| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 5, October 2022, pages 249-258
Cancer Progression Is not Different in Mice of Different Gender Inoculated With Cells of the Triple-Negative 4T1 Breast Cancer Model
Exsal Manuel Albores-Mendeza, b, Rocio Guadalupe Casanas-Pimentelb , Indira Raquel Reyes-Chaconc, Juan Maldonado Cubasd
, Jaime Lopez-Cruza, Jorge Alberto Rincon-Huertac, Alejandro Camacho-Ibarraa, Eduardo San Martin-Martinezb, e
aCE.MI.C.SA. - Escuela Militar de Graduados de Sanidad, Universidad del Ejercito y Fuerza Aerea, Batalla de Celaya 202, Lomas de Sotelo, Ciudad de Mexico, C.P. 11200, Mexico
bInstituto Politecnico Nacional, Centro de Investigacion en Ciencia Aplicada y Tecnologia Avanzada, Unidad Legaria, Calzada Legaria 694, Irrigacion, Miguel Hidalgo, Ciudad de Mexico, C.P. 11500, Mexico
cCentro de Estudios Navales en Ciencias de la Salud, Coapa, Ex-Ejido de San Pablo Tepetlapa, Ciudad de Mexico, C.P. 04800, Mexico
dUniversidad La Salle, Grupo de Investigacion en Procesamiento Digital de Senales Biomedicas, Vicerrectoria de Investigacion, Benjamin Franklin 45, Condesa, Cuauhtemoc, Ciudad de Mexico, C.P. 06140, Mexico
eCorresponding Author: Eduardo San Martin-Martinez, Instituto Politecnico Nacional, Centro de Investigacion en Ciencia Aplicada y Tecnologia Avanzada, Unidad Legaria, Calzada Legaria 694, Irrigacion, Miguel Hidalgo, Ciudad de Mexico, C.P. 11500, Mexico
Manuscript submitted July 24, 2022, accepted August 15, 2022, published online October 22, 2022
Short title: Male Murine Breast Cancer
doi: https://doi.org/10.14740/wjon1517
| Abstract | ▴Top |
Background: Breast cancer in men is a rare and poorly studied disease, and its treatment is based on women breast cancer studies. However, clinical outcome is not the same in men and women. Basic studies and clinical trials in animal models provide detailed information on cancer, origin, development, cell signaling pathways, sites of metastasis, and target molecules. It is necessary to explore the biology of breast cancer in male animal models that allow observing their similarity.
Methods: The triple-negative 4T1 breast cancer model was developed in both male and female mice and studied weekly during 4 weeks. For that, twenty 8-week-old female and male BALB/c mice were used. Sixteen mice (eight males and eight females) were inoculated into the second left thoracic mammary pad with 20,000 4T1 cells, resuspended in 20 µL phosphate-buffered saline (PBS). All samples were processed for immunodetection, characterized histopathologically and immunohistochemically.
Results: In this work, we describe the development of a triple-negative 4T1 breast cancer model in male BALB/c mice. Breast tumors were characterized histopathologically at different time points and corresponded to a moderately differentiated invasive ductal carcinoma, estrogen receptor ER-/progesterone receptor PR-/human epidermal growth factor receptor 2 HER2-/Ki67+, with histological grade II (moderately differentiated; a solid mass with occasional duct formation and moderate to severe nuclear pleomorphism), infiltrating the adipose and muscular tissue, and metastasis to lungs. From the results, we did not observe differences in the time of tumor development, necrosis, color change of tumor tissue, and lung metastasis between male and female mice. Even though we did not find histological differences, response to treatment and molecular signaling may be different.
Conclusions: The histogenesis of male breast tumors was similar to that of female BALB/c mice. The histological and immunohistochemical characteristics of male tumors also match the features reported for stage IV human breast cancer of men and women. The murine male breast cancer model described here can be a significant tool to explore the molecular mechanisms involved in male breast cancer tumorigenesis and metastasis and may bring new approaches for clinical treatment of triple-negative breast cancer in men.
Keywords: Breast cancer; Histopathological; Immunohistochemical; Animal model; TNBC; BALB/c mice; 4T1; Male
| Introduction | ▴Top |
Breast cancer is the most common type of cancer and the one that causes the highest number of deaths in women worldwide [1]. In contrast, this type of cancer is rare in men [2], accounting for approximately 1% of all breast cancer diagnoses in the world [3]. The management of male breast cancer is based on studies conducted on women or female animal models. However, triple-negative breast cancer in male patients is likely to be at a more advanced stage at the time of diagnosis, often with worse outcomes than in women. In addition, cancer treatments in men are commonly extrapolated from those used to treat postmenopausal women with breast cancer without evidence to support this extrapolation from women to men [4]. Studies addressing the biology and management of breast cancer in men (e.g., studies that correlate prognostic factors with treatment outcomes) are limited, and often lack statistical power [2, 5-7]; data about risk factors, optimal treatment, and short-term and long-term outcomes in men are also unknown [8].
Clinical studies report different characteristics of the disease in men and women [2]. For example, breast cancer in men has a lower survival rate and a higher mortality than in women. This type of cancer occurs at an older age in men and the lobular cancer is very rare compared to women. Male breast cancer cases have higher levels of detectable estrogen receptors (ERs) and progesterone receptors (PRs) and lower probability of being positive for human epidermal growth factor receptor 2 (HER2) than female breast cancer cases, therefore, the luminal subtype is more common. Moreover, HER2-positive cases of cancer in men have a higher mortality rate than in women. In women, cases of triple-negative breast cancer account for 11% of cases, while in men they represent less than 1% of cases. About 99% of breast cancer cases in men are ER-positive and more than 96% of cases are androgen receptor-positive and BRCA2 mutations are more common in men than in women. The causes of these differences in tumor biology are unknown. However, it has been suggested that these differences may be related to distinct biological processes in men and women influencing tumorigenesis, mainly hormonal variations related to androgens, estrogens, and progestins. Genomic differences in breast cancer cases have also been evidenced between men and women; for example, in ER- and PR-positive male breast cancers mutations that are common in ER- and PR-positive female breast cancer cases (e.g., PIK3CA and TP53) are less likely to be identified in men [3, 8]. Molecular studies show that gene expression patterns and the susceptibility to develop breast cancer are sex-dependent in mice (BALB/c and C57BL/6) [9], supporting the idea that the biology of breast cancer may be different in men and women. Despite these observations, some authors have suggested that breast cancer studies performed on females are suitable for the study of breast cancer in men [10]. It is thus unclear whether female-based studies are suitable for the management and treatment of male breast cancer, and it is thus necessary to understand the biology of breast cancer in men.
