| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Case Report
Volume 11, Number 1, February 2020, pages 37-40
Breast Metastasis From Castrate-Resistant Prostatic Adenocarcinoma Mimicking as a Second Primary: A Case Report
Valerie Limaa, b, Felycette Gay Martinez-Lapusa, Kenny Jun Demegilloa
aDepartment of Internal Medicine, Davao Doctors Hospital, Davao City, Philippines
bCorresponding Author: Valerie Lima, Department of Internal Medicine, Davao Doctors Hospital, Davao City, Philippines
Manuscript submitted January 6, 2020, accepted January 18, 2020
Short title: Breast Metastasis From Prostate Carcinoma
doi: https://doi.org/10.14740/wjon1258
| Abstract | ▴Top |
The occurrence of breast metastasis from prostate carcinoma and primary breast carcinoma in men may cause a diagnostic dilemma. This report aims to present a patient diagnosed with metastatic castrate-resistant prostatic adenocarcinoma who developed breast metastasis mimicking as a second primary. A 57-year-old male patient presented with a breast mass while undergoing hormonal therapy for prostatic adenocarcinoma. The initial histopathologic diagnosis of the breast specimen was an infiltrating ductal breast carcinoma. The breast mass enlarged after four cycles of docetaxel. Immunostaining with prostate-specific antigen (PSA) and prostate-specific acid phosphatase (PSAP) was done on the breast specimen revealing a negative PSA and a moderately staining PSAP. These stains confirmed the diagnosis of a breast metastasis from prostatic adenocarcinoma. The differentiation between primary breast carcinoma and breast metastasis from prostate carcinoma is crucial. Hence, immunohistochemistry staining should be utilized for diagnosis and appropriate management.
Keywords: Breast metastasis; Prostate cancer; Castrate-resistant prostate cancer; Prostate-specific antigen; Prostate-specific acid phosphatase
| Introduction | ▴Top |
Male breast cancer and breast metastasis from prostate cancer are uncommon [1, 2]. Despite having opposite risk factors, studies have shown a direct relationship of prostate cancer with breast cancer due to the similarities in hormonal and genetic mechanisms [3]. Moreover, there is a significant increased risk of breast cancer in patients with prostate cancer who received estrogen therapy [4]. Likewise, hormonal treatment for prostate cancer has also been found to increase blood supply to the breast, thus resulting in gynecomastia and promoting metastasis. Hence, in prostate cancer patients who present with a breast lump, a primary breast carcinoma should be differentiated from breast metastasis from prostate carcinoma to provide the most appropriate treatment.
| Case Report | ▴Top |
A 57-year-old male patient was diagnosed with prostatic carcinoma with bone metastasis in 2016. His prostatic specific antigen (PSA) level was 20 ng/mL, Gleason grade 9 (5+4). His bone scan showed multiple bone metastasis, hence he was treated with leuprolin acetate and bicalutamide. While on leuprolin acetate, a palpable 3 × 3 cm mass on the subareolar area of the right breast was noted with a benign sonomammographic finding. An elevated PSA of 66 ng/mL and a testosterone level of 0.025 ng/mL were also noted. He was considered to have castration-resistant disease, thus systemic therapy was initiated with abiraterone acetate.
However, there was an increase in the size of the breast mass (Fig. 1). Core needle biopsy of the mass showed a poorly differentiated triple (estrogen receptor (ER), progesterone receptor (PR) and HER2 neu (HER2neu) receptor) negative infiltrating ductal carcinoma (Fig. 2a, b). Metastatic workup with a computed tomography (CT) scan showed an enhancing ovoid right breast mass measuring 8.6 × 7.2 × 6.2 cm with suggestive sites of multiple metastases in the lungs, liver, pancreas, urinary bladder wall and lymph nodes. He was managed with palliative docetaxel chemotherapy for a double primary case of castration-resistant prostatic carcinoma and infiltrating ductal carcinoma.
