| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Case Report
Volume 14, Number 6, December 2023, pages 570-574
Catch the Calcium: T-Cell Histiocyte-Rich B-Cell Lymphoma Presenting as Hypercalcemia
Richard K. Okekea, g, Gabriella A. Harmonb, Ijeoma G. Okekea, Jake W. Schulerc, Sahana J. Sangappad, Jonathan S. Harmone, Evgeniya Angelovaf, Xiu Sunf, Angelo A. Chinnicia
aDepartment of Medicine, Jersey Shore University Medical Center, Hackensack Meridian Health, Neptune, NJ, USA
bDepartment of Hematology/Oncology, Jersey Shore University Medical Center, Hackensack Meridian Health, Neptune, NJ, USA
cSaint George’s University School of Medicine, Grenada, West Indies
dHackensack Meridian School of Medicine, Nutley, NJ, USA
eDepartment of Medicine, Einstein Medical Center Montgomery, East Norriton, PA, USA
fDepartment of Pathology, Jersey Shore University Medical Center, Hackensack Meridian Health, Neptune, NJ, USA
gCorresponding Author: Richard K. Okeke, Department of Medicine, Jersey Shore University Medical Center, Hackensack Meridian Health, Neptune, NJ 07753, USA
Manuscript submitted June 27, 2023, accepted September 14, 2023, published online October 21, 2023
Short title: THRLBCL Presenting as Hypercalcemia
doi: https://doi.org/10.14740/wjon1610
| Abstract | ▴Top |
T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) is an extremely rare and aggressive subtype of diffuse large B-cell lymphoma (DLBCL) that typically presents in middle-aged patients and carries a poor prognosis. Hypercalcemia presenting as the initial manifestation of the disease is rare, with only one other case reported in the literature. We report a case of a 90-year-old male who presented with progressive lethargy and unintentional weight loss. Initial workup showed elevated serum calcium of 14.6 mg/dL, corrected for albumin, and creatinine of 1.51 mg/dL. He had a suppressed iPTH of 6.3 pg/mL and normal PTHrP (13 pg/mL). Computed tomography (CT) scan of the abdomen and pelvis was performed to rule out underlying malignancy, which showed splenomegaly and enlarged retrocrural and porta hepatis lymph nodes. Bone marrow biopsy was performed to evaluate for hematological malignancy, which revealed findings diagnostic of THRLBCL. While rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) is one of the mainstay therapies for DLBCL and has been shown to have comparable outcomes in THRLBCL, there are documented concerns with its toxicity profile limiting the ability of older patients (60 years and older) to complete therapy. Our patient was treated with R-mini-CHOP, which is much better tolerated in this patient demographic. R-mini-CHOP features decreased doses of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) with the conventional dose of rituximab. This case discusses a rare subtype of non-Hodgkin lymphoma presenting with a unique manifestation of hypercalcemia. We highlight the importance of thorough investigation for causes of hypercalcemia as well as the efficacy and tolerability of R-mini-CHOP in this elderly patient demographic.
Keywords: Diffuse large B-cell lymphoma; Hypercalcemia; T-cell/histiocyte-rich large B-cell lymphoma; R-mini-CHOP; Malignancy
| Introduction | ▴Top |
Diffuse large B-cell lymphoma (DLBCL) is the most common type of non-Hodgkin lymphoma (NHL) in Western countries, accounting for about 30% of all NHL cases worldwide [1]. DLBCL is a heterogeneous group of neoplasms composed of transformed large B-cells with vesicular nuclei, prominent nucleoli, basophilic cytoplasm, and usually a high proliferation rate [2]. T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) is a rare and aggressive histological subtype of DLBCL that accounts for < 5% of cases [3]. The World Health Organization (WHO) defines THRLBCL as < 10% malignant B cells in the setting of abundant reactive T cells and histiocytes [1]. THRLBCL most often presents in middle-aged patients, with a median age of 57 years, and is predominant in males. Most cases of THRLBCL are diagnosed at advanced stages (stages III/IV) and present with high rates of extranodal malignancy, frequently involving the bone marrow, liver, and spleen [4, 5]. Historically, hypercalcemia has a low incidence in NHL. However, DLBCL is the histological subtype most commonly associated with hypercalcemia. The prevalence of hypercalcemia at diagnosis of DLBCL has been reported to be 23%. Hypercalcemia at diagnosis of DLBCL has also been reported to be associated with poor prognostic factors such as elevated lactate dehydrogenase (LDH), low levels of albumin, frequent extranodal involvement, and advanced stage [6, 7]. Hypercalcemia is an infrequent presenting symptom of THRLBCL, with only one case reported in the literature involving a 69-year-old male [8]. This paper describes the second case of THRLBCL manifesting as severe hypercalcemia involving an elderly 90-year-old patient.
