| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Case Report
Volume 9, Number 2, April 2018, pages 56-61
A Case Series of Two Patients Presenting With Pericardial Effusion as First Manifestation of Non-Small Cell Lung Cancer With BRAF Mutation and Expression Of PD-L1
Muhammad Muftia, f, Steven Chinga, Sassan Farjamib, c, Sharareh Shahangiand, Serap Sobnoskye
aDepartment of Medicine, St. Mary Medical Center, Long Beach, CA, USA
bDepartment of Hematology/Oncology, St. Mary Medical Center, Long Beach, CA, USA
cPacific Shores Medical Group, Long Beach, CA, USA
dCritical Care Unit, St. Mary Medical Center, Long Beach, CA, USA
eDepartment of Cardiology, St. Mary Medical Center, Long Beach, CA, USA
fCorresponding Author: Muhammad Mufti, St, Mary Medical Center, GME, 1050 Linden Ave., Long Beach, CA 90813, USA
Manuscript submitted March 2, 2018, accepted March 28, 2018
Short title: Pericardial Effusion With Non-Small Cell Lung Cancer
doi: https://doi.org/10.14740/wjon1092w
| Abstract | ▴Top |
Lung cancer is the number one cause of cancer-related deaths in the United States. Involvement of pericardium occurs once cancer has progressed to stage IV which can cause massive effusion in the pericardial sac. This can lead to cardiac tamponade which can be fatal very quickly if untreated. The following is a two patient case series in which both patients presented with large pericardial effusion. The first patient sought medical attention due to new onset palpitations and was found to have hemorrhagic pericardial effusion and pulmonary embolism (PE). The second patient presented with shortness of breath. Investigations revealed that she had pericardial and pleural effusions along with multiple metastases throughout the body. Both patients ended up with a diagnosis of non-small cell lung cancer (NSCLC) with BRAF mutation. One patient had V600E mutation; other patient had a variant p.D594N mutation. Both patients also had expression of PD-L1.
Keywords: NSCLC; Pericardial effusion; BRAF V600E; BRAF c.1780G>A; BRAF p.D594N; PD-L1; Non-small cell lung cancer; Adenocarcinoma of the lung
| Introduction | ▴Top |
Lung cancer is the second most common cancer in both males and females in the United States and the most common cause of cancer-related deaths [1]. BRAF mutations have been found in about one to 1-4% of all non-small cell lung cancers (NSCLC) [2-3]. BRAF mutations in lung cancer have been identified as V600E (50%), G649A (39%), D594G (11%) [3]. The new targeted therapy against BRAF V600E makes that mutation important. Treatment of lung cancer with BRAF V600E mutation with BRAF and MEK inhibitors has led to a better prognosis. In this case series, we will present two patients presenting with massive pericardial effusion as the presenting complaint of BRAF positive lung cancer.
| Case Reports | ▴Top |
Case 1
A 63-year-old female presented to the emergency department with 2 days history of palpitations, shortness of breath and chest pain. She also reported chest heaviness with deep inspiration with both ambulation and at rest. Her past medical history was significant for hypertension controlled with medications. She denied any history of smoking. Electrocardiogram (EKG) performed in the emergency department showed patient to be in supraventricular tachycardia with a heart rate of about 170 beats per min (Fig. 1) and blood pressure 123/83 mm Hg. Pulse oximetry showed 97% saturation on room air. On examination, she was in mild distress. Cardiac auscultation revealed normal S1, and S2 heart sounds without any gallops. No jugular venous distension was noted. Lungs were clear to auscultation.
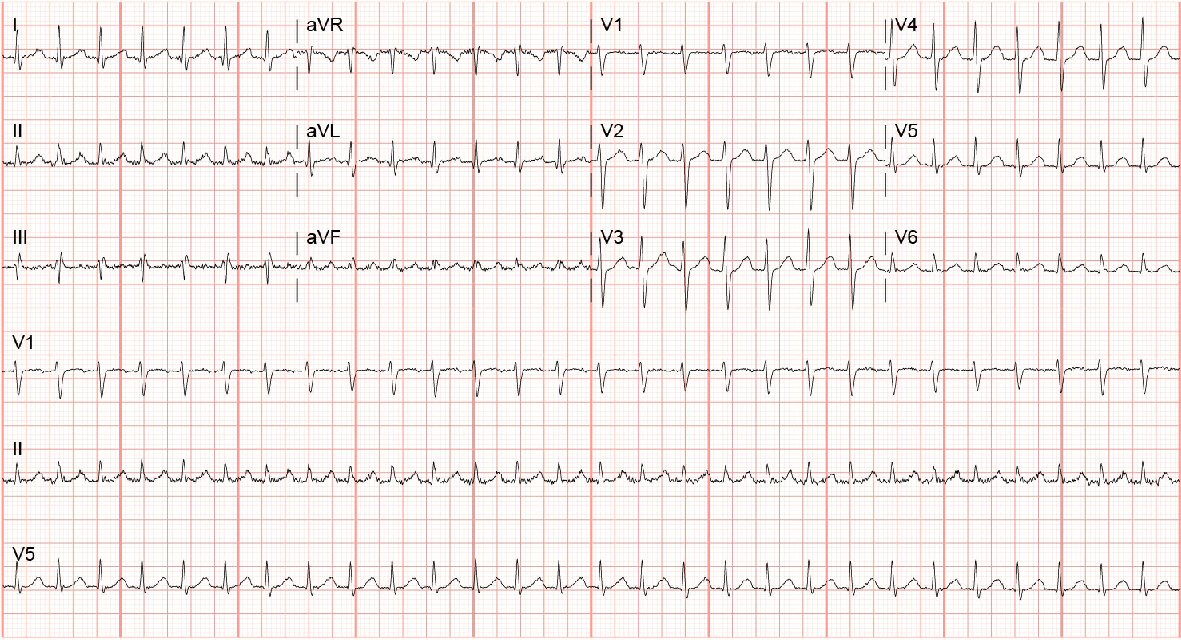 Click for large image | Figure 1. EKG showing supraventricular tachycardia. |
The patient was given adenosine 6 mg followed by 12 mg dose two times without a change in rhythm. The patient was then given metoprolol 50 mg orally once and metoprolol 5 mg intravenously three times. Telemetry data showed that patient would go in and out of this rhythm even with the administration of the medications. Due to the arrhythmias refractoriness, the patient was started on amiodarone. On imaging, the initial chest X-ray was unremarkable for any acute changes and only showed pulmonary vascular congestion with cardiomegaly (Fig. 2).