Studies addressing the biology of breast cancer and treatment development strongly depend on available in vitro and in vivo models [11, 12]. Thus far, the female 4T1 mouse model has offered researchers several advantages for the study of female breast cancer: tumors develop at the correct anatomical site (the breast) in immunocompetent animals, the efficiency of tumor development is high (100% according to our experience and the available literature), spontaneous metastasis is observed at the same sites where women with breast cancer develop metastases [11]; in addition, this animal model efficiently emulates the triple-negative female breast cancer in stage IV, which is a highly aggressive type of breast cancer both in men and women [8, 13].
Typically, the 4T1 breast cancer model involves the inoculation of 6 - 15, 5- to 8-week-old female BALB/c with a certain number of 4T1 tumor cells, which are injected subcutaneously in the mammary gland [11-15]. Similar to women, triple-negative breast cancer in men is molecularly characterized by a lack of the expression of hormone receptors (ER, PR) and the HER2.
We hypothesized that male BALB/c could be used to develop a male triple-negative 4T1 breast cancer model that could be specifically applied to the study of breast cancer biology and therapeutic strategies in men. The novel male triple-negative 4T1 breast cancer model that we describe here may help to strengthen, or possibly even to redirect, the current management of male breast cancer, which is largely based on studies performed on females.
| Materials and Methods | ▴Top |
The present study was developed following international guidelines for the care and use of laboratory animals, NOM-062 (Official Mexican Standard NOM-062-ZOO-1999, Technical specifications for the production, care, and use of laboratory animals), and approved by the Internal Committee for the Care and Use of Laboratory Animals of the Military School of Medicine (Mexico City).
Cell culture
The culture of the 4T1 cell line was carried out following the protocol suggested by the ATCC. RPMI-1640 medium (ATCC, Cat. No. 30-2001), supplemented with 10% fetal bovine serum (ATCC, Cat. No. 30-2020) was used. The cells were cultured in a humidified atmosphere at 37 °C and 5% CO2. Once 80% of confluence was reached, the cells were treated with trypsin and washed with phosphate-buffered saline (PBS) to be subsequently resuspended in PBS for inoculation. Cell count was performed using the Muse Cell Analyzer equipment (Merck Millipore, Muse Count & Viability Assay Kit, Cat. No. MCH100102).
Experimental procedure
We used 20 BALB/c 8-week-old female and male mice (20 - 25 g in weight, Rismart S.A. de C.V). To compare the tumor behavior in both sexes, animals were kept in ventilated racks with light and dark cycles of 12 h, and water and food ad libitum (laboratory rodent diet 5001, Lab Diet, USA). Sixteen mice (eight males and eight females) were inoculated in the pad of the second left thoracic breast with 20,000 4T1 cells (purchased from ATCC, Cat. No. CRL-2539, passage number 3), resuspended in 20 µL of PBS. Four mice constituted the control group (two males and two females) and were injected only with 20 µL of PBS in the second left thoracic breast.
After inoculation, mice were sacrificed in groups of four per week (two males and two females), thus obtaining the groups of first, second, third, and fourth weeks. One mouse of the control group was sacrificed every week. All mice were sacrificed by cervical dislocation after sedation with ketamine 50 mg/kg. Mice were dissected, then the primary tumor and organs that are often metastasis targets (lung, liver, heart, and kidney) [11, 12, 15], were extracted for analysis. Each tumor was measured with a Vernier caliper, and its volume was calculated with the formula reported by Richtig et al [16]. All samples were dehydrated and embedded in paraffin blocks.
Immunohistochemistry
The paraffin blocks of control sample tissues and the tumors and organs of inoculated mice were cut to 3 µm thickness with a microtome and subjected to histopathological analysis. All samples were processed for immunodetection, with antibodies against the PR from clone BSB2 (Cat. No. BSB 2126), ER from clone RBT11 (Cat. No. BSB 5491), Ki67 from clone EP5 (Cat. No. BSB 5708), and HER2 from clone RBT-HER2 (Cat. No. BSB 2035); all antibodies were obtained from BIO SB. Muscle (PR+), ovary (ER+), amygdala (Ki67+), and breast cancer (HER2+) sample tissues were used as controls for each antibody. Samples were de-waxed with xylol and rehydrated ethanol at decreasing concentrations (100%, 70%, 60%, 30%, and 0%). Antigenic retrieval was carried out for 10 min at 98 °C with sodium citrate pH 6. Samples were subsequently washed three times with distilled water. Then, endogenous peroxidase blocking was performed for 5 min with a hydrogen peroxide solution (1:20), followed by three washes with distilled water.
Samples were blocked for 10 min with bovine serum albumin (ScyTek Cat. No. 52912). Subsequently, samples were incubated with the corresponding antibodies for 1 h. Samples were washed with × 1 Tris buffered saline (TBS) and incubated with polymer-based biotin-free horseradish peroxidase for 45 min, and then washed with × 1 TBS. The detection of the target antigens was carried out with diaminobenzidine for 3 min, followed by washing with tap water. Counterstaining was performed with Harris hematoxylin for 1 min, followed by rinsing with tap water and washing with lithium carbonate. Samples were dehydrated with ethanol at increasing concentrations (70%, 80%, 96%, and 100%), washed with xylol, and finally, mounted using synthetic resin (Entellan). Each slide was observed under a microscope at × 10, × 20, and × 40 magnification. Photographs of each slide, stained with hematoxylin and eosin and immunohistochemistry, were taken with an Olympus CH30 microscope.
Statistical analysis
A two-way analysis of variance (ANOVA) test, followed by Dunnett’s multiple comparison tests, was performed to analyze the tumor growth per week. Mann-Whitney tests were used to study the association between tumor volume and necrosis. Relationships between necrosis and color change or lung metastasis development were established through Fisher’s exact tests. A two-way ANOVA statistical analysis was used to study the effect of time (weeks post-injection) and the sex of the animals on necrosis development and color change. Chi-square tests were used to determine the relationship between the time (weeks post-injection) and the presence of metastasis in different organs. All statistical analyses were performed using the software GraphPad PRISM v7.0.
| Results | ▴Top |
Breast tumor growth in male BALB/c mice
The 4T1 breast cancer model established in male BALB/c mice revealed similar histological features in male and female mice. Tumor growth was recorded from the first-week post-injection (Fig. 1a, b) in mice of both sexes. The size of the tumor increased after the first week (Fig. 1). The time elapsed after inoculation was a significant factor determining the volume of the tumor. The volume of the tumor increased considerably after the third and fourth week post-inoculation. The largest tumors were observed at the fourth week. At each time point, tumor volume was very similar in male and female mice (Fig. 2). The average volume of the tumor was 0.054 ± 0.025 cm3 at the first week, 0.138 ± 0.035 cm3 at the second week, 1.345 ± 0.240 cm3 at the third week, and 9.430 ± 0.415 cm3 at the fourth week.
