| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 3, June 2022, pages 117-125
Association Between CD204-Expressed Tumor-Associated Macrophages and MGMT-Promoter Methylation in the Microenvironment of Grade 4 Astrocytomas
Maher Kurdia, b, l , Yousef Katibc, Eyad Faizod, Basem Bahakeeme, Alaa Alkhotanif, Shadi Alkhayyatg, Ahmed A. Najjarh, Riffat Mehboobi, Taher F. Halawaj, Bassam M.J. Addask, Koloud Albrikyb, Sahar Hakamyb
aDepartment of Pathology, Faculty of Medicine in Rabigh, King Abdulaziz University, Jeddah, Saudi Arabia
bNeuromuscular Unit, Roya Specialized Medical Laboratories, Jeddah, Saudi Arabia
cDepartment of Radiology, Faculty of Medicine, Taibah University, Almadinah Almunawwarah, Saudi Arabia
dDivision of Neurosurgery, Department of Surgery, Faculty of Medicine, Tabuk University, Tabuk, Saudi Arabia
eDepartment of Internal Medicine, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
fDepartment of Pathology, Faculty of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
gDepartment of Internal Medicine, Faculty of Medicine, King Abdulaziz University and King Abdulaziz University Hospital, Jeddah, Saudi Arabia
hCollege of Medicine, Taibah University, Almadinah Almunawwarah, Saudi Arabia
iLahore Medical Research Center, LLP, Lahore, Pakistan
jDepartment of Pediatrics, Faculty of Medicine, King Abdulaziz University, Rabigh, Saudi Arabia
kDepartment of Surgery, Faculty of Medicine, King Abdulaziz University, Jeddah, Saudi Arabia
lCorresponding Author: Maher Kurdi, Department of Pathology, Faculty of Medicine in Rabigh, King Abdulaziz University, Jeddah, Saudi Arabia
Manuscript submitted March 15, 2022, accepted April 30, 2022, published online May 10, 2022
Short title: CD204 and MGMT in Grade 4 Astrocytoma
doi: https://doi.org/10.14740/wjon1473
| Abstract | ▴Top |
Background: Tumor-associated macrophages (TAMs) are principal immune cells in glioma microenvironment which support tumor growth and proliferation. Our aim in this study was to assess the relationship between CD204-expressed TAMs and O6-methylguanine-DNA methyltransferase (MGMT)-promoter methylation in World Health Organization (WHO) grade 4 astrocytomas, and its impact on patient’s clinical outcome.
Methods: The expression of CD204+ TAMs was quantitively assessed on 45 samples of WHO grade 4 astrocytomas using immunohistochemistry. MGMT-promoter methylation was tested by methylation techniques. The relationship between TAMs, MGMT-promoter methylation, and recurrence-free interval (RFI) was statistically analyzed.
Results: There were 10 cases (22.2%) with isocitrate dehydrogenase (IDH)-mutant grade 4 astrocytoma and 35 cases (77.8%) with IDH-wildtype glioblastoma. MGMT-promotor was methylated in 18 cases (40%), unmethylated in 15 cases (33%), and the remaining 12 cases showed no MGMT status because of nucleic acid degradations. The expression of CD204+ TAMs was high in 32 cases (71.7%) and low in 13 cases (28.8%). The relationship between IDH1 mutation and CD204+ TAM expression was insignificant (P = 0.93). However, the significant difference was found between MGMT methylation and CD204+ TAMs expression (P = 0.01), in which CD204+ TAMs were diffusely expressed in MGMT-methylated cases. There was no significant difference in RFI between CD204+ TAMs expression, MGMT-promoter methylation and treatment modalities.
Conclusions: Grade 4 astrocytomas with diffusely expressed CD204+ TAMs are usually associated with MGMT-promoter methylation. Although this association is unclear, CD204+ TAMs may neutralize the effect of MGMT-DNA protein to loss its function, which contributes to tumor progression. This relationship had no significant impact on the patient’s clinical outcome after different treatment modalities.