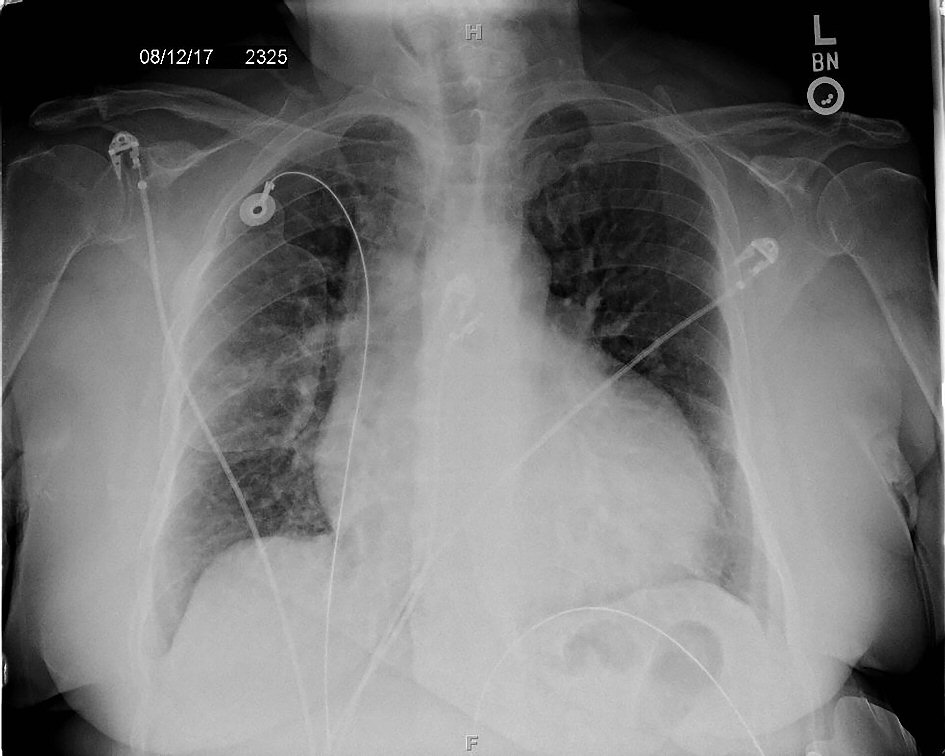 Click for large image | Figure 2. Posterior-anterior chest X-ray showing enlarged cardiac silhouette and vascular congestion. |
After admission to the medical floor, patient blood pressure started to drop to 97/65 mm Hg, and her oxygen requirements went up. She required 2 L of oxygen via nasal cannula to keep oxygen saturation above 94%. Due to concern for pulmonary embolus (PE), a stat computed tomography (CT) angiogram chest was ordered, and it revealed multiple pulmonary emboli bilaterally, a large pericardial effusion, 7mm nodule in the right upper lobe and mediastinal adenopathy (Fig. 3a-c).