| Case Report | ▴Top |
A 90-year-old male with a past medical history of paroxysmal atrial fibrillation, heart failure with preserved ejection fraction (HFpEF), premature ventricular contractions (PVCs), permanent pacemaker (PPM) placement for non-transmission syndrome of fascicular parasystole, sick sinus syndrome, chronic bradycardia presented with a 3-week history of lethargy. He presented 3 weeks earlier to our hospital with substernal chest pain and shortness of breath and was admitted with non-transmission PVC syndrome. He subsequently had a PPM implanted placed by the cardiologist. After discharge, he became progressively lethargic and his activities of daily living markedly decreased, prompting a visit to his primary care physician (PCP). During the encounter, his calcium level was noted to be elevated at 15 mg/dL. His PCP advised him to return to our hospital for further management. In the emergency room, he looked fatigued, pale, and dry. In the past 5 months, he reported constipation, decreased appetite, polyuria, and an unintentional 40-pound weight loss. He denied abdominal pain, bone pain, unusual mood changes, diarrhea, nausea, vomiting, use of any calcium supplements, new medications, and a history of cancer. Labs were significant for pancytopenia, which is chronic for the patient. The repeat calcium adjusted for albumin was 14.6 mg/dL. His creatinine level was elevated at 1.51 mg/dL, a 50% increase from his baseline. He was given a total of 2 L of normal saline and placed on maintenance fluid at 50 mL per hour. A one-time dose of pamidronate was given for symptomatic hypercalcemia. His calcium level normalized after 3 days. A further workup was performed to determine the etiology of his hypercalcemia. Intact parathyroid hormone (iPTH) was suppressed at < 6.3, ionized calcium elevated at 1.99 mmol/L, and phosphorus was within normal limits. A serum protein electrophoresis (SPEP) test to rule out multiple myeloma was negative. Parathyroid hormone-related peptide (PTHrP) was normal at 13 pg/mL. Workup for anemia was consistent with anemia of chronic disease. Computed tomography (CT) scan of the chest revealed calcified mediastinal lymph nodes and noncalcified 15 × 11 mm pre-tracheal lymph node. CT scan of the abdomen and pelvis revealed splenomegaly measuring 16.4 cm, enlarged left retrocrural lymph node measuring 2.5 × 1.3 cm, enlarged porta hepatis lymph node measuring 3.8 × 2.5 cm, and air within the urinary bladder and its wall. JAK2 V617 mutation was negative. Fluorescence in situ hybridization (FISH) for myelodysplastic syndrome and BCR/ABL fusion performed on peripheral blood was negative. A leukemia disorder profile done by flow cytometry analysis of peripheral blood revealed no immunophenotypic evidence of lymphoproliferative disorder or increased blasts. A bone marrow core biopsy at the left iliac crest was performed to evaluate for an underlying hematologic malignancy. Flow cytometry analysis from the bone marrow aspirate did not yield any diagnostic immunophenotypic abnormalities. However, the bone marrow biopsy (Fig. 1) revealed large interstitial and paratrabecular lymphoid aggregates composed predominantly of small lymphocytes and scattered large, atypical lymphocytes with irregular multilobed nuclei. The large, atypical cells by immunohistochemistry (IHC) were positive for paired box 5 (PAX5, strong), octamer transcription factor-2 (OCT-2), CD79a, CD20, while negative for CD3, CD20, CD30, CD68, and Epstein-Barr virus-encoded small RNA (EBER). The small lymphocytes in the lymphoid aggregates were predominantly CD3-positive T-cells admixed with CD68-positive histiocytes and occasional small B-cells (CD20+/PAX5+/OCT-2+). Cytogenetics showed normal male karyotype. The bone marrow core biopsy and aspirate smears were reviewed by the pathology team in the Department of Pathology and Laboratory Medicine at Jersey Shore University Medical Center. These findings confirmed the diagnosis of THRLBCL. The patient was discharged to home 6 days later when the hypercalcemia resolved, and his lethargy improved.