Keywords: Astrocytoma; Tumor-associated macrophages; CD204; MGMT methylation
| Introduction | ▴Top |
Despite the palliative treatment of World Health Organization (WHO) grade 4 astrocytomas, it remains the deadliest cancer in the body with a median survival time of less than 18 months [1]. Because of this unfavorable prognosis, the need to explore new therapeutic approaches becomes crucial. According to the 2021 WHO classification of central nervous system (CNS) tumors and European Association of Neuro-oncology (EANO) guidelines, WHO grade 4 astrocytoma is classified into isocitrate dehydrogenase (IDH)-mutant and IDH-wildtype; however, IDH-wildtype astrocytoma is isolated for glioblastoma [2, 3].
The microenvironment of astrocytoma consists of different cellular lineages including tumor cells, immune cells and non-immune cells that infiltrate as tumor niches [4]. Tumor-associated macrophages (TAMs) are considered as essential immune cells in this microenvirment, which assist and control tumor cells’ proliferation and modulation [1, 4]. Nevertheless, the recruitment and the reprogramming between tumor cells and TAMs are still not well explained. Two types of TAMs were identified: 1) M1-polarized TAMs “anti-tumor” and 2) M2-polarized TAMs “anti-inflammatory”. M2-polarized TAMs have an immune modulatory effect that stimulates tumor cells’ proliferation and leads to formation of metastatic niches [5, 6]. When TAMs encircle tumor cells, they inhibit T-cell cytotoxic function, and consequently the tumor cells escape the immune system. This may cause TAMs aggregating in the microenvironment with no T-cell development [7]. Preclinical studies have suggested that these regulatory T cells (Tregs) are essential for promoting tumor immunosuppression parallelly with TAMs, by which they are strongly associated with vascular endothelial growth factor (VEGF) production [8, 9]. Tregs depletion significantly downregulates the expression of immune suppressive molecules, such as B7-H1 on TAM, and reduces tumor growth [8, 9].
Some clinically reported data indicated that large numbers of TAM, such as CD163, CD204, and CD206, were associated with poor outcome in different types of cancers such as skin melanoma and carcinoma of breast, urinary bladder, ovary and lung [10]. Theses receptors are considered as crosstalk between cancer cells and TAMs for circulating tumor cells in the blood [11]. One of the recently explored markers of M2-polarized TAMs in the WHO grade 4 astrocytoma microenvironment is CD204 [12-14]. CD204 is involved in the process of tumor phagocytosis and the production of reactive oxygen species [12, 14]. Its expression in tumor microenvironment was found to be involved in the process of glioblastoma immunomodulatory system [15, 16].
Other than CD204-linked TAMs, several molecular biomarkers have been identified as prognosticators for patients with high-grade astrocytoma. Amongst these, O6-methylguanine-DNA methyltransferase (MGMT)-promoter methylation is a biomarker for good clinical outcome [17, 18]. MGMT is a DNA repair protein that reverses alkylation at the O6 position of guanine, thereby neutralizing the cytotoxic effects of temozolomide (TMZ) alkylating agent. A lack of MGMT repair contributes to the progression of cancers through the accumulation of DNA mutations [19, 20]. Because grade 4 astrocytomas started to develop resistant to TMZ treatment in MGMT-methylated tumors, the need to explore new therapeutic targets to increase sensitivity of tumor cells to TMZ becomes essential [21].
Although the relationship between the immune check point registry and IDH1 mutation has been explored, the association between immune check point receptors, receptor blockers or current immunotherapies has never been investigated in correlation with MGMT-promoter methylation [22]. In our study, we investigated for the first time the relationship between the CD204-expressed TAMs and MGMT gene promoter methylation in the microenvironment of WHO grade 4 astrocytomas. We also explored the prognostic impact of these biomarkers on patient’s clinical outcome after different treatment modalities.
| Materials and Methods | ▴Top |
This study has been approved by Biomedical Ethics Committee at King Abdulaziz University (HA-02-J-008) to authorize using patient samples in research, which complies with the guidelines of the “System of ethics of research” prepared by the King Abdulaziz City for Science and Technology and approved by Royal Decree No. M/59 on August 24, 2010.