 Click for large image | Figure 1. A non-movable, non-tender breast mass with nipple retraction on the right anterior chest measuring 8 × 7 cm. |
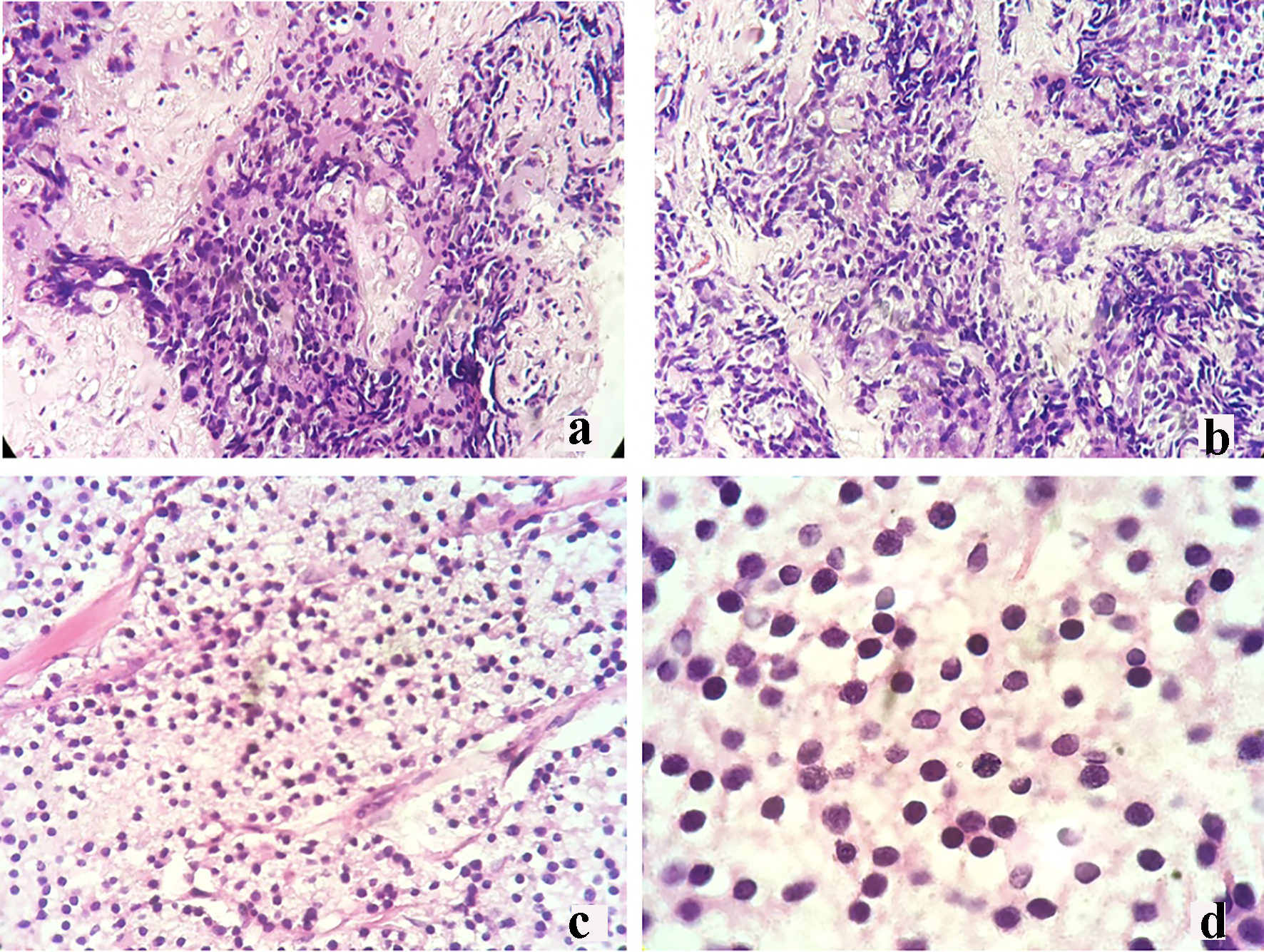 Click for large image | Figure 2. Core needle biopsy of the right breast mass revealing infiltrating ductal carcinoma (a: × 20; b: × 40). Mastectomy breast mass biopsy revealing poorly differentiated malignant round cell neoplasm (c: × 20; d: × 40). |
After two cycles of docetaxel, there was noticeable decrease in the swelling of the breast. PSA was down to 45 ng/mL. However, after an additional two more cycles of docetaxel, there was an increase in the size of the breast mass with darkening of the underlying skin. Repeat CT scan showed an increase in the size of the breast mass with stable findings of the rest of the metastatic lesions. Bone scan showed widespread metastatic lesions. PSA was 68.99 ng/mL. Palliative modified radical mastectomy was performed.