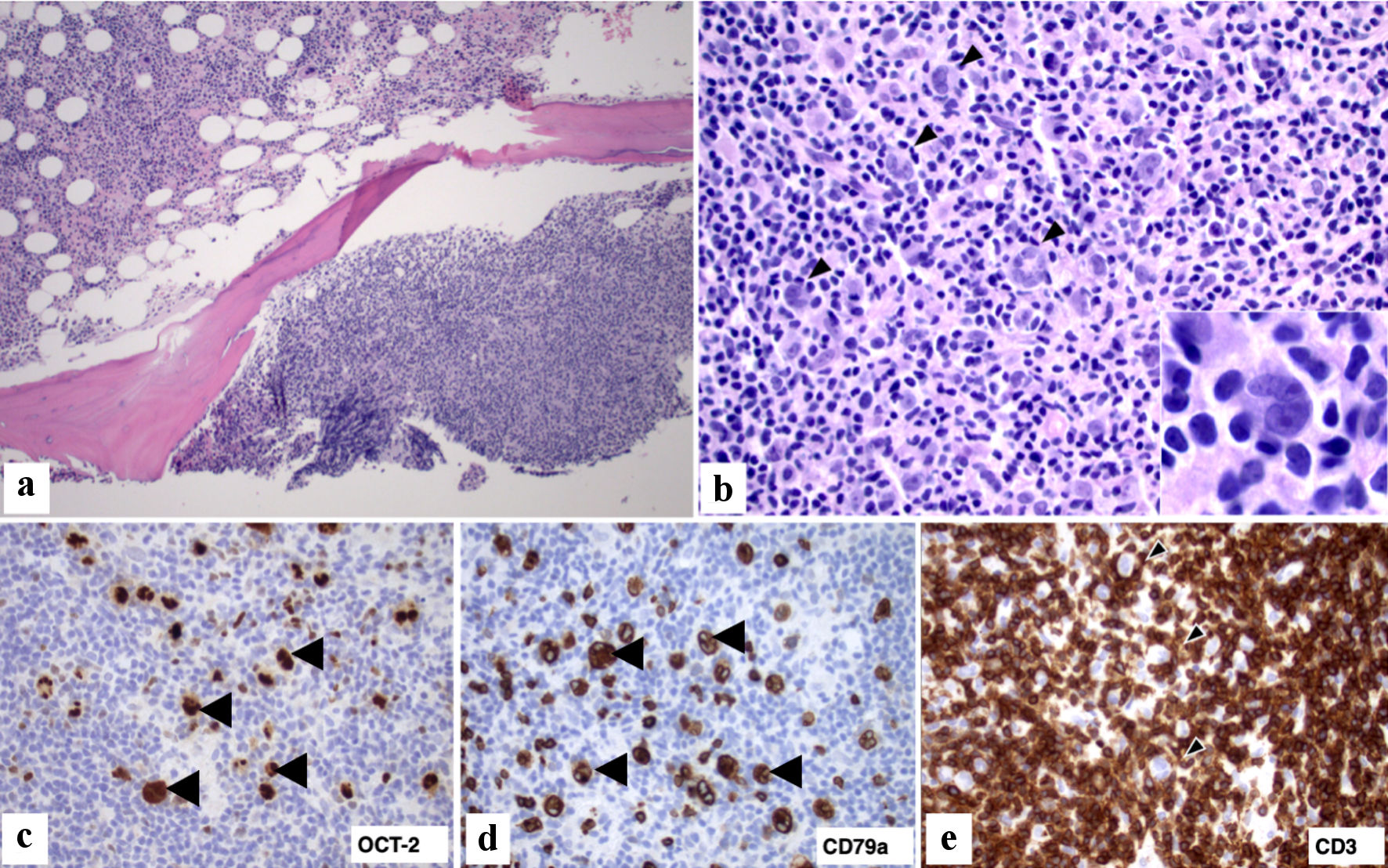 Click for large image | Figure 1. Bone marrow biopsy with involvement by large B-cell lymphoma. (a) Marrow core biopsy with nodular involvement by lymphoma (right lower corner) and hypercellular for age marrow with trilineage hematopoiesis (left upper corner) (H&E stain, × 100). (b). Lymphoma infiltrate composed predominantly of small lymphoid cells and occasional histiocytes intermixed with scattered large (arrowhead) lymphoid cells (H&E, × 400); Inset: The large cells have irregular nuclei with prominent nucleoli and are surrounded by small lymphocytes (H&E, × 1,000). (c, d) Large lymphoma cells express B-cell markers OCT-2 and CD79a (arrowhead, × 400). (e) Immunohistochemical stain for CD3 highlights numerous small lymphocytes in the lymphoma infiltrate. There is focal rosette formation (arrowhead) of small CD3+ T lymphocytes around the scattered large lymphoma cells (× 400). H&E: hematoxylin and eosin; OCT-2: octamer transcription factor-2. |
When the diagnosis of THRLBCL was confirmed by our pathology team, the patient’s PCP and hematologist/oncologist advised him to return to the hospital for a chemotherapy Port-A-Cath placement. Due to the patient’s advanced age and risk of significant toxicity, he was initiated on a reduced-dosage chemotherapy regimen consisting of rituximab (375 mg/m2), cyclophosphamide (400 mg/m2), doxorubicin (12.5 mg/m2), vincristine (0.5 mg), and oral prednisone (40 mg/m2), R-mini-CHOP therapy. He received four cycles of R-mini-CHOP along with pegfilgrastim for primary prophylaxis. He tolerated all cycles thus far without complications. The patient continues to follow up closely with his hematologist/oncologist for routine monitoring. Significant clinical improvement following treatment is illustrated in Tables 1 and 2.