Patients sampling
Our study included 45 patients histologically diagnosed as WHO grade 4 astrocytoma after radical surgical resection, in the period between 2015 and 2018 (Table 1). Patients’ information were collected from the hospital archives which included patient’s age during diagnosis, gender, tumor location, and the results of IDH1R132H mutation and MGMT methylation. Recurrence-free interval (RFI) was estimated from the beginning of post-surgical therapy to the possible first day of recurrence. The entire cases included in this study were associated with tissue necrosis, microvascular proliferation, ATRX loss and intact 1p19q (Table 2). The histopathological diagnoses were re-evaluated based on 2021 WHO classification of CNS tumors [2, 3]. Standard radiotherapy was given as a total dose of 60 Gy, and the post-surgical chemotherapy regime followed the Stupp’s protocol [23]. TMZ was given at 150 - 200 mg/m2 for 5 days for 6 - 12 cycles. All patients involved in this project died.
 Click to view | Table 1. Demographic Data of 45 Cases of WHO Grade 4 Astrocytoma Enrolled in the Study |
 Click to view | Table 2. Patients’ Data Enrolled in This Study |
Tissue samples
Formalin-fixed paraffin-embedded (FFPE) tissue blocks and slides of 45 patients, diagnosed with WHO grade 4 astrocytomas, were collected. Sections stained with H&E, ATRX and IDH1R132H were examined by consultant pathologist (MK) to reassess the histological diagnosis based on the 2021 WHO classification of CNS tumors (Table 2). Additional one slide of each 45 blocks was stained for anti-CD204 antibody.
Immunohistochemistry (IHC) protocol
Anti-CD204 antibody (Rabbit polyclonal, Abcam, Cat# 217843,) directed against human antibody, was used in the IHC assay of the entire 45 FFPE sections. The procedure was done by using a Ventana detection Kit (Ultra-View) that was processed in GX automated immunostainer from Ventana (Tuscon, AZ, USA). The protocol comprised of deparaffinization with EZ Prep at 75 °C, heat pre-treatment in a cellular medium for 60 min followed by an optimum incubation for 20 min at 37 °C. The antibody was adjusted using a dilution of 1:300. The slides were counterstained with hematoxylin II and bluing reagent for 30 min. The positive control was histological sections containing macrophages.
IHC assessment
Quantitative assessment of CD204 expression in WHO grade 4 astrocytomas
Anti-CD204 stains TAMs in the microenvironment of astrocytoma. Each histological section was screened at low power field (× 10) using light microscopy (Digital Tele-Path Technology using Grundium Ocus × 40, Finland) and focal non-necrotic area was elected to manually count the cells at high magnification (× 40). Cells expressing anti-CD204 were considered as CD204-positive (CD204+ TAMs). The total cells were defined as cells with both stained TAMs and non-stained TAMs. The cell that did not express CD204 included neoplastic astrocytic cells, lymphocytes, and other types of neurological cells. The labelling index (LI) was assessed through the following equation:
Two staining patterns were defined: high expression and low expression, based on the density of the staining. The assessment of CD204 expression matches to what has been described by Kurdi et al protocol [15]. The expression was considered high when CD204+ TAMs were expressed in more than 40% of the cellular microenvironment. The expression was considered low when CD204+ TAMs were expressed in less than 40% of the cellular microenvironment (Fig. 1a-d).