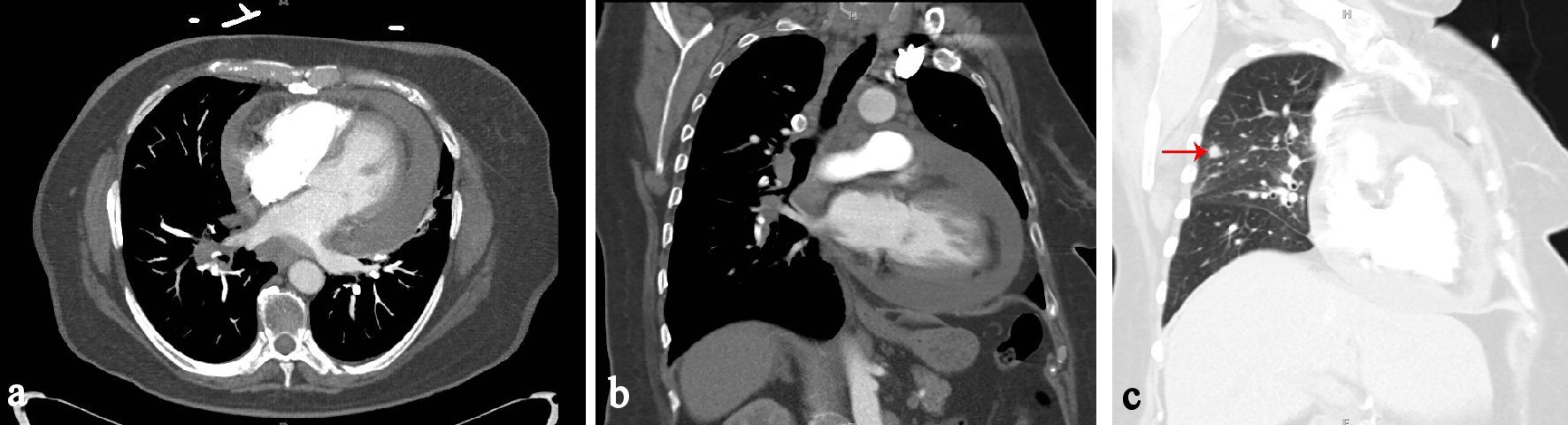 Click for large image | Figure 3. (a) CT chest axial view showing pericardial effusion. (b) CT chest coronal view showing pericardial effusion. (c) CT chest coronal view in lung window showing 7 mm nodule in right upper lobe (red arrow). |
The patient was started on low molecular weight heparin for acute PE. An echocardiogram was ordered which showed ejection fraction of 60-65%, apical diastolic collapse, and large pericardial effusion (Fig. 4). Cardiothoracic surgery was consulted, for urgent subxiphoid pericardial window and placement of a chest tube. About 800 mL of hemorrhagic fluid was removed. Analysis of the fluid revealed malignant cells positive for TTF1 and CK7 tumor markers indicative of primary lung adenocarcinoma. Immunohistochemical staining was performed on the sample, and it was positive for BRAF V600E mutation and PD-L1 expression.
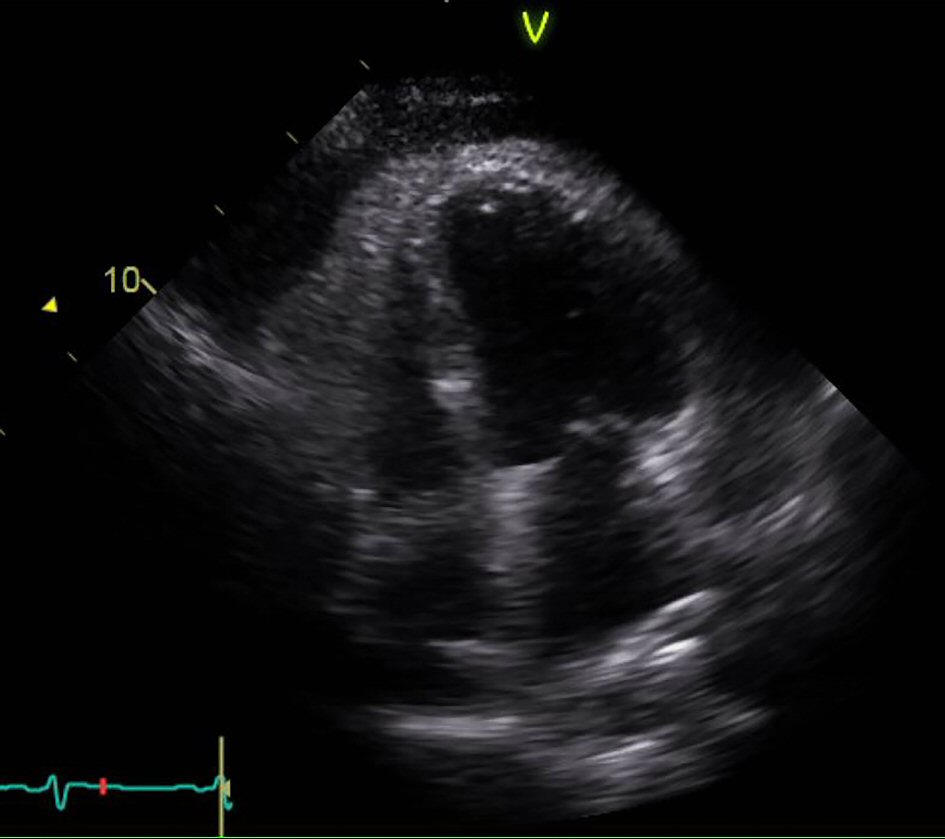 Click for large image | Figure 4. Echocardiogram apical view showing right ventricular collapse in diastole. |
The patient was started systemic therapy with dabrafenib and trametinib. The patient was discharged after clinical improvement and removal of a chest tube. She was given follow-up outpatient with oncologist and cardiologist.
Case 2
A 66-year-old Caucasian woman with no significant past medical history presented to the emergency department with a 2-month history of progressively worsening dyspnea. The dyspnea was worse with exertion or laying down flat. She also complained of a productive cough, fatigue, and weight loss. She denied any sick contacts or recent travel. The patient has not been seen by a primary care physician for many years. She had 25 pack-years smoking history. She also endorsed occasional marijuana and cocaine use. Family history was notable for lung cancer in her mother.