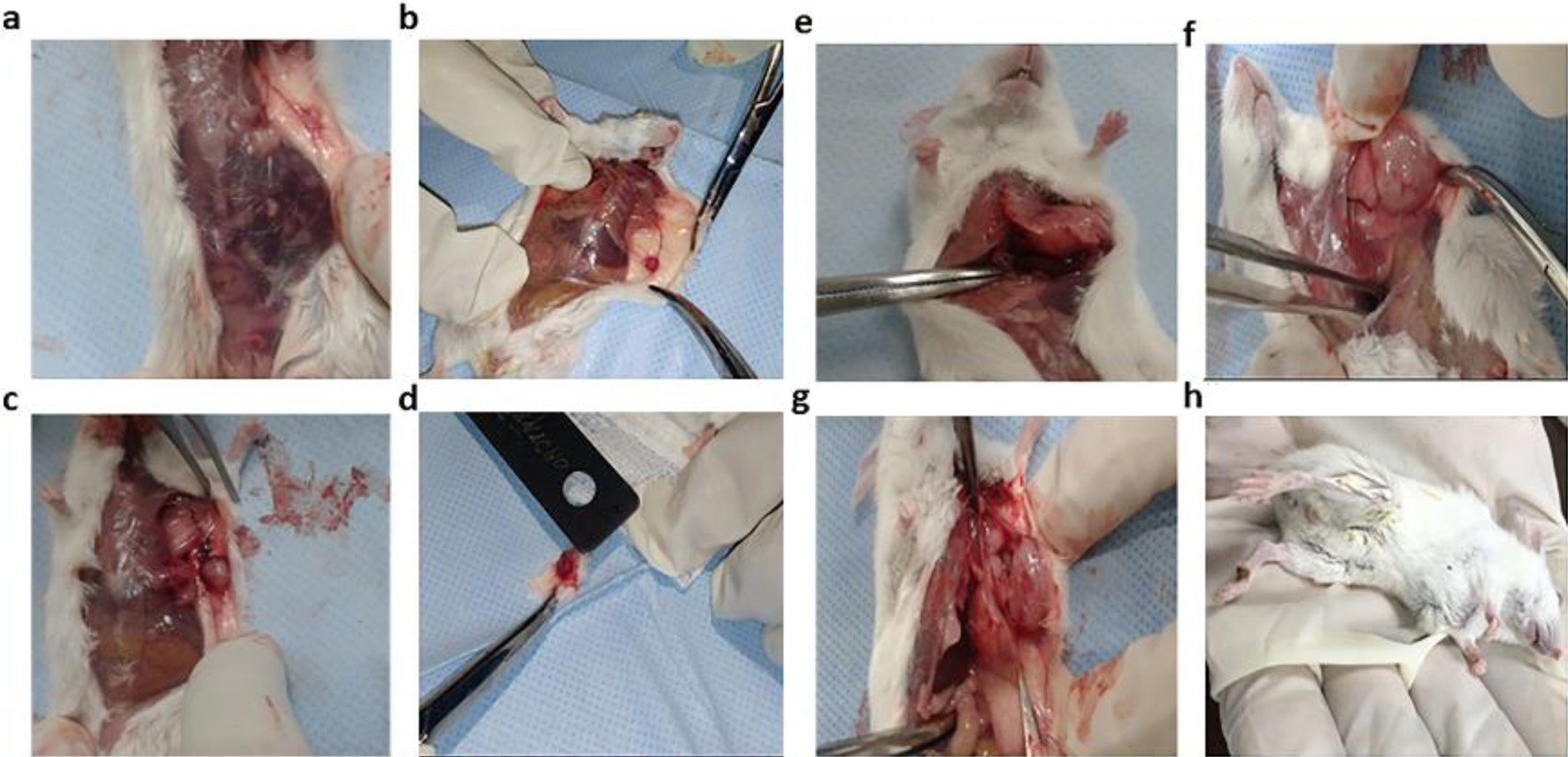 Click for large image | Figure 1. Macroscopic changes observed as the breast tumor grew in male BALB/c mice after injection with 4T1 cells: (a, b) first-week animals; (c, d) second-week animals; (e, f) third-week animals; (g, h) fourth-week animals. The tumor was clinically observable from the first week. The largest tumors were obtained at the fourth week. |
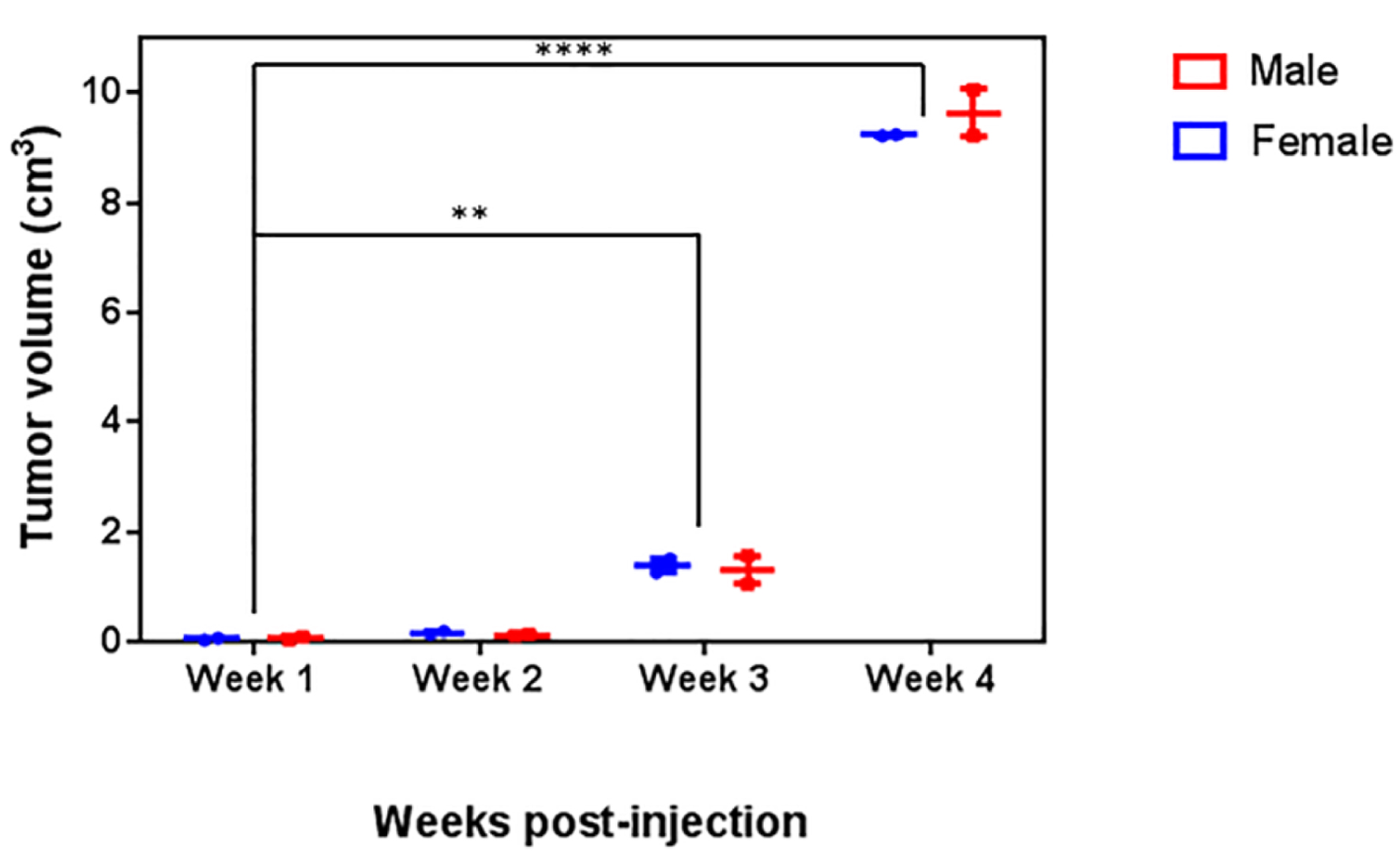 Click for large image | Figure 2. Analysis of tumor volume. Male and female BALB/c mice were inoculated with 4T1 cells. Tumor volume was recorded for 4 weeks. A two-way ANOVA followed by a Dunnet’s multiple comparisons test was carried out to compare the tumor growth; no significant differences in the tumor volume between male and female mice were observed (P = 0.1966) at any time point. |
Histological changes during breast tumor growth in male BALB/c mice
During tumor development, we observed two important histological features that were common for both sexes: necrosis (Fig. 3a) and color change of the tumor tissue (Fig. 3b); these two features were studied over time. At the first week of tumor development, mild inflammation and edema were observed at the site of inoculation in both sexes. In the second week, 50% of the inoculated animals presented necrosis in about 5% of the tumor mass. At the third week, all the inoculated animals presented coagulative necrosis in 30% to 40% of the tumor mass. The tumors observed at the fourth week had already invaded the skin and deep tissues, with extensive coagulative necrosis, and ulceration of the skin.
