients had a solitary (61%) and unilobar (70%) LM and objectively responded to medical treatment (57%). Most patients (61%) underwent intraoperative local destruction (n = 2) or a minor hepatectomy (n = 8) possibly associated with local destruction (n = 4). Severe morbidity and 90-day mortality rates were 13% and 4.3%, respectively (one patient died from postoperative liver failure during the early period of the study). R0 resection was achieved in 16 patients (70%). Four patients (17%) received adjuvant chemotherapy.
 Click to view | Table 1. Characteristics of the 23 Patients |
Recurrence was diagnosed in 13 patients (56%) with a median delay of 11 months (range, 6 - 27 months); recurrences were located in the liver in 11 patients, and one patient with EGC developed a unique lung metastasis that was treated by local destruction. Overall, 12 patients (52%) died of disease recurrence (Table 2). After the liver resection, the median OS was 24 months without a statistical difference when considering the primary tumor site; 1, 3-, and 5-year OS were 70%, 23%, and 23%, respectively (Fig. 1).
 Click to view | Table 2. Oncological Outcomes of the 23 Patients |
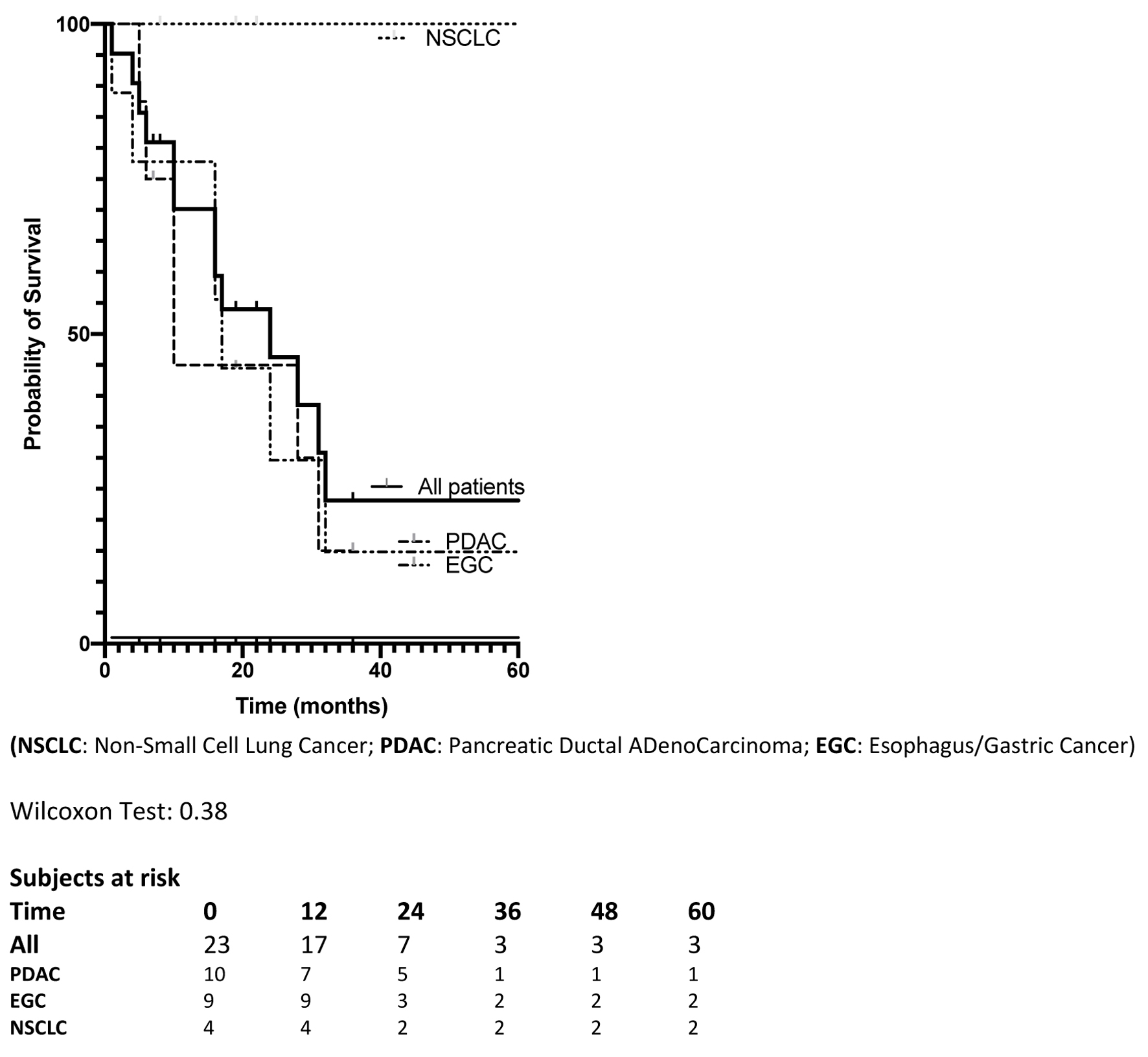 Click for large image | Figure 1. Overall survival of the 23 patients, and according to primary tumor site, since liver surgery. |
| Discussion and Conclusions | ▴Top |
Our study showed that selected patients with LM of MKs benefit from resection as the median OS after liver resection was 2 years.