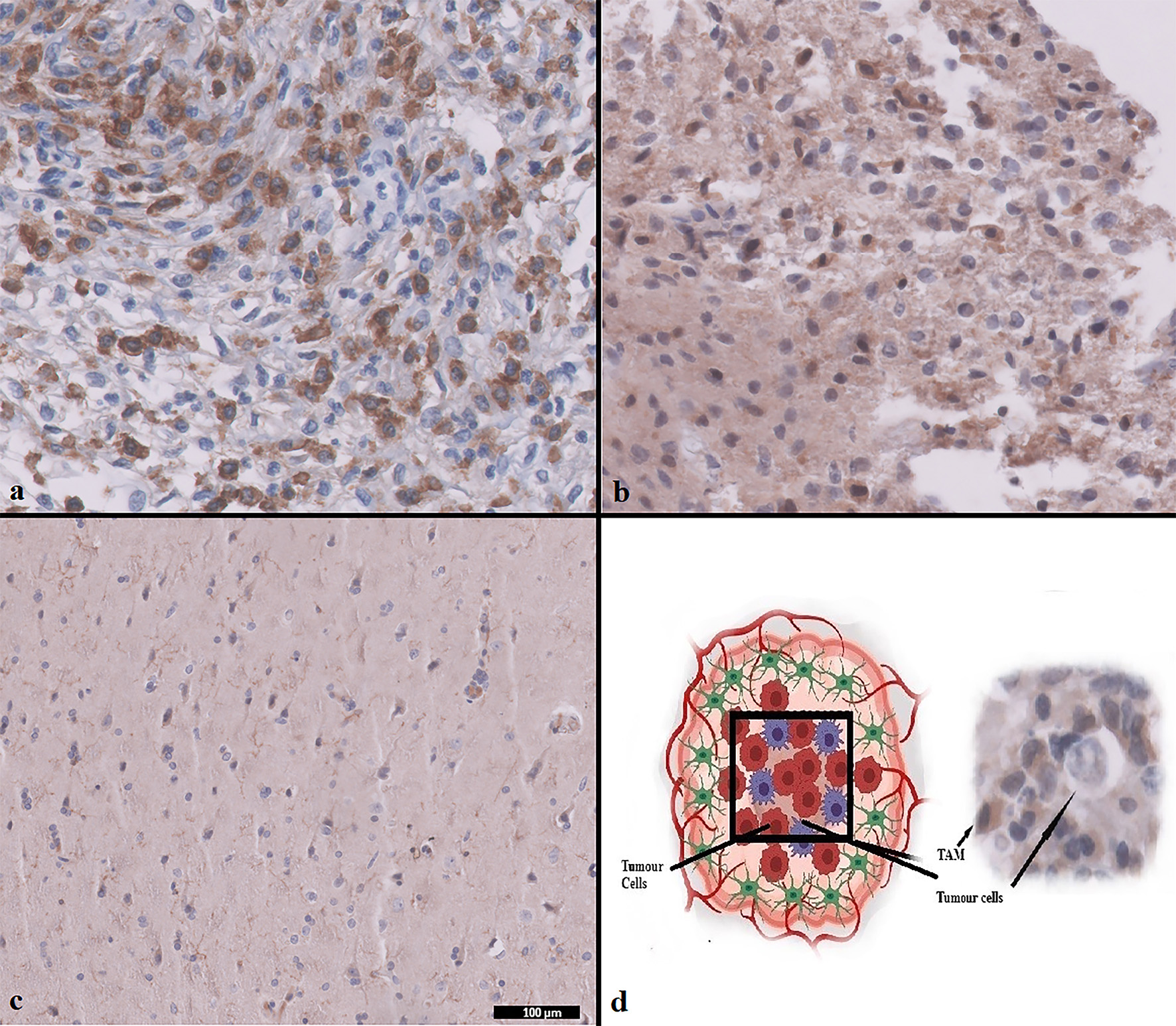 Click for large image | Figure 1. CD204+ TAMs expression in WHO grade 4 astrocytoma using IHC. (a) CD204+ TAMs high expression. (b) CD204+ TAMs low expression. (c) Normal brain control. (d) Diagram shows the relationship between TAMs and tumor cells. Magnification (× 100 µm). TAMs: tumor-associated macrophages; WHO: World Health Organization; IHC: immunohistochemistry. |
Assessment of IDH1R132H expression in WHO grade 4 astrocytomas
Sections in which > 10% of neoplastic glial cells positively stained with IDH1R132H were defined as IDH1-mutant [24]. Tumors with negative IDH1 staining were not DNA-sequenced due to the redundant amount of the tissue.
Assessment of MGMT-promoter methylation
MGMT gene promoter methylation was assessed by using one of the two different methods: methylation specific-polymerase chain reaction (MS-PCR) and pyrosequencing using Qiagen. The techniques were chosen based on the institution’s protocol. Both techniques started with DNA extraction using FFPE kit. DNA concentration and purity were assessed using a NanoDrop spectrophotometer.
For first method (MS-PCR), DNA concentration was standardized to 60 ng and transformed using EpiTect bisulfate Kit from Qiagen. The forward and reverse primers were targeted to the methylated and unmethylated exon of the human MGMT gene which matches the protocol described by Esteller et al [21] (Table 3). Thermal cycling started at 95 °C for 2 min followed by 40 - 45 cycles of half minute and half minute at 52 °C and 72 °C. The PCR products were visualized using gel electrophoresis. Samples with both methylated and non-methylated products were recorded as MGMT-methylation positive.