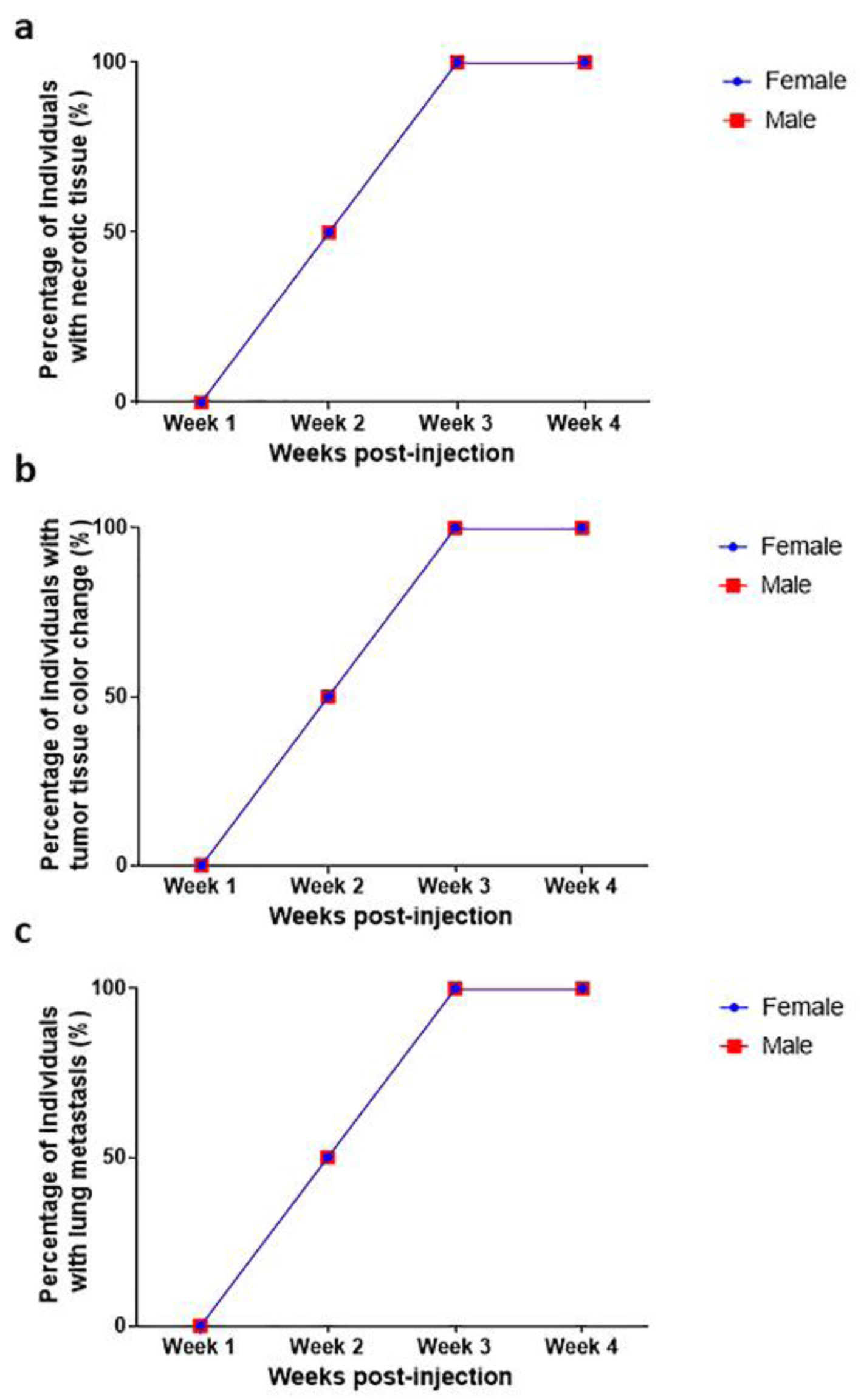 Click for large image | Figure 3. Macroscopic changes during tumor development (a) necrosis, (b) color change and (c) lung metastasis. Necrosis, color change, and lung metastasis were observed from the second week of tumor development; 100% of the animals had necrosis, color change, and lung metastasis after the third week. No statistical differences were observed between female and male individuals (P > 0.9999). |
Sites of metastasis in male BALB/c mice
In both male and female mice, the lung was the most affected organ. A Chi-square analysis was carried out to determine if there was a relationship between the time of tumor evolution and the presence of metastasis to different organs; this analysis confirmed that the lung is the most frequently metastasized organ, especially after the third week (P = 0.029). A two-way ANOVA statistical analysis showed no significant differences in the frequency of lung metastasis (Fig. 3c) between sexes (P > 0.9999). We found a correlation between necrosis development and lung metastasis (P = 0.0076, Fig. 4c). Metastatic lesions were also observed in perirenal adipose tissue (mesentery), skeletal muscle, and bone, while in the liver, kidney, and heart, no metastasis was observed.