Physical examination in the emergency room revealed a cachectic female in moderate respiratory distress who was alert and oriented. She was afebrile with a pulse of 106 beats per min, blood pressure of 119/76 mm Hg, respiratory rate of 24 breaths per min, and oxygen saturation of 99% on 2 L oxygen via nasal cannula. No jugular venous distension was noted. S1 and S2 heart sounds were distant with no audible murmurs, rubs, or gallops. Lung sounds were severely decreased in the right mid and lower fields but otherwise normal in the other fields. No edema, clubbing, or cyanosis was observed in the extremities.
Laboratory work up revealed leukocytosis (WBC 15.8 K/uL), hyponatremia 128 mmol/L, hypochloremia 92 mmol/L, and lactic acidosis 2.94 mEq/L. B-type natriuretic peptide and troponin were within normal limits. Arterial blood gas on 2 L oxygen revealed a pH of 7.45, a partial carbon dioxide pressure of 30 mm Hg, a partial oxygen pressure of 92 mm Hg, and bicarbonate of 23.1 mEq/L. A 12-lead electrocardiogram showed sinus tachycardia (Fig. 5). Chest radiograph revealed a cavitary lesion with an air-fluid level in the right upper lobe and an enlarged cardiac silhouette (Fig. 6). CT of chest, abdomen, and pelvis with contrast was performed, and it revealed a right upper lobe necrotic mass, pericardial effusion, and right pleural effusion along with right middle lobe medial segment collapse (Fig. 7a-d). Extensive mediastinal adenopathy and bilateral adrenal masses were also noted. Echocardiogram revealed left ventricular ejection fraction of > 70% with right ventricular and right atrial diastolic collapse (Fig.8 a-b).