 Click to view | Table 3. Primers for MS-PCR Used for the Assessment of MGMT-Promoter Methylation |
For second method (pyrosequencing), MGMT Pyro Kit from Qiagen was utilized to evaluate the methylation at four CpG sites on human MGMT gene. After DNA extraction and optimization, the Therascreen MGMT-PyroKit and PyroMark sequencer were both employed to evaluate the MGMT methylation. The control was built-in as a positive control for sequencing reaction. This procedure matches the protocol described by Pangopoulos et al [25].
Statistical analysis
To explore the relationship between CD204-expressed TAMs, MGMT-promoter methylation, and IDH1R132H mutation, the analyses were processed using a Fisher’s exact test. Kaplan-Meier curve (KMC) was used to compare the distribution of RFI among WHO grade 4 astrocytoma cases with different CD204+ TAMs expressions. A P-value of < 0.05 was considered statistically significant. All statistical analyses in this study were performed using IBM SPSS1 ver. 24 (SPSS Inc., Chicago, IL, USA).
| Results | ▴Top |
Our study included 45 patients diagnosed as WHO grade 4 astrocytoma based on 2021 WHO classification of CNS tumors. Around 22% (n = 10) of cases were less than 55 years and 78% (n = 35) of the cases were more than 55 years (Tables 1 and 2). Ten samples showed IDH1 mutation and referred as IDH-mutant astrocytomas, while 35 samples showed no IDH1 mutation and referred as glioblastoma. MGMT was methylated in 18 cases (40%) and 15 cases (33%) had unmethylated MGMT-promoter. For the remaining 12 samples, MGMT methylation status could not be determined due to nuclei acid degradation. The expression of CD204+ TAMs was evaluated in the whole 45 patients (Tables 1and 2). Around 57% (n = 26) of patients received combined radiotherapy and chemotherapy, 37% patients (n = 17) received radiotherapy, and two patients were reluctant to receive any adjuvant because of their comorbidities. Around 57% (n = 15) patients received TMZ alone, and the remaining 42% (n = 11) patients received TMZ and other adjuvant chemotherapies such as bevacizumab, irinotecan, lomustine, or etoposide. The median RFI was 1 year and 2 months (Table 2).
Relationship between CD204+ TAMs and MGMT-promoter methylation
The relationship between MGMT-promoter methylation status and CD204+ TAMs expression status was statistically significant (P = 0.01) (Table 4). Approximately, 71.6% (n = 15) of WHO grade 4 astrocytoma cases with high CD204+ TAMs expression were associated with MGMT-promoter methylation, while 28.5% (n = 6) of the cases were found in astrocytoma with unmethylated MGMT-promoter. Cases with low expression of CD204+ TAM (75%, n = 9) was associated with unmethylated MGMT-promoter. Hence, CD204+ TAMs increase when MGMT-promoter is methylated.
 Click to view | Table 4. Relationship Between CD204+ TAMs and MGMT-Promoter Methylation |
Relationship between CD204+ TAMs and IDH mutation
The relationship between IDH mutation and CD204+ TAMs expression was statistically insignificant (P = 0.93) (Table 5). Nevertheless, CD204+ TAMs were overexpressed in IDH-wildtype cases (78.2%) more than IDH1-mutant cases (21.8%).
 Click to view | Table 5. Relationship Between CD204+ TAMs and IDH Mutation |
Relationship between CD204+ TAMs, MGMT-promoter methylation, with the type of treatment modalities and RFI
There was no significant difference statistically found in the recurrence interval between CD204+ TAMs (high and low expression) and MGMT-promoter methylation (P = 0.95 and P = 0.09) (Fig. 2a, b) (Table 6). Furthermore, no statistically significant relationship was also identified in the RFI between CD204+ TAMs expression (mainly high expression), MGMT-promoter methylation and the treatment modalities or specific chemotherapeutic agents respectively (P = 0.06 and P = 0.9) (Fig. 2c, d).