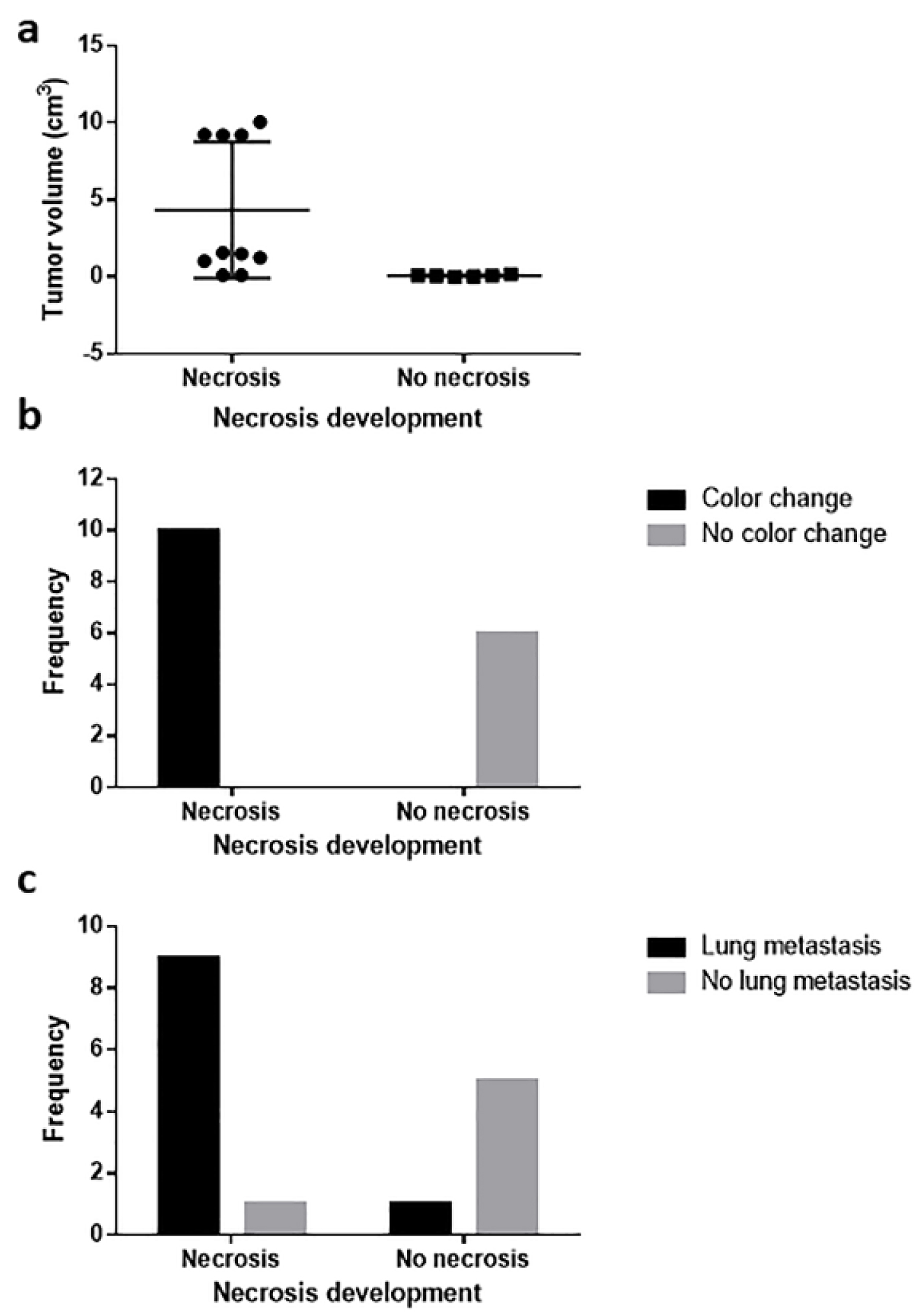 Click for large image | Figure 4. Relationship between necrosis development and (a) tumor volume, (b) tissue color change, and (c) lung metastasis. Tumor volume correlated with necrosis development, and necrosis was related to tissue color change and lung metastasis. |
In mice of both sexes, the tumor observed at the first week post-inoculation corresponded to a ductal carcinoma infiltrating the adipose and muscular tissue. Histopathological analysis showed ducts with epithelial hyperplasia, containing cells with nuclear pleomorphism and hyperchromasia, loss of the normal nucleus-cytoplasm ratio, mitosis, and atypia; these features corresponded to a premalignant lesion (florid ductal hyperplasia with atypia). At the second, third, and fourth weeks, the tumors maintained the same histological characteristics of the first week; however, from the second week, the primary tumors could be macroscopically observed in mice of both sexes. Histological characteristics of tumors are shown in Figure 5.