 Click to view | Table 1. CMP From the Initial ED Visit, Prior to Beginning R-Mini-CHOP Therapy, After Completing R-Mini-CHOP Therapy, and the Most Recent Results on File |
 Click to view | Table 2. CBC From the Initial ED Visit, Prior to Beginning R-Mini-CHOP Therapy, After Completing R-Mini-CHOP Therapy, and the Most Recent Results on File |
| Discussion | ▴Top |
This case discusses the significance of thorough investigation for unusual causes of hypercalcemia, irrespective of a patient’s given age. This 90-year-old male had mildly elevated calcium levels in the past but was deemed insignificant and dismissed as secondary to the use of over-the-counter antacids. Further investigation was initiated when symptoms developed in conjunction with a calcium level greater than 15 and unintentional 40-pound weight loss. There is a wide differential of etiologies for hypercalcemia, with primary hyperparathyroidism and hypercalcemia of malignancy being some of the most common pathologic entities [9-11]. Some studies suggest that hypercalcemia of malignancy occurs in up to 44.1% of patients diagnosed with cancer and is more prevalent in patients with advanced stages [10, 12]. About 15% of patients with NHL will be affected by hypercalcemia during their disease course [13]. Symptoms secondary to hypercalcemia include lethargy, abdominal pain, nausea, vomiting, constipation, anorexia, cognitive dysfunction, and polyuria [9, 13].
The mechanisms by which hypercalcemia of malignancy manifests are well documented. The most common mechanism includes malignant tumor secretion of PTHrP, which acts on osteoblasts to enhance the production of nuclear factor-κB (RANK) and its respective ligand (RANK-L). The downstream effect of this interaction is a subsequent increase in osteoclast activity and bone resorption, thus translating to an increased level of calcium in the blood [10, 13]. The second most common mechanism, accounting for about 20% of cases, is via metastasis of malignant cells into the bone marrow. This generates an inflammatory response with downstream stimulation of osteoclast activity. The third mechanism of hypercalcemia of malignancy includes tumor production of calcitriol (1,25-dihydroxycholecalciferol). This causes an increased intestinal absorption of calcium and phosphate along with decreased renal calcium excretion [9, 10, 13].
While patients with cancer diagnoses are often affected by hypercalcemia throughout their clinical course, hypercalcemia manifesting at the time of diagnosis is much less common. In a retrospective case-control study, Abadi et al found that merely 18% of patients with DLBCL had hypercalcemia at the time of diagnosis [7]. Our patient was discovered to have hypercalcemia at the time of diagnosis of an extremely rare subtype of DLBCL, THRLBCL, which accounts for 1-3% of all DLBCLs [13]. THRLBCL is distinguished by < 10% malignant B cells with a rich background of reactive T lymphocytes and histiocytes [4]. While it may appear morphologically similar to nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL), THRLBCL has a much more aggressive course and worse overall prognosis [14-16]. It typically affects middle-aged men (median age of 40 years) who often present with advanced-stage disease, frequently involving the spleen, liver, and bone marrow [4, 17-19]. To our knowledge, this is the first reported case of THRLBCL in a 90-year-old patient with hypercalcemia manifesting at the time of diagnosis. Unique to the few cases in the literature with hypercalcemia as the initial manifestation of THRLBCL, our patient had a normal PTHrP in conjunction with a low iPTH and an unremarkable SPEP.