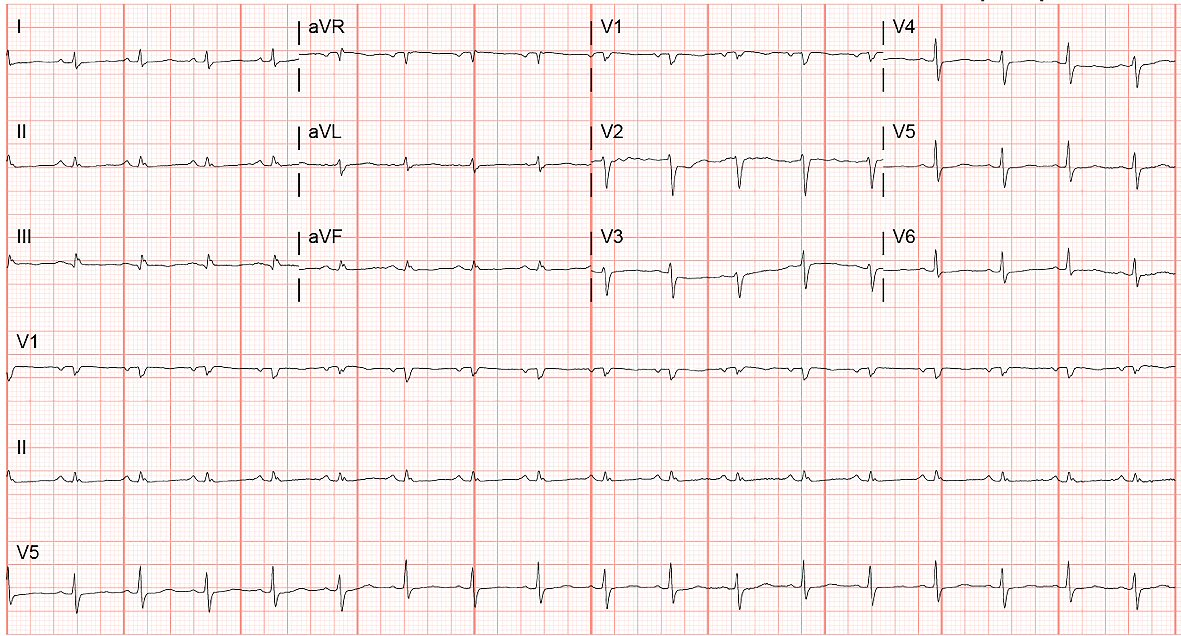 Click for large image | Figure 5. EKG showing sinus tachycardia. |
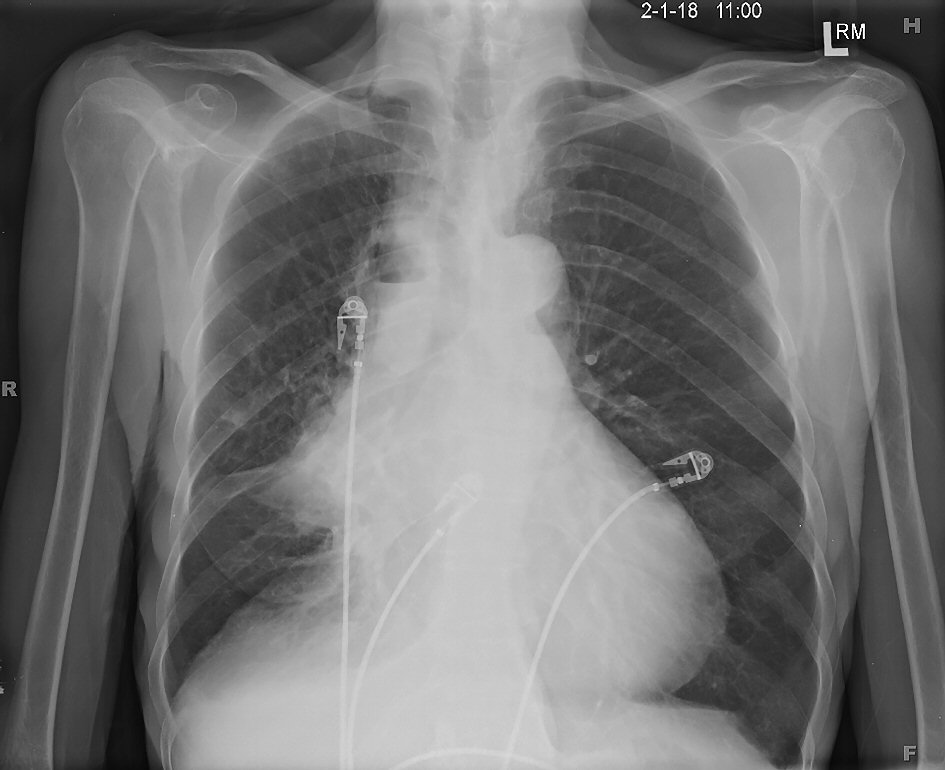 Click for large image | Figure 6. Posterior-anterior chest X-ray showing cavitary lesion with an air-fluid level in the right upper lobe and an enlarged cardiac silhouette. |
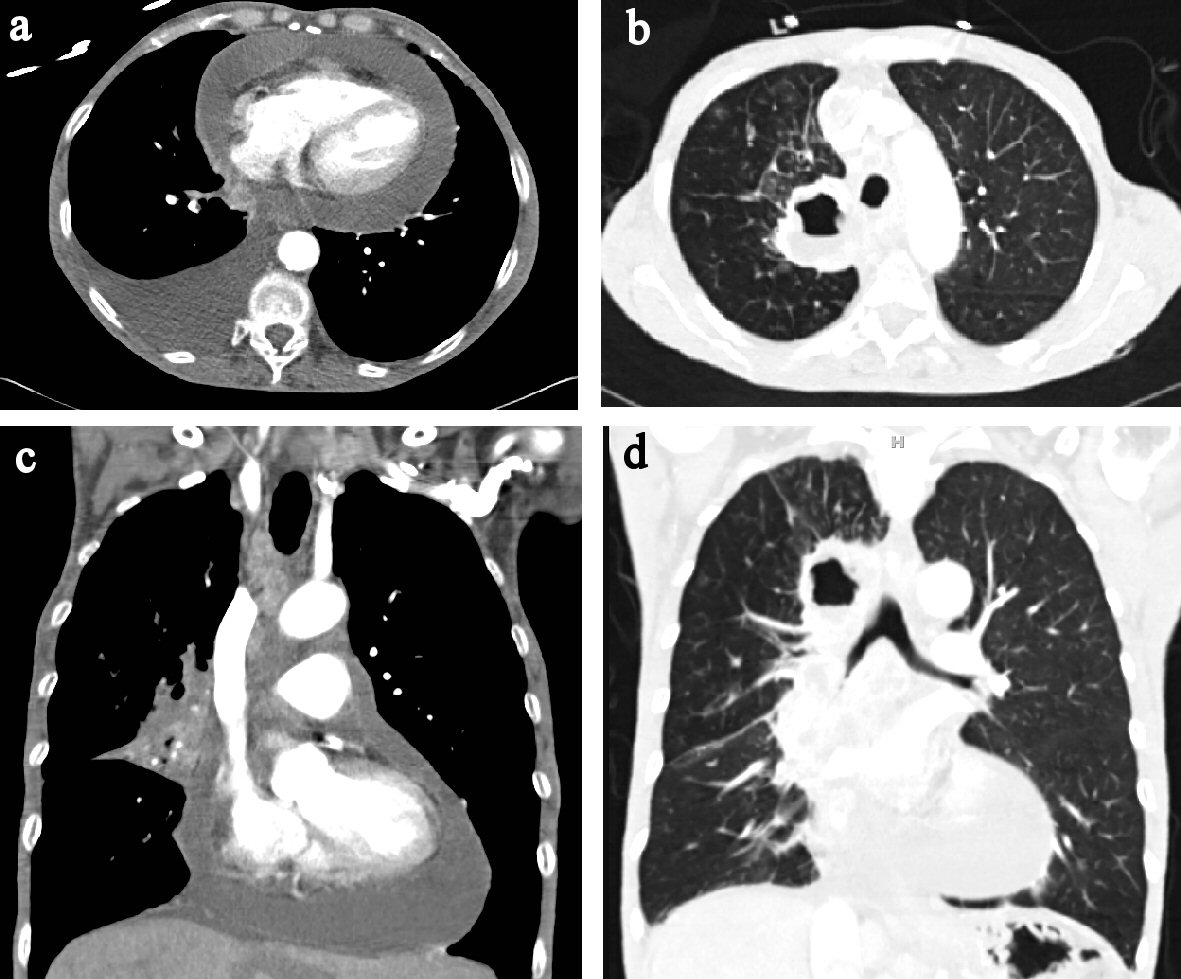 Click for large image | Figure 7. (a) CT chest axial view showing pericardial effusion. (b) CT chest axial view showing in pulmonary window showing air filled cavity with air-fluid level. (c) CT chest coronal view showing pericardial effusion. (d) CT chest coronal view in pulmonary window. |
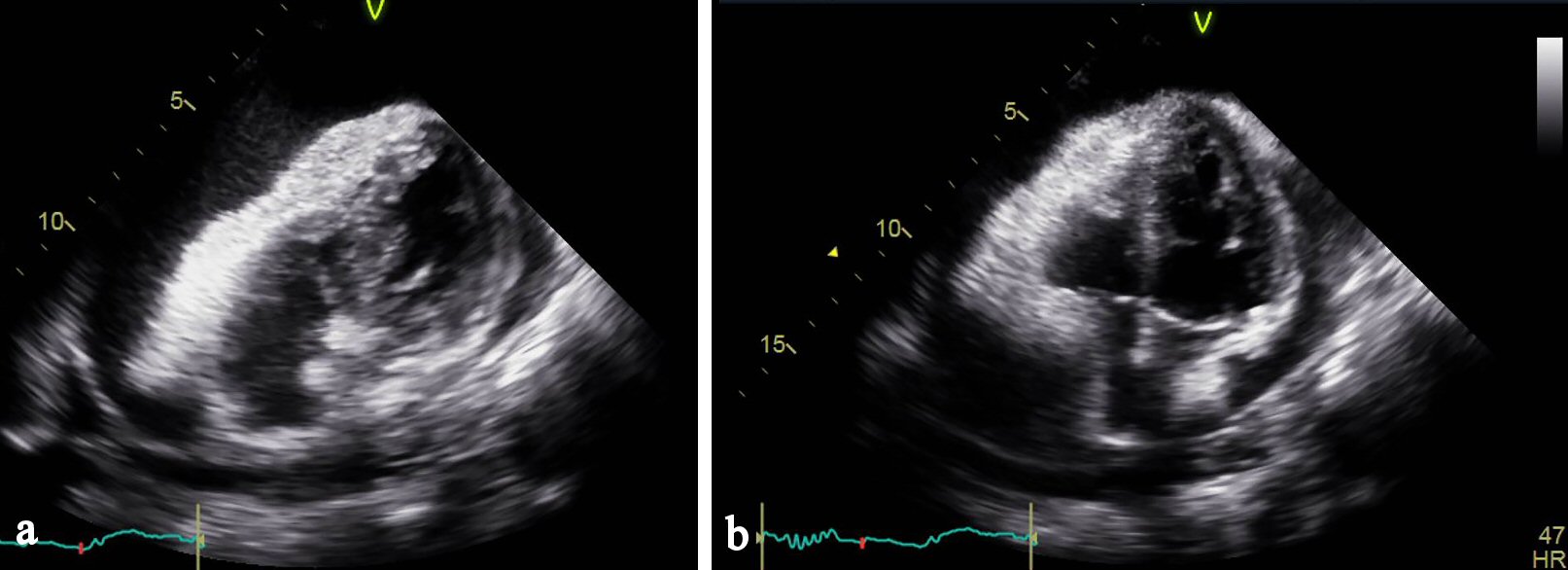 Click for large image | Figure 8. (a) Echocardiogram apical view showing right ventricular collapse in diastole. (b) Echocardiogram apical view showing right atrial collapse in diastole. |
The patient underwent emergent pericardiocentesis and placement of pigtail catheter to evacuate pericardial and pleural fluid, respectively, for therapeutic relief and fluid analysis. Approximately 850 mL of serosanguineous fluid was removed from the pericardial cavity. Later on, a pericardial window was placed by cardiothoracic surgery. Cytology studies indicated primary lung adenocarcinoma with positivity for Napsin-A and TTF-1. Further analysis revealed high expression of PD-L1 and positive BRAF c.1780G>A (p.D594N) mutation. Blood cultures and acid-fast bacilli smear performed on admission were negative for any