Oncologists and surgeons are reluctant to consider liver resection in patients with metastatic MKs because of two reasons. First, MKs are rarely confined to one organ and medical teams are worried of metastasis in other organs despite an exhaustive preoperative imaging workup. Therefore, the benefit of surgery for LM is considered questionable; it could even cause rapid metastasis of the disease in the event of a poor postoperative course. Second, the therapeutic arsenal in MKs is not evolved as other solid tumors (i.e., colorectal cancer, breast cancer, etc.).
However, few patients with metastatic MKs seem to have a less aggressive disease course. These patients probably have a particular tumor biology that could not be detected early due to the lack of current knowledge. Consequently, we could only discuss liver resection based on criteria that are a surrogate of this favorable tumor biology and that answer the oncologists on their reluctance as mentioned above.
When considering the first reason, PS is a major criterion in our decision-making process. Obviously, the patient’s clinical status has to be optimal to minimize the risk of poor postoperative courses. However, the clinical status also reflects the impact of the disease on the patient: a poor PS (> 1) and/or a significant weight loss (> 10%) probably underestimates the spread of the disease even if the imaging did not show any metastatic site other than the liver. Consequently, we argue that the clinical status is the first criterion to be considered to further discuss liver resection in patients who have an exhaustive imaging staging that excluded extrahepatic disease. Together with the clinical status, “prolonged” (unknown cut-off) follow-up helps identify other metastatic locations in most patients. In our series, only one patient developed a solitary extrahepatic recurrence that reinforced our preoperative supposition of a disease solely located in the liver. However, most patients showed a recurrence in the liver that highlighted our poor ability to identify very small LM despite the improvement of liver imaging, mainly with MRI and PET-CT.
If the patient is surgically fit according to its PS, comorbidities, and follow-up, several factors could help answer the second reason. Tumor response to chemotherapy is crucial to discuss in all patients with solid tumors as resection in patients with tumor progression during chemotherapy remains exceptional even for favorable etiologies [6]. Thus, in patients with MKs, stabilization or progression during chemotherapy is frequent, and good responders are rarely identified, representing only 1% of our liver resection procedures over two decades. This small sample size prevents any strong conclusions or recommendations. However, these patients can have an interesting survival as previously reported [7-18].
The appropriateness of resection cannot be determined without comparison with a group of patients with similar conditions who are pursuing medical treatment. This represents the major limitation of our study as we cannot compare the prognosis with patients who received medical treatment in a matching analysis or discuss the approximate duration of the expected prognosis if the medical treatment is continued under the same conditions. Indeed, patients in whom continued medical treatment or resection can be considered are very few. Therefore, 1) there is no randomized study that has compared the two approaches, and 2) as we routinely propose a surgical attitude, we cannot provide data on patients with same criteria that received only medical treatment. However, the literature reports series of fit patients receiving exclusive medical treatment and whose survival is less than the 24 months that we observed with our interventionist attitude [19-21]. We can therefore maintain that the survival of resected patients is not inferior to that reported in these series.
But the advantage of resection is to allow a break in medical treatments which undeniably generate significant side effects and alter the quality of life. Our study is also limited by its retrospective design and the heterogeneity of the perioperative medical treatments delivered during the long inclusion period. Despite these drawbacks, it is reasonable to suggest liver resection in the qualified patients. While genetic markers can identify the disease accurately and at an early stage, criteria such as good clinical condition, response to treatment, and long observation period can help multidisciplinary staff perform local resection or destruction in patients. Oncologists must consider this strategy as it can avoid prolonged medical treatment that is often poorly tolerated.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
All study participants provided informed consent.
Author Contributions
AAF contributed to the study design and wrote the manuscript. AP contributed to data collection and data analysis. JE contributed to data analysis and reviewed the manuscript. JG contributed to data collection and reviewed the manuscript. JRD reviewed the manuscript. OT contributed to the study design and wrote the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
MK: major killer; PDAC: pancreatic ductal adenocarcinoma; NSCLC: non-small cell lung cancer; EGC: esophagus/gastric cancer; LM: liver metastasis; OS: overall survival; PS: performance status; MRI: magnetic resonance imaging; PET-CT: positon emission tomography-computed tomography
| References | ▴Top |
- National Cancer Institute. Cancer Type. Available at https://www.cancer.gov/types.