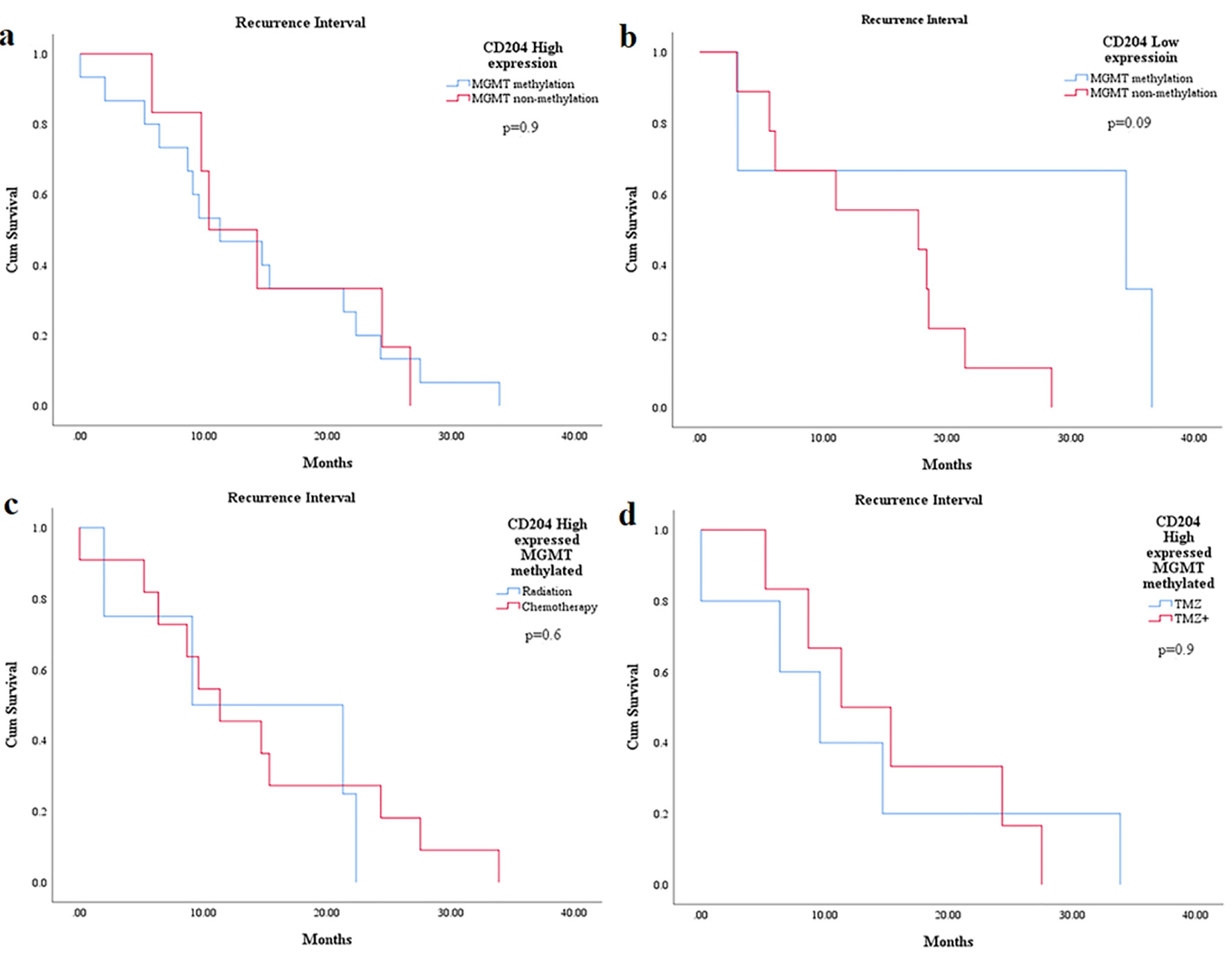 Click for large image | Figure 2. The relationship between CD204+ TAMs expression and MGMT-promoter methylation with RFI in WHO grade 4 astrocytoma patients. KM graphs show no statistically significant difference in RFI between CD204+ TAMs expression and MGMT-promoter methylation using different treatment modalities. TAMs: tumor-associated macrophages; MGMT: O6-methylguanine-DNA methyltransferase; RFI: recurrence-free interval; WHO: World Health Organization; KM: Kaplan-Meier. |
 Click to view | Table 6. Relationship Between CD204+ TAMs, MGMT methylation and RFI |
| Discussion | ▴Top |
Several molecular biomarkers have been identified as prognosticators in high-grade astrocytomas. Amongst these, genetic biomarkers such as IDH mutation and MGMT gene promoter methylation were considered essential targets for patients’ treatment and prognosis [17, 18]. In the tumor microenvironment, TAMs and tumor-infiltrating lymphocytes (TILs) are considered as central immune-modulatory cells that are distributed as tumor niches where treatment-resistant is localized. M2-polarized TAMs, one of the subclasses of TAMs, behave as an immunomodulator to stimulate tumor growth or suppress tumoricidal effect of TILs [5]. One of the recently explored TAMs receptor is CD204, a macrophage scavenger receptor 1 (MSR1) [12, 14]. Kurdi et al found that CD204 is highly expressed in glioblastoma patients and associated with a reduced expression of CD4+ TILs in the tumor microenvironment [15]. They also found that insignificant relationship between IDH mutation and CD204+ TAMs expression [15]. Nonetheless, the association of CD204+ TAMs and MGMT-promoter methylation was significantly explored in our results. We found that around 71% of WHO grade 4 astrocytomas with elevated expression of CD204+ TAMs were associated with MGMT-promoter methylation. Consequently, CD204+ TAMs in WHO grade 4 astrocytomas become dense when MGMT-promoter is methylated. This relationship has never been broadly explicated in the literatures. The only explanation here is that CD204+ TAMs may neutralize the effect of MGMT-DNA protein to loss its function which contributes into the progression of cancers. Nevertheless, we revealed that the relationship between CD204+ TAMs and MGMT-promoter methylation had no significant impact on tumor recurrence (Fig. 2a, b). In excess, this association also had no significant impact on RFI amid all types of treatment modalities (Fig. 2c, d). This irrelevance might be related to the limited number of samples in our study.