acute infection. The patient’s shortness of breath improved during the hospital stay, and her electrolyte abnormalities were corrected. She was discharged to a skilled nursing facility once she was hemodynamically stable. She was given outpatient follow-up with an oncologist for treatment of stage IV non-small cell lung cancer.
| Discussion | ▴Top |
Lung cancer is the second most common cancer in both males and females in the United States and the most common cause of cancer-related deaths [1]. Most commonly reported symptom is a cough. Other symptoms include dyspnea hemoptysis and chest pain. Pericardial effusion occurs after cancer has progressed to stage IV. Only about 26% of hemorrhagic pericardial effusions are secondary to malignancy [4], and up to 10% of pericardial effusions are the first manifestation of previously unknown malignancy [5].
Our first patient presented with this new onset of palpitations and chest pain on deep inspiration. Initial imaging like posterior-anterior and lateral view chest X-rays were negative for any underlying mass or hilar adenopathy. EKG did not show any low voltages or electrical alternans. The presence of tachycardia with ventricular arrhythmia and increasing oxygen requirements compelled the primary team to look for pulmonary embolus which led to the discovery of the large pericardial effusion. For the second patient, the concern for malignancy was high from the beginning due to per physical appearance and presence of metastatic lesions on CT scans. Both Patients had BRAF mutations along with PD-L1 expression.