- Ghidini M, Petrillo A, Salati M, Khakoo S, Varricchio A, Tomasello G, Grossi F, et al. Surgery or locoregional approaches for hepatic oligometastatic pancreatic cancer: myth, hope, or reality? Cancers (Basel). 2019;11(8):1095.
doi pubmed - Fernandez RAS, Lau RWH, Ho JYK, Yu PSY, Chow SCY, Wan IYP, Ng CSH. Evidence for surgical resections in oligometastatic lung cancer. J Thorac Dis. 2019;11(Suppl 7):S969-S975.
doi pubmed - Salati M, Valeri N, Spallanzani A, Braconi C, Cascinu S. Oligometastatic gastric cancer: An emerging clinical entity with distinct therapeutic implications. Eur J Surg Oncol. 2019;45(8):1479-1482.
doi pubmed - Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213.
doi pubmed - Vigano L, Darwish SS, Rimassa L, Cimino M, Carnaghi C, Donadon M, Procopio F, et al. Progression of Colorectal Liver Metastases from the End of Chemotherapy to Resection: A New Contraindication to Surgery? Ann Surg Oncol. 2018;25(6):1676-1685.
doi pubmed - Sakamoto Y, Ohyama S, Yamamoto J, Yamada K, Seki M, Ohta K, Kokudo N, et al. Surgical resection of liver metastases of gastric cancer: an analysis of a 17-year experience with 22 patients. Surgery. 2003;133(5):507-511.
doi pubmed - Shirabe K, Shimada M, Matsumata T, Higashi H, Yakeishi Y, Wakiyama S, Ikeda Y, et al. Analysis of the prognostic factors for liver metastasis of gastric cancer after hepatic resection: a multi-institutional study of the indications for resection. Hepatogastroenterology. 2003;50(53):1560-1563.
- Roh HR, Suh KS, Lee HJ, Yang HK, Choe KJ, Lee KU. Outcome of hepatic resection for metastatic gastric cancer. Am Surg. 2005;71(2):95-99.
doi pubmed - Sakamoto Y, Sano T, Shimada K, Esaki M, Saka M, Fukagawa T, Katai H, et al. Favorable indications for hepatectomy in patients with liver metastasis from gastric cancer. J Surg Oncol. 2007;95(7):534-539.
doi pubmed - Yano T, Haro A, Yoshida T, Morodomi Y, Ito K, Shikada Y, Shoji F, et al. Prognostic impact of local treatment against postoperative oligometastases in non-small cell lung cancer. J Surg Oncol. 2010;102(7):852-855.
doi pubmed - Yano T, Okamoto T, Haro A, Fukuyama S, Yoshida T, Kohno M, Maehara Y. Local treatment of oligometastatic recurrence in patients with resected non-small cell lung cancer. Lung Cancer. 2013;82(3):431-435.
doi pubmed - Petrelli F, Coinu A, Cabiddu M, Ghilardi M, Borgonovo K, Lonati V, Barni S. Hepatic resection for gastric cancer liver metastases: A systematic review and meta-analysis. J Surg Oncol. 2015;111(8):1021-1027.
doi pubmed - Kinoshita T, Kinoshita T, Saiura A, Esaki M, Sakamoto H, Yamanaka T. Multicentre analysis of long-term outcome after surgical resection for gastric cancer liver metastases. Br J Surg. 2015;102(1):102-107.
doi pubmed - Martella L, Bertozzi S, Londero AP, Steffan A, De Paoli P, Bertola G. Surgery for liver metastases from gastric cancer: a meta-analysis of observational studies. Medicine (Baltimore). 2015;94(31):e1113.
doi pubmed - Tachezy M, Gebauer F, Janot M, Uhl W, Zerbi A, Montorsi M, Perinel J, et al. Synchronous resections of hepatic oligometastatic pancreatic cancer: Disputing a principle in a time of safe pancreatic operations in a retrospective multicenter analysis. Surgery. 2016;160(1):136-144.
doi pubmed - Oki E, Tokunaga S, Emi Y, Kusumoto T, Yamamoto M, Fukuzawa K, Takahashi I, et al. Surgical treatment of liver metastasis of gastric cancer: a retrospective multicenter cohort study (KSCC1302). Gastric Cancer. 2016;19(3):968-976.
doi pubmed - Hackert T, Niesen W, Hinz U, Tjaden C, Strobel O, Ulrich A, Michalski CW, et al. Radical surgery of oligometastatic pancreatic cancer. Eur J Surg Oncol. 2017;43(2):358-363.
doi pubmed - Hanaki T, Sunaguchi T, Goto K, Morimoto M, Murakami Y, Shishido Y, Miyatani K, et al. The significance of surgical intervention for metasynchronous liver metastasis in gastric cancer: a single-centre analysis. Anticancer Res. 2022;42(4):2177-2184.
doi pubmed - Ren Y, Dai C, Zheng H, Zhou F, She Y, Jiang G, Fei K, et al. Prognostic effect of liver metastasis in lung cancer patients with distant metastasis. Oncotarget. 2016;7(33):53245-53253.
doi pubmed - Yamanaka M, Hayashi M, Yamada S, Sonohara F, Takami H, Inokawa Y, Shimizu D, et al. A possible definition of oligometastasis in pancreatic cancer and associated survival outcomes. Anticancer Res. 2021;41(8):3933-3940.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