Because TAMs encircle cancer cells and inhibits T-cell cytotoxic function, tumor cells will escape the immune system with less tumor cells killed by TILs. Indeed, the immune check point targets, anti-CD204 receptor, will be an effective immunomodulator that can prevent TAMs role, evolute TILs and increase sensitization of glioma cells to chemotherapeutic agents. Cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4), programmed cell death-1 receptor (PD-1), and T-cell inhibitory receptor (TIM-3) were all found to perform suppressor effect by interacting with their receptors on tumor cells or TAMs [22, 26]. The influence of these check point modulators has never been studied in high-grade astrocytomas with MGMT-promoter methylation.
One limitation that should be admitted in our research is that the whole number of analyzed samples is relatively low. Despite this limitation, this is the first study that correlates MGMT- promoter methylation and CD204 biomarkers in WHO grade 4 astrocytomas.
Conclusions
Our study emphasized that the expression of CD204+ TAMs in WHO grade 4 astrocytomas increases when MGMT-promoter is methylated. CD204+ TAMs may also neutralize the effect of MGMT-DNA protein to loss its function, which contributes to tumor progression. This mechanism targets a key approach to suppress TAMs to increase tumor cells sensitivity to chemotherapeutic agents.
Acknowledgments
Special thanks to the laboratory team at center of excellence in genomic medicine research at King Fahad Medical Research Center in King Abdulaziz University.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained.
Author Contributions
MK: idea, writing, study design and data, and histological analysis; YK: data provider, writing, and analysis; EF: data entry, tissue collection, and writing; BB: writing and editing; AK: study design, writing, and editing; SK: writing and editing; AN: writing and editing; RM: statistical analysis and histological analysis; TH: writing and editing; BA: writing and editing; KB: writing and editing; SH: interpreted data and histological revision. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon request.
| References | ▴Top |
- Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462-477.
doi pubmed - Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231-1251.
doi pubmed - Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170-186.
doi pubmed - Chen Z, Hambardzumyan D. Immune microenvironment in glioblastoma subtypes. Front Immunol. 2018;9:1004.
doi pubmed - Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14-20.
doi pubmed - Ye XZ, Xu SL, Xin YH, Yu SC, Ping YF, Chen L, Xiao HL, et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGFbeta1 signaling pathway. J Immunol. 2012;189(1):444-453.
doi pubmed - Corrales L, Matson V, Flood B, Spranger S, Gajewski TF. Innate immune signaling and regulation in cancer immunotherapy. Cell Res. 2017;27(1):96-108.