The breast specimen biopsy was reported as a poorly differentiated malignant round cell neoplasm with considerations of metastatic prostatic adenocarcinoma versus invasive ductal carcinoma (Fig. 2c, d). The specimen was sent for immunohistochemistry (IHC) staining for PSA and prostate-specific acid phosphatase (PSAP). Results revealed a negative PSA (Fig. 3b) and a moderately stained PSAP report (Fig. 3d). Final pathologic report of the breast specimen concluded a breast metastasis from prostatic adenocarcinoma.
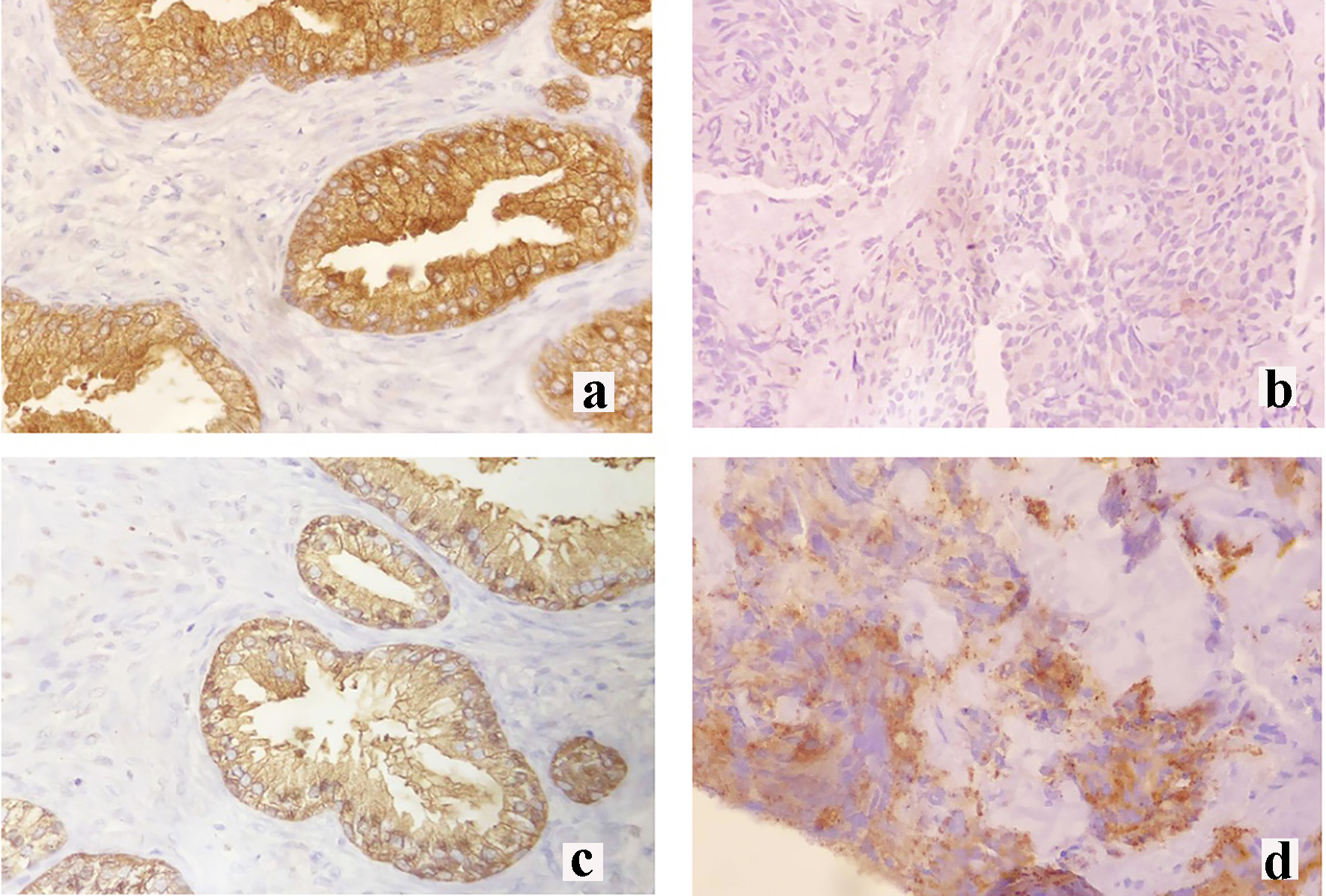 Click for large image | Figure 3. Immunohistochemical staining of the right breast mass. (a) Prostate-specific antigen (PSA) control staining, (b) negative PSA staining, (c) prostate-specific acid phosphatase (PSAP) control staining and (d) moderate PSAP staining. |
| Discussion | ▴Top |
Hormonal therapy for prostate carcinoma has shown to increase the blood supply to the breast predisposing to metastasis by producing an imbalance of estrogen levels through the initial increased secretion of luteinizing hormones, thereby increasing testosterone and estrogen levels [1, 5]. Likewise, the alteration of estrogen to androgen ratios has also been found to increase the risk of primary male breast cancer [3]. Our patient received both anti-androgen (bicalutamide) and hormonal (leuprolin acetate) therapy which increased the likelihood of either breast metastasis from the prostate or a primary male breast cancer. The differentiation of these two disease entities is essential since hormonal treatment of prostate cancer is contraindicated in breast cancer [5].