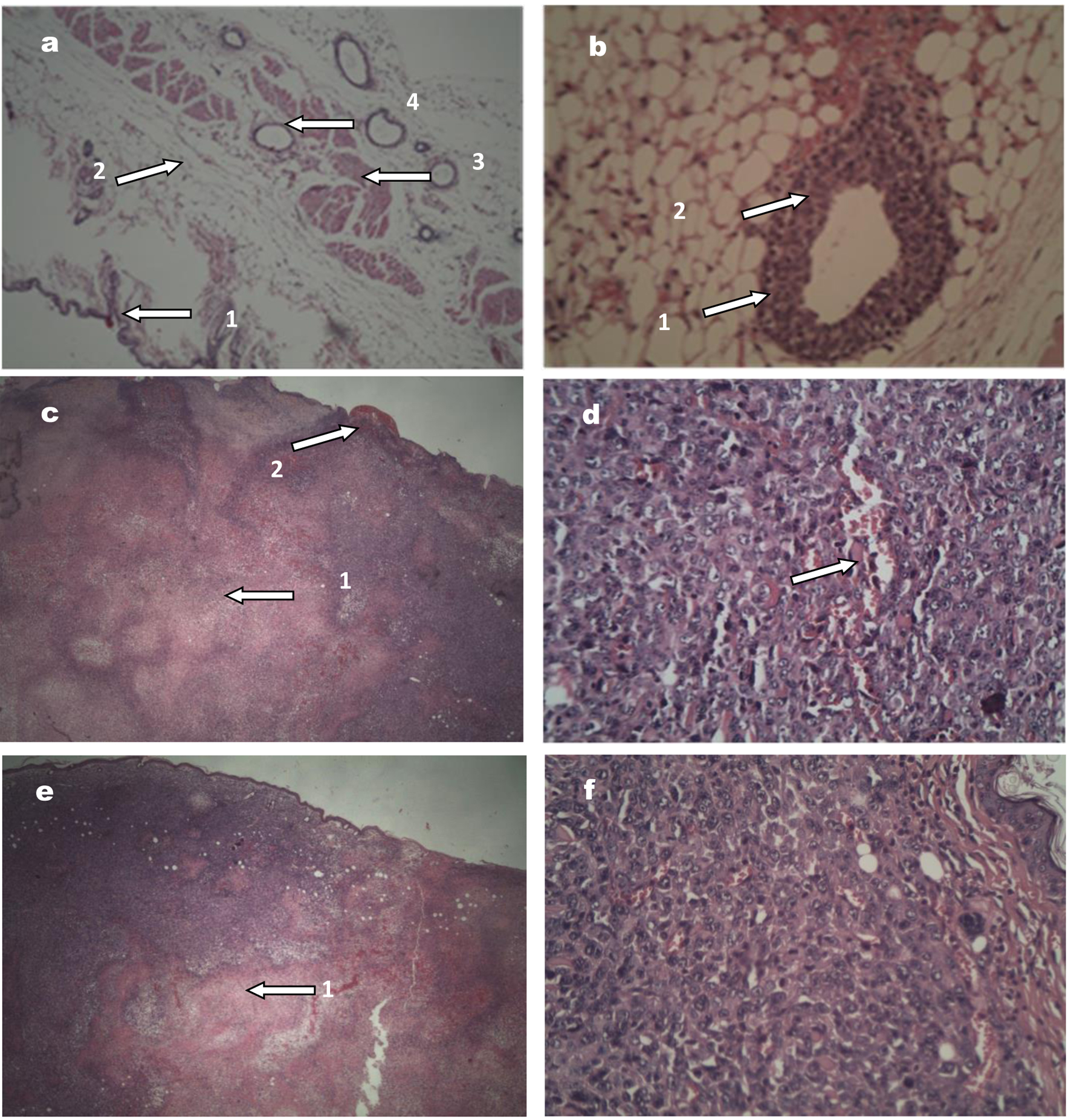 Click for large image | Figure 5. Images of tumors stained with hematoxylin and eosin. (a) Normal breast tissue (× 10; × 20 and × 40), showing (1) healthy skin, (2) fatty tissue, (3) muscle tissue, and (4) mammary duct with a cell monolayer. (b) Tumor tissue (× 40) corresponding to a premalignant lesion: (1) the duct is observed with various epithelial cell layers, (2) modifications in the nuclei. (c) Female mouse tumor tissue (× 20), showing (1) area of central necrosis and (2) skin ulceration. (d) Female mouse tumor tissue (× 40), the tumor in more advanced stages, with an area of lymphovascular infiltration. (e) and (f) correspond to tumor tissue in male mouse, × 20 and × 40, respectively, with similar features to the female tumor tissue. |
Immunohistochemistry analysis showed that the molecular expression patterns in the tumors of both male and female animals corresponded to ER-/PR-/HER2-/Ki67+ (Fig. 6), which corroborates that for both sexes the tumor had triple-negative features [13].
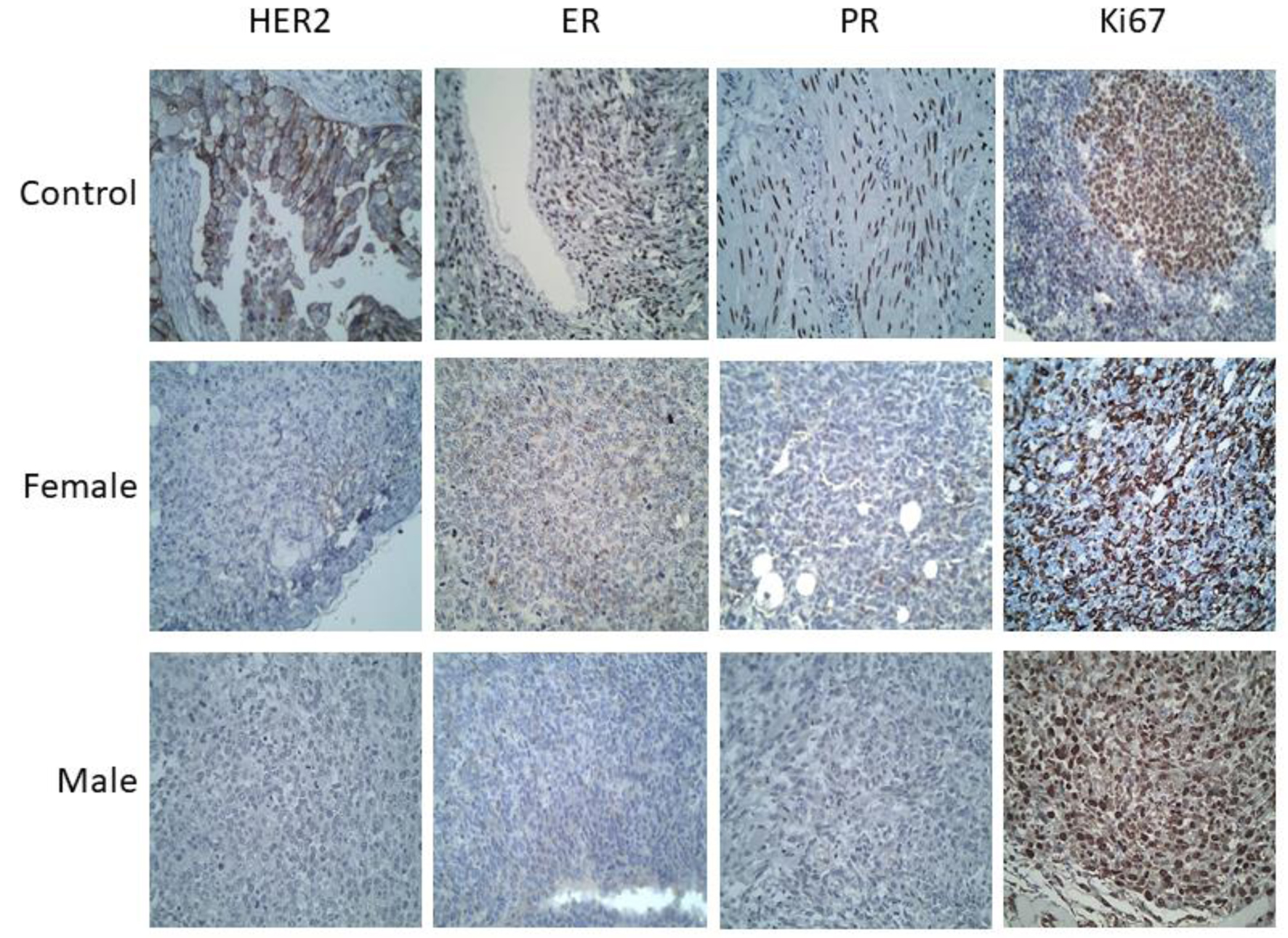 Click for large image | Figure 6. Immunohistochemistry of the breast tumors developed in male and female mice (images at × 40 magnification). Positive controls used for each antibody were: HER2+ (breast cancer), Ki67+ (amygdala), ER+ (ovary) and PR+ (muscle). The tumors in both cases (male and female mice) were positive for Ki67, and negative for the hormone receptors: ER and PR, as well as for HER2, in all samples from the first to the fourth week. |
| Discussion | ▴Top |
In this work, we clearly show that it is possible to develop a 4T1 breast cancer model in male BALB/c mice. This male breast cancer model was similar to the female breast cancer model of mice in terms of tumor histogenesis, necrosis, tumor volume, tissue color change, and sites of metastasis.