BRAF, a serine kinase, is a part of a family of kinases ARAF, BRAF and CRAF. It is involved in the cell signaling pathway of mitogen-activated protein kinases (MAPK). RAF was first discovered in 1983 [6] and subsequently in 2002 it was found to be present in human tumor cells [7]. This is a part of the mitogen-activated pathway which upon receiving a signal from growth factor activates a cascade which goes from RAS/RAF/MEK/ERK [8]. Activation of this signaling cascade leads to the cell nucleus and triggers replication. Mutations in the kinase domain of exon 15 (c.1799T>A) is labeled as BRAF V600E. Other sites of mutations are on exon 11. This causes uncontrolled activation of the pathway leading to explosive cell growth in tumor cells. Once this pathway was understood, drugs were developed to block it. The first set of drugs which showed improved overall survival and progression-free survival compared to standard of care included vemurafenib [9] and dabrafenib [10] in metastatic melanomas. They were later tested in patients with lung cancer as they can also express this mutation and a survival benefit was found. These drugs do lose their efficacy as tumors develop resistance to these treatments. Postulated mechanisms include mutation of RAS to NRAS which can activate CRAF or activation of MEK through PI3K/AKT/mTOR pathway in melanomas [11]. Combination of MEK inhibitors with BRAF inhibitors can have shown better outcomes than with BRAF inhibitors alone. Other targeted drugs are also being developed to block the alternate pathways. Second generation BRAF inhibitors are also being developed which can be more selective and have a better side effect profile.
The classic V600E mutation is seen in about 55% of the BRAF mutated NSCLC in the Catalog of Somatic Mutations in Cancer (COSMIC database) which was present in our first patients’ NSCLC. The second patient had a mutation that is not commonly seen. The COSMIC database query reveals (March 17, 2018) that p.D594N (missense mutation) has only be isolated in three samples of lung tissue. No case reports could be found on this variant of BRAF mutation, and this makes it challenging to come up with a treatment strategy with the current standard of care approved for V600E mutation only. Literature review reveals that dabrafenib has activity against codon 600 mutation but activity against non-codon 600 mutation is unclear [12]. Zheng et al studied 1,027 patients with BRAF mutations to check for kinase activity and found that 37% of the BRAF mutations were non-V600E. About 15% of their study group had a BRAF kinase-impaired mutation. The most common kinase-impaired mutations in their study were p.D594G and p.D594N, and they showed an association with concomitant KRAS or NRAS mutations. Lung cancers patients showed significantly higher incidences of kinase-impaired or kinase-unknown mutants [13]. They also noticed that kinase-impaired mutants were more common in lung cancer subset than colorectal or melanoma [13]. Marchetti et al studied 1,046 NSCLC patients for BRAF mutations and concluded that V600E mutation was more prevalent in females, it was independent of the smoking status of the patient, and it was frequently associated with aggressive tumor cells with micropapillary features. While on the other hand non-V600E mutations were only seen in smokers [14]. Currently, there are studies underway to look more broadly for drugs that can be effective against BRAF mutations in general and not just the V600E mutation. A few mentions would include LXH254 and LTT462 (currently going phase 1 clinical trial for treating advanced or metastatic KRAS or BRAF) (clinicalTrials.gov Identifier: NCT02974725) and AUY922 in EGFR, HER2, or BRAF-mutated; ALK, ROS1, or RET-rearranged NSCLC (clinicalTrials.gov Identifier: NCT01922583).
Another interesting fact is that both patients had BRAF mutation and massive malignant pericardial effusion on presentation. Mechanisms underlying malignant pericardial effusions are not completely understood. It can occur due to blockage of the lymphatics or angiogenesis related to cancer cells leading to disorganized leaky vessels [15]. Some of these patients can have elevated levels of VEGF in these cavities promoting effusions. In some patients, VEGF inhibitors have been used to control these effusions [16]. There have been isolated reports of malignant effusions with BRAF mutations including Langerhans cell histiocytosis [17], Erdheim-Chester disease [18]. Gautschi et al [19] presented a case of BRAF V600E adenocarcinoma with pleural and pericardial effusions. None of these case reports discussed the possibility of the association of the BRAF with the propensity of the tumor cells to cause malignant effusions. Recently, Αgalioti T et al hypothesized that KRAS could be involved in the production of malignant pleural effusion and they were able to induce it in mice. Furthermore, they showed that it was mediated by KRAS dependent CCL2 signaling which recruited myeloid cells in pleural space [20]. More studies need to be conducted with a bigger sample size to check for correlation of pericardial effusion to BRAF mutation.
Our first patient final diagnosis was NSCLC with BRAF V600E mutation and PD-L1 expression and was given standard of care dabrafenib and trametinib. The second patient also had NSCLC but with a variant of BRAF, a non-V600E mutation (p.D594N) and PD-L1 expression. It was initially planned that she should get PD-L1 targeted therapy as she did not have the V600E mutation but later, based on tumor board recommendations, it was decided that she should be treated with dabrafenib and trametinib (not current standard of care). The second patient is supposed to follow up with the oncologist in an outpatient setting for initiation of the treatment. Currently, studies are being done to assess using BRAF and MEK inhibitors along with PD-L1 ligands for patients with cancer positive for both and some studies have shown promising results [21].