A family history of breast cancer places a person at risk for developing breast cancer [6]. Hence, genetic factors play a major role in the development of breast cancer with the most frequently found mutation on BRCA gene 1 and 2 [7]. A study conducted in a tertiary hospital in the Philippines estimated a 5.1% prevalence rate of BRCA mutations among Filipino women [8]. However, there are limited data on the prevalence of BRCA mutations among Filipino men. Therefore, BRCA gene screening in Filipino men with low risk for BRCA mutations is not a cost-effective tool to aid in the diagnosis of breast cancer in underprivileged settings. Our patient had low risk factors for BRCA gene mutations, hence was not tested.
In contrast, prostate carcinoma most commonly metastasizes to the bone, liver, lungs and lymph nodes [1]. Breast metastasis from prostate carcinoma is rare [1, 2, 5]. Non-mammary malignancies that metastasize to the breast are also clinically observed in only 2% of all breast tumors [1, 2]. In its rarity, only one case of prostatic carcinoma metastasizing to the breast has been reported in Hong Kong and China [5].
High-grade neoplasms, such as poorly differentiated prostatic adenocarcinomas, may show similar histologic features to primary breast carcinoma [9]. Our patient presented with an initial histopathologic finding of infiltrating ductal carcinoma on core needle biopsy that did not respond to docetaxel. This posed a diagnostic dilemma. IHC staining is an informative and economical tool to obtain a final diagnosis in low resource settings. The essential IHC stains for breast carcinoma are for ER, PR and HER2neu. Male breast cancer patients present with 85-90% and 92-96% positivity for ER and PR, respectively [3]. Furthermore, HER2neu determination provides an important guide in the management of breast cancer [3]. Therefore, a high index of suspicion for breast metastasis is warranted in a patient diagnosed with prostatic adenocarcinoma and an ER, PR, HER2neu negative IHC stain despite a histopathologically confirmed breast carcinoma.