In our model, primary tumors could be observed macroscopically after the first week post-inoculation, both in male and female mice. We also found that the growth of the breast tumor exhibited a similar rate in male and female mice, with no significant differences in tumor volume between sexes at any time point. These results are in keeping with previous reports showing that, in female BALB/c mice, tumors were macroscopically observed 1 week after inoculation with a similar number of 4T1 cells [11, 12]. Thus, the time of tumor development in our murine male breast cancer model is consistent with available studies performed on females.
During tumor development, we observed three important histopathological features that were common for both sexes: necrosis, color change of the tumor tissue, and lung metastasis. We found that, both in male and female mice, necrosis increased as the volume of the tumor increased, and that necrosis was also directly associated with tissue color change. These features were evident after the second week post-inoculation, and after the third week these features were present in 100% of the animals, regardless of their sex. Necrosis and color change had not been reported previously, whereas lung metastasis is a frequent observation in the female model, after animals are injected with a wide range of 4T1 cell numbers [11, 12].
In terms of metastasis, the lung was the most affected organ, both in male and female mice in our study. We observed a correlation between necrosis and lung metastasis. These results are in keeping with a report by Gregorio et al [12], who found that, in female BALB/c mice both, the time necessary to develop a palpable tumor, and the sites of metastasis are a function of the number of inoculated cells [12]; they also observed the highest frequency of metastasis in the lung. It is worth noting that, in the present study, a similar number of inoculated cells resulted in metastasis to the lung in male mice. The accelerated growth and high metastatic potential (particularly to the lungs) of tumors in our male breast cancer model are consistent with stage IV human breast cancer [13].
In the present work, the tumors of both male and female mice corresponded to a ductal carcinoma grade II (moderately differentiated; a solid mass with occasional duct formation and moderate to severe nuclear pleomorphism), infiltrating the adipose and muscular tissue. Immunohistochemistry analysis showed that both male and female tumors had triple-negative features (ER-/PR-/HER2-/Ki67+) as expected. Our histological data are in keeping with data reported by Tao et al [15], who reported alteration of the tissue architecture and hyperchromatic nuclei. These histological and immunohistochemical characteristics, together with the presence of metastasis, match the features reported for human stage IV breast cancer in men and women [13].
The validation of an animal model for the study of cancer biology and pharmacological response in the design of therapeutic schemes or in the development of new drugs is of great importance [17]. In this study, it is noteworthy that the model generated in male mice satisfactorily emulates the behavior of male breast cancer in humans, from the histopathological point of view; the model is orthotopic and uses immunocompetent animals, which gives to it some advantages. It is also necessary to remember that it is a syngeneic mouse model with murine cells implanted in mice, which may imply some disadvantages. Therefore, its application will depend on the interest of the specific research. Understanding the advantages and limitations of breast cancer models is critical for investigators exploring breast cancer therapies [17].
Orthotopic models have proven to be more suitable for tumor development by allowing higher growth rates and more effectively emulating the evolution of breast cancer in humans compared to models developed in implantation sites other than the breast, such is the case subcutaneous and intravenous implantation [18, 19].
Within the orthotopic models, those developed by xenotransplantation of tumor cells derived from patients have gained strength and clinical relevance because they retain the tumor heterogeneity, gene expression, and similar response to treatment of the patient, thus, they give specific information about the tumor under study and treatment [19, 20]. However, they have the disadvantage of being developed in immunodeficient host strains; therefore, they omit immunological interactions between the organism and tumor cells, which also has an important role on tumor development [18]. These models currently have limited utilization for investigation on cancer immunotherapies such as immune checkpoint blockade therapy [20].
It is worth noting that in this work we observed no differences in the time of tumor development, necrosis, color change of the tumor tissue, and lung metastasis, between male and female mice. This similarity is somewhat surprising because under normal conditions, the development and the function of mammary tissue are different in males and females. We chose a triple-negative model because, in men, triple-negative breast cancer is highly aggressive and has worse prognosis. The lack of differences between males and females for all the variables that we measured may be partly related to the absence of hormonal markers. However, the fact that in men breast cancer (particularly triple-negative breast cancer) is more aggressive than in women suggests that the underlying biology of this type of cancer is different in men and women. Thus, we cannot rule out the existence of molecular differences between males and females in our breast cancer model, even if the somewhat general features of male tumor development and metastasis that we analyzed were quite similar between sexes. This may be particularly important, considering that we did not delve deep into the analysis of the molecular mechanisms involved in tumor development and metastasis. As a whole, our murine male model is robust, as its features are in line with the available literature on both mice and humans. In a similar model of breast cancer (induced with luminescent 4T1 cells) developed in female BALB/c mice, it was shown that even the cell implantation method generates significant differences in gene expression patterns, even though animals of the same sex were used. Moreover, the authors also observed in this orthotopic model that the course of the disease is modified if the second or fourth mouse mammary pad is chosen for cell implantation [18]. Therefore, it is essential that this model be molecularly validated to determine its similarity with respect to human breast cancer in men and determine its usefulness for the study of cancer biology and the prediction of pharmacological response in humans. This fact reinforces the importance, not only of molecularly studying the model that we have presented, but also of joining efforts for the development of specific studies of breast cancer for the male sex, because the biological environment has a significant impact on tumor development, histopathologic features, sites of metastases, and survival [18].