THRLBCL is a rare, aggressive subtype of DLBCL that shares similar treatment options as DLBCL. R-CHOP is one of the mainstay systemic antineoplastic therapies for DLBCL and has been shown to have comparable outcomes in THRLBCL [4, 14, 15, 20-23]. Despite its efficacy, there are documented concerns with its toxicity and adverse effect profile limiting the ability of older patients (over 60 years of age) to complete therapy. For this reason, the R-mini-CHOP regimen has been administered for patient demographics like our case patient. R-mini-CHOP features a decreased dose of CHOP chemotherapy in conjunction with the conventional dose of rituximab [15, 23, 24]. Peyrade et al conducted a prospective, multicenter, single-arm, phase 2 study of patients over 80 years of age with a diagnosis of DLBCL, in which they received R-mini-CHOP therapy. Study results showed a 2-year overall survival of 59%, 2-year progression-free survival of 47%, and improved quality of life, highlighting R-mini-CHOP therapy as both efficacious and safe [15, 20]. Another prospective, multicenter, single-arm, phase 2 study featured R-mini-CHOP in a similar patient demographic with the addition of ibrutinib, a Bruton tyrosine kinase inhibitor. This study showed an increased 2-year overall survival from 59% to 68%, with similar improvements in quality of life among survivors [15, 20, 23, 24]. While our patient received the R-mini-CHOP therapy, these studies highlight the importance of adjusting treatment regimens with consideration to adverse effect profile, tolerability, and changes to quality of life, especially when working with elderly patients who are diagnosed with this rare, aggressive malignancy.
Conclusions
In conclusion, we report a unique presentation of a rare clinicopathologic entity. DLBCL is the most common type of NHL, with THRLBCL being an extremely rare, aggressive subtype. There are very few documented cases of THRLBCL manifesting as severe hypercalcemia. Furthermore, a literature search revealed no previously reported cases of THRLBCL manifesting as severe hypercalcemia in an elderly patient. We thus highlight the importance of including THRLBCL in the differential diagnosis and workup in all patients with severe, unexplained hypercalcemia, irrespective of a patient’s age. We additionally aim to educate on the use of R-mini-CHOP therapy in the elderly population as well as discuss its tolerability and efficacy versus traditional R-CHOP therapy. With varying clinical presentations and the need for detailed morphological and immunohistochemical analysis, THRLBCL is a difficult diagnosis to make. However, this aggressive malignancy carries a poor prognosis and therefore requires prompt recognition and initiation of treatment in the appropriate scenario.
Acknowledgments
The authors extend their sincere gratitude to the Department of Pathology at Jersey Shore University Medical Center, Hackensack Meridian Health, for their invaluable support.
Financial Disclosure
The authors declare that no financial funding or grant were made.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Verbal informed consent was obtained from the patient for this case report.
Author Contributions
Each author of this case report has made meaningful contributions to the design, analysis, and interpretation of the data presented. The following is a brief summary of each author’s specific contributions. RKO contributed to case selection and case presentation. GAH contributed to planning and drafting the manuscript. IGO contributed to case discussion and edition of the manuscript. JWS contributed to case discussion and case planning. SJS contributed to case introduction and abstract. JSH contributed to reviewing and finalizing the manuscript. EA and XS contributed to the histological slide review and interpretation. AAC contributed to revision, proofreading, editing, and final approval.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375-2390.
doi pubmed pmc - Martelli M, Ferreri AJ, Agostinelli C, Di Rocco A, Pfreundschuh M, Pileri SA. Diffuse large B-cell lymphoma. Crit Rev Oncol Hematol. 2013;87(2):146-171.
doi pubmed - Sukswai N, Lyapichev K, Khoury JD, Medeiros LJ. Diffuse large B-cell lymphoma variants: an update. Pathology. 2020;52(1):53-67.
doi pubmed - Kommalapati A, Tella SH, Go RS, Nowakowski GS, Goyal G. T cell/histiocyte-rich large B cell lymphoma: incidence, demographic disparities, and long-term outcomes. Br J Haematol. 2019;185(1):140-142.
doi pubmed - Pittaluga S, Jaffe ES. T-cell/histiocyte-rich large B-cell lymphoma. Haematologica. 2010;95(3):352-356.
doi pubmed pmc - Gauchy AC, Kanagaratnam L, Quinquenel A, Gaillard B, Rodier C, Godet S, Delmer A, et al. Hypercalcemia at diagnosis of diffuse large B-cell lymphoma is not uncommon and is associated with high-risk features and a short diagnosis-to-treatment interval. Hematol Oncol. 2020;38(3):326-333.