The determination of prostate carcinoma metastasizing to the breast involves the use of PSA and PSAP IHC staining. PSA shows a detection rate of 81% but there is loss of its expression in high grade or metastatic prostatic carcinoma [10]. Hence, for PSA negative cases, PSAP staining is recommended since a positive PSAP stain will suggest a prostatic primary tumor [5]. PSAP staining detects 10% of PSA negative cases and its combination increases the detection rate for primary prostatic carcinoma to 60% [10]. Our patient presented with a poorly differentiated malignant neoplasm that was positive for PSAP stain on repeat breast biopsy and this supplemented the diagnosis of a higher-grade prostatic tumor. However, these stains are not available in the Philippines. Nevertheless, it is important to test for PSA and PSAP to establish a diagnosis of breast metastasis from prostatic adenocarcinoma.
In resource-limited settings, IHC staining is a practical tool to utilize in order to clinch the correct diagnosis. When presented with a diagnostic dilemma of a breast mass in a patient with prostatic adenocarcinoma, it is prudent to test for PSA and PSAP IHC staining to differentiate a primary breast carcinoma from breast metastasis.
Acknowledgments
We thank Mr. William Lima for the assistance in transporting the breast specimen for immunohistochemistry staining and Dr. Teodora Amor Evora, Dr. Jeannine Erika Tarongoy and Dr. Stephen Hermosa for comments that greatly improved the manuscript.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
All authors have participated sufficiently in the intellectual concent, conception and design of this work or the analysis and interpretation of the information available, as well as the writing of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Mandaliya H, Sung J, Hill J, Samali R, George M. Prostate cancer: cases of rare presentation and rare metastasis. Case Rep Oncol. 2015;8(3):526-529.
doi pubmed - Njiaju UO, Truica CI. Metastatic prostatic adenocarcinoma mimicking inflammatory breast carcinoma: a case report. Clin Breast Cancer. 2010;10(1):E3-5.
doi pubmed - Lee UJ, Jones JS. Incidence of prostate cancer in male breast cancer patients: a risk factor for prostate cancer screening. Prostate Cancer Prostatic Dis. 2009;12(1):52-56.
doi pubmed - Thellenberg C, Malmer B, Tavelin B, Gronberg H. Second primary cancers in men with prostate cancer: an increased risk of male breast cancer. J Urol. 2003;169(4):1345-1348.
doi pubmed - Cheng CW, Chan LW, Ng CF, Chan CK, Tse MK, Lai MM. Breast metastasis from prostate cancer and interpretation of immunoreactivity to prostate-specific antigen. Int J Urol. 2006;13(4):463-465.
doi pubmed - Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, Bartlett JM, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28(12):2114-2122.
doi pubmed - Gargiulo P, Pensabene M, Milano M, Arpino G, Giuliano M, Forestieri V, Condello C, et al. Long-term survival and BRCA status in male breast cancer: a retrospective single-center analysis. BMC Cancer. 2016;16:375.
doi pubmed - Laudico A, Redaniel MT, Mirasol-Lumague MR, Mapua CA, Uy GB, Pukkala E, Pisani P. Epidemiology and clinicopathology of breast cancer in metro Manila and Rizal Province, Philippines. Asian Pac J Cancer Prev. 2009;10(1):167-172.
- Bombonati A, Lerwill M. Metastasis to and from the breast. Surg Pathol Clin. 2012;5(3):719-747.
doi pubmed - Kristiansen I, Stephan C, Jung K, et al. Sensitivity of HOXB13 as a diagnostic immunohistochemical marker of prostatic origin in prostate cancer metastases: comparison to PSA, prostein, androgen receptor, ERG, NKX3. 1, PSAP, and PSMA. Int J Mol Sci. 2017;18(6):1151.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