Conclusions
BRAF remains a fascinating target for treatment of cancers with targeted agents. Combination of these agents that block multiple steps in the MAPK pathway has shown better outcomes and seems to be the way treatments are going to be designed in the future. Further work needs to be done to understand the variants of BRAF mutation. Role of BRAF in producing massive effusions is unclear at the moment and warrants a bigger study pool.
| References | ▴Top |
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
doi pubmed - Cardarella S, Ogino A, Nishino M, Butaney M, Shen J, Lydon C, Yeap BY, et al. Clinical, pathologic, and biologic features associated with BRAF mutations in non-small cell lung cancer. Clin Cancer Res. 2013;19(16):4532-4540.
doi pubmed - Paik PK, Arcila ME, Fara M, Sima CS, Miller VA, Kris MG, Ladanyi M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol. 2011;29(15):2046-2051.
doi pubmed - Atar S, Chiu J, Forrester JS, Siegel RJ. Bloody pericardial effusion in patients with cardiac tamponade: is the cause cancerous, tuberculous, or iatrogenic in the 1990s? Chest. 1999;116(6):1564-1569.
doi pubmed - Ben-Horin S, Bank I, Guetta V, Livneh A. Large symptomatic pericardial effusion as the presentation of unrecognized cancer: a study in 173 consecutive patients undergoing pericardiocentesis. Medicine (Baltimore). 2006;85(1):49-53.
doi pubmed - Rapp UR, Goldsborough MD, Mark GE, Bonner TI, Groffen J, Reynolds FH, Jr., Stephenson JR. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983;80(14):4218-4222.
doi pubmed - Macaluso M, Russo G, Cinti C, Bazan V, Gebbia N, Russo A. Ras family genes: an interesting link between cell cycle and cancer. J Cell Physiol. 2002;192(2):125-130.
doi pubmed - Ritt DA, Abreu-Blanco MT, Bindu L, Durrant DE, Zhou M, Specht SI, Stephen AG, et al. Inhibition of Ras/Raf/MEK/ERK pathway signaling by a stress-induced phospho-regulatory circuit. Mol Cell. 2016;64(5):875-887.
doi pubmed - Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507-2516.
doi pubmed - Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380(9839):358-365.
doi - Posch C, Moslehi H, Feeney L, Green GA, Ebaee A, Feichtenschlager V, Chong K, et al. Combined targeting of MEK and PI3K/mTOR effector pathways is necessary to effectively inhibit NRAS mutant melanoma in vitro and in vivo. Proc Natl Acad Sci U S A. 2013;110(10):4015-4020.
doi pubmed - Carter J, Tseng LH, Zheng G, Dudley J, Illei P, Gocke CD, Eshleman JR, et al. Non-p.V600E BRAF mutations are common using a more sensitive and broad detection tool. Am J Clin Pathol. 2015;144(4):620-628.
doi pubmed - Zheng G, Tseng LH, Chen G, Haley L, Illei P, Gocke CD, Eshleman JR, et al. Clinical detection and categorization of uncommon and concomitant mutations involving BRAF. BMC Cancer. 2015;15:779.
doi pubmed - Marchetti A, Felicioni L, Malatesta S, Grazia Sciarrotta M, Guetti L, Chella A, Viola P, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J Clin Oncol. 2011;29(26):3574-3579.
doi pubmed - Chen D, Zhang Y, Shi F, Zhu H, Li M, Luo J, Chen K, et al. Intrapericardial bevacizumab safely and effectively treats malignant pericardial effusion in advanced cancer patients. Oncotarget. 2016;7(32):52436-52441.
doi pubmed - Chen D, Zhang Y, Shi F, Li M, Zhu H, Kong L, Yu J. Sustained response of malignant pericardial effusion to intrapericardial bevacizumab in an advanced lung cancer patient: a case report and literature review. Onco Targets Ther. 2015;8:2767-2770.
pubmed - Gholami N. Pericardial Effusion in Langerhans Cell Histiocytosis: A Case Report. Iran Red Crescent Med J. 2016;18(6):e25604.
doi pubmed - Tran KD, Ayala-Haedo J, Fischer O, Dubovy SR, Wester ST. Sustained efficacy of targeted therapy with vemurafenib in BRAF(V600e) - Mutated Erdheim - Chester Disease. Clin Surg. 2016;1:1230.
- Gautschi O, Pauli C, Strobel K, Hirschmann A, Printzen G, Aebi S, Diebold J. A patient with BRAF V600E lung adenocarcinoma responding to vemurafenib. J Thorac Oncol. 2012;7(10):e23-24.
doi pubmed - Agalioti T, Giannou AD, Krontira AC, Kanellakis NI, Kati D, Vreka M, Pepe M, et al. Mutant KRAS promotes malignant pleural effusion formation. Nat Commun. 2017;8:15205.
doi pubmed - Homet Moreno B, Mok S, Comin-Anduix B, Hu-Lieskovan S, Ribas A. Combined treatment with dabrafenib and trametinib with immune-stimulating antibodies for BRAF mutant melanoma. Oncoimmunology. 2016;5(7):e1052212.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