doi pubmed - Fecci PE, Sweeney AE, Grossi PM, Nair SK, Learn CA, Mitchell DA, Cui X, et al. Systemic anti-CD25 monoclonal antibody administration safely enhances immunity in murine glioma without eliminating regulatory T cells. Clin Cancer Res. 2006;12(14 Pt 1):4294-4305.
doi pubmed - Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, Kanojia D, et al. CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016;76(19):5671-5682.
doi pubmed - Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14(12):717-734.
doi pubmed - Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer. 2019;18(1):64.
doi pubmed - Rohrer L, Freeman M, Kodama T, Penman M, Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990;343(6258):570-572.
doi pubmed - Ohtaki Y, Ishii G, Nagai K, Ashimine S, Kuwata T, Hishida T, Nishimura M, et al. Stromal macrophage expressing CD204 is associated with tumor aggressiveness in lung adenocarcinoma. J Thorac Oncol. 2010;5(10):1507-1515.
doi pubmed - Ichimura T, Morikawa T, Kawai T, Nakagawa T, Matsushita H, Kakimi K, Kume H, et al. Prognostic significance of CD204-positive macrophages in upper urinary tract cancer. Ann Surg Oncol. 2014;21(6):2105-2112.
doi pubmed - Kurdi M, Alghamdi B, Butt NS, Baeesa S. The relationship between CD204 (M2)-polarized tumour-associated macrophages (TAMs), tumour-infiltrating lymphocytes (TILs), and microglial activation in glioblastoma microenvironment: a novel immune checkpoint receptor target. Discov Oncol. 2021;12(1):28.
doi pubmed - Yuan Y, Zhao Q, Zhao S, Zhang P, Zhao H, Li Z, Du Y, et al. Characterization of transcriptome profile and clinical features of a novel immunotherapy target CD204 in diffuse glioma. Cancer Med. 2019;8(8):3811-3821.
doi pubmed - Binabaj MM, Bahrami A, ShahidSales S, Joodi M, Joudi Mashhad M, Hassanian SM, Anvari K, et al. The prognostic value of MGMT promoter methylation in glioblastoma: A meta-analysis of clinical trials. J Cell Physiol. 2018;233(1):378-386.
doi pubmed - Thon N, Eigenbrod S, Grasbon-Frodl EM, Lutz J, Kreth S, Popperl G, Belka C, et al. Predominant influence of MGMT methylation in non-resectable glioblastoma after radiotherapy plus temozolomide. J Neurol Neurosurg Psychiatry. 2011;82(4):441-446.
doi pubmed - Kurdi M, Butt NS, Baeesa S, Alghamdi B, Maghrabi Y, Bardeesi A, Saeedi R, et al. Prognostic value of TP53 expression and MGMT methylation in glioblastoma patients treated with temozolomide combined with other chemotherapies. J Neurooncol. 2021;152(3):541-549.
doi pubmed - Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol. 2002;20(9):2388-2399.
doi pubmed - Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59(4):793-797.
- July J, Patricia D, Gunawan PY, Setiajaya H, Ginting TE, Putra TP, Wuisan Z, et al. Clinicopathological associations and prognostic values of IDH1 gene mutation, MGMT gene promoter methylation, and PD-L1 expressions in high-grade glioma treated with standard treatment. Pan Afr Med J. 2020;36:309.
doi pubmed - Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996.
doi pubmed - Takano S, Tian W, Matsuda M, Yamamoto T, Ishikawa E, Kaneko MK, Yamazaki K, et al. Detection of IDH1 mutation in human gliomas: comparison of immunohistochemistry and sequencing. Brain Tumor Pathol. 2011;28(2):115-123.
doi pubmed - Panagopoulos I, Gorunova L, Leske H, Niehusmann P, Johannessen LE, Staurseth J, Oino N, et al. Pyrosequencing analysis of MGMT promoter methylation in meningioma. Cancer Genomics Proteomics. 2018;15(5):379-385.
doi pubmed - Li G, Wang Z, Zhang C, Liu X, Cai J, Wang Z, Hu H, et al. Molecular and clinical characterization of TIM-3 in glioma through 1,024 samples. Oncoimmunology. 2017;6(8):e1328339.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