doi pubmed - Abadi U, Peled L, Gurion R, Rotman-Pikielny P, Raanani P, Ellis MH, Rozovski U. Prevalence and clinical significance of hypercalcemia at diagnosis in diffuse large B-cell lymphoma. Leuk Lymphoma. 2019;60(12):2922-2926.
doi pubmed - Conte GA, Harmon JS, Le ML, Sun X, Schuler JW, Levitt MJ, Chinnici AA, et al. Hypercalcemia in T-cell/histiocyte-rich large B-cell lymphoma: an unusual presentation of a rare disease and literature review. World J Oncol. 2019;10(6):231-236.
doi pubmed pmc - Mirrakhimov AE. Hypercalcemia of malignancy: an update on pathogenesis and management. N Am J Med Sci. 2015;7(11):483-493.
doi pubmed pmc - Feldenzer KL, Sarno J. Hypercalcemia of malignancy. J Adv Pract Oncol. 2018;9(5):496-504.
pubmed pmc - Endres DB. Investigation of hypercalcemia. Clin Biochem. 2012;45(12):954-963.
doi pubmed - Burt ME, Brennan MF. Incidence of hypercalcemia and malignant neoplasm. Arch Surg. 1980;115(6):704-707.
doi pubmed - Trindade J, Pinheiro L, Lucas M, Victorino RMM. Severe hypercalcemia due to large B-cell non-Hodgkin Lymphoma. European Journal of Internal Medicine. 2013;24(suppl 1):e172.
doi - Siricilla M, Irwin L, Ferber A. A case of chemotherapy-refractory "THRLBCL like Transformation of NLPHL" successfully treated with lenalidomide. Case Rep Oncol Med. 2018;2018:6137454.
doi pubmed pmc - Peyrade F, Jardin F, Thieblemont C, Thyss A, Emile JF, Castaigne S, Coiffier B, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2011;12(5):460-468.
doi pubmed - Gloghini A, Carbone A. T-cell/histiocyte rich large B-cell lymphoma. Atlas Genet Cytogenet Oncol Haematol. 2016. http://atlasgeneticsoncology.org/haematological/1764/t-cell-histiocyte-rich-large-b-cell-lymphoma.
- Tousseyn T, De Wolf-Peeters C. T cell/histiocyte-rich large B-cell lymphoma: an update on its biology and classification. Virchows Arch. 2011;459(6):557-563.
doi pubmed - Bouabdallah R, Mounier N, Guettier C, Molina T, Ribrag V, Thieblemont C, Sonet A, et al. T-cell/histiocyte-rich large B-cell lymphomas and classical diffuse large B-cell lymphomas have similar outcome after chemotherapy: a matched-control analysis. J Clin Oncol. 2003;21(7):1271-1277.
doi pubmed - Aki H, Tuzuner N, Ongoren S, Baslar Z, Soysal T, Ferhanoglu B, Sahinler I, et al. T-cell-rich B-cell lymphoma: a clinicopathologic study of 21 cases and comparison with 43 cases of diffuse large B-cell lymphoma. Leuk Res. 2004;28(3):229-236.
doi pubmed - Kim YS, Ji JH, Ko YH, Kim SJ, Kim WS. Matched-pair analysis comparing the outcomes of T cell/histiocyte-rich large B cell lymphoma and diffuse large B cell lymphoma in patients treated with rituximab-CHOP. Acta Haematol. 2014;131(3):156-161.
doi pubmed - Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, Klasa R, et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood. 2007;109(5):1857-1861.
doi pubmed - Olaniyi JA, Oluwasola AO, Ibijola A. Therapy outcome of a T-cell-rich-B-Cell lymphoma (TCRBCL) patient with R-CHOP in Ibadan, Nigeria: a case report. Mediterr J Hematol Infect Dis. 2011;3(1):e2011008.
doi pubmed pmc - Silva RNF, Mendonca EF, Batista AC, Alencar RCG, Mesquita RA, Costa NL. T-cell/histiocyte-rich large B-cell lymphoma: report of the first case in the mandible. Head Neck Pathol. 2019;13(4):711-717.
doi pubmed pmc - Verner E, Johnston A, Pati N, et al. Efficacy of Ibrutinib, rituximab and mini-CHOP in very elderly patients with newly diagnosed diffuse large B cell lymphoma: primary analysis of the Australasian Leukaemia & Lymphoma Group NHL29 Study. Presented at: 2021 ASH Annual Meeting and Exposition. 2021. Atlanta, Georgia. Abstract 304.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