More studies are needed to develop suitable animal models for the study of breast cancer. We are far from having ideal models, but within the available alternatives, it is necessary to visualize the relevance of selecting the appropriate model for each study. In addition, the development of new models that provide new approaches for the development of successful therapies for the treatment of breast cancer is necessary [17].
The murine male breast cancer model described here can be a significant tool to explore the molecular mechanisms involved in male breast cancer tumorigenesis and metastasis. The information resulting from future studies will be highly relevant for the development of therapeutic strategies specifically designed for men that are not based on studies conducted on female subjects.
Conclusions
We have successfully developed a murine male cancer model in BALB/c mice injected with 4T1 cells. Our male breast cancer model was similar to the female breast cancer model of mice in terms of tumor histogenesis, necrosis, tumor volume, tissue color change, and sites of metastasis, and is consistent with human stage IV breast cancer. The histological and immunohistochemical characteristics of male tumors also match the features reported for stage IV human breast cancer of men and women. We observed no differences in the time of tumor development, necrosis, color change of the tumor tissue, and metastasis, between male and female mice, but we cannot rule out the existence of molecular differences underlying tumor development and metastasis in males and females. Thus, future molecular studies are needed to better understand this model.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by the Secretaria de Investigacion y Posgrado del Instituto Politecnico Nacional and the National Council of Science and Technology (CONACYT) through the Catedras CONACYT program (grant number 1728).
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Conceptualization: Eduardo San Martin-Martinez. Methodology: Exsal Manuel Albores-Mendez, Rocio Guadalupe Casanas-Pimentel. Acquisition of data: Indira Raquel Reyes-Chacon, Jaime Lopez-Cruz. Formal analysis and investigation: Exsal Manuel Albores-Mendez, Indira Raquel Reyes-Chacon, Jaime Lopez-Cruz, Jorge Alberto Rincon-Huerta, Alejandro Camacho-Ibarra. Writing original draft preparation: Juan Maldonado Cubas, Exsal Manuel Albores-Mendez. Writing review, critical feedback, and editing: Eduardo San Martin-Martinez, Rocio Guadalupe Casanas-Pimentel. Resources and supervision: Eduardo San Martin-Martinez.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Parsa Y, Mirmalek SA, Kani FE, Aidun A, Salimi-Tabatabaee SA, Yadollah-Damavandi S, Jangholi E, et al. A review of the clinical implications of breast cancer biology. Electron Physician. 2016;8(5):2416-2424.
doi pubmed - Konduri S, Singh M, Bobustuc G, Rovin R, Kassam A. Epidemiology of male breast cancer. Breast. 2020;54:8-14.
doi pubmed - Sabih QA, Young J, Takabe K. Management of male breast cancer: the journey so far and future directions. World J Oncol. 2021;12(6):206-213.
doi pubmed - Yao N, Shi W, Liu T, Siyin ST, Wang W, Duan N, Xu G, et al. Clinicopathologic characteristics and prognosis for male breast cancer compared to female breast cancer. Sci Rep. 2022;12(1):220.
doi pubmed - Cardoso F, Bartlett JMS, Slaets L, van Deurzen CHM, van Leeuwen-Stok E, Porter P, Linderholm B, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol. 2018;29(2):405-417.
doi pubmed - Liu N, Johnson KJ, Ma CX. Male breast cancer: an updated surveillance, epidemiology, and end results data analysis. Clin Breast Cancer. 2018;18(5):e997-e1002.
doi pubmed - Abdelwahab Yousef AJ. Male Breast Cancer: Epidemiology and Risk Factors. Semin Oncol. 2017;44(4):267-272.
doi pubmed - Ghani S, Sochat M, Luo J, Tao Y, Ademuyiwa F. Characteristics of male triple negative breast cancer: A population-based study. Breast J. 2020;26(9):1748-1755.
doi pubmed - Mukherjee S, Sainis KB, Deobagkar DD. F1 hybrids of BALB/c and C57BL/6 mouse strains respond differently to low-dose ionizing radiation exposure. J Genet. 2014;93(3):667-682.
doi pubmed - Willsher PC, Leach IH, Ellis IO, Bourke JB, Blamey RW, Robertson JF. A comparison outcome of male breast cancer with female breast cancer. Am J Surg. 1997;173(3):185-188.
doi - Pulaski BA, Ostrand-Rosenberg S. Mouse 4T1 breast tumor model. Curr Protoc Immunol. 2000;39(1):20.2.1-20.2.16.
doi pubmed - Gregorio AC, Fonseca NA, Moura V, Lacerda M, Figueiredo P, Simoes S, Dias S, et al. Inoculated cell density as a determinant factor of the growth dynamics and metastatic efficiency of a breast cancer murine model. PLoS One. 2016;11(11):e0165817.
doi pubmed - Kaur P, Nagaraja GM, Zheng H, Gizachew D, Galukande M, Krishnan S, Asea A. A mouse model for triple-negative breast cancer tumor-initiating cells (TNBC-TICs) exhibits similar aggressive phenotype to the human disease. BMC Cancer. 2012;12:120.
doi pubmed - Paschall AV, Liu K. An orthotopic mouse model of spontaneous breast cancer metastasis. J Vis Exp. 2016;114:e54040.
doi pubmed - Tao K, Fang M, Alroy J, Sahagian GG. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer. 2008;8:228.
doi pubmed - Richtig E, Langmann G, Mullner K, Richtig G, Smolle J. Calculated tumour volume as a prognostic parameter for survival in choroidal melanomas. Eye (Lond). 2004;18(6):619-623.
doi pubmed - Rashid OM, Takabe K. Animal models for exploring the pharmacokinetics of breast cancer therapies. Expert Opin Drug Metab Toxicol. 2015;11(2):221-230.
doi pubmed - Rashid OM, Nagahashi M, Ramachandran S, Dumur C, Schaum J, Yamada A, Terracina KP, et al. An improved syngeneic orthotopic murine model of human breast cancer progression. Breast Cancer Res Treat. 2014;147(3):501-512.
doi pubmed - Okano M, Oshi M, Butash A, Okano I, Saito K, Kawaguchi T, Nagahashi M, et al. Orthotopic implantation achieves better engraftment and faster growth than subcutaneous implantation in breast cancer patient-derived xenografts. J Mammary Gland Biol Neoplasia. 2020;25(1):27-36.
doi pubmed - Kawaguchi T, Foster BA, Young J, Takabe K. Current update of patient-derived xenograft model for translational breast cancer research. J Mammary Gland Biol Neoplasia. 2017;22(2):131-139.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


