| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Case Report
Volume 14, Number 4, August 2023, pages 309-315
A Case of BRCA2-Pathogenic Variant Breast Cancer With Metachronous Endometrial Cancer and Pancreatic Cancer
Masanori Oshia, Akimitsu Yamadaa, b, f, Aki Kimuraa, Toshiaki Kataokac, Noritoshi Kobayashid, Yasushi Ichikawad, Shoji Yamanakac, Satoshi Fujiic, e, Itaru Endoa
aDepartment of Breast Surgery, Yokohama City University Graduate School of Medicine, Yokohama, Kanagawa, Japan
bDepartment of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine, 3-9 Fukuura, Kanazawa-ku, Yokohama, Kanagawa 236-0004, Japan
cDepartment of Pathology, Yokohama City University Graduate School of Medicine, Yokohama, Kanagawa, Japan
dDepartment of Oncology, Yokohama City University Graduate School of Medicine, Yokohama, Japan
eDepartment of Molecular Pathology, Yokohama City University Graduate School of Medicine, Yokohama, Japan
fCorresponding Author: Akimitsu Yamada, Department of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine, Kanazawa-ku, Yokohama, Kanagawa 236-0004, Japan.
Manuscript submitted July 5, 2023, accepted July 21, 2023, published online August 4, 2023
Short title: A Case of MPMNs With BRCA2-Pathogenic Variant
doi: https://doi.org/10.14740/wjon1658
| Abstract | ▴Top |
Since the popularization of cancer screening and an improvement in treatment over the last two decades, multiple primary malignant neoplasms (MPMNs) have been increasingly reported. We report a patient who developed metachronous MPMNs in the breast, the endometrium, and the pancreas over a period of 13 years. A 42-year-old woman was first diagnosed with breast cancer and underwent breast-conserving surgery with adjuvant radiation therapy and endocrine therapy. Four years after breast surgery, she was diagnosed with endometrial cancer and underwent a laparoscopic modified radical hysterectomy with bilateral oophorectomy with pelvic lymph node dissection followed by adjuvant chemotherapy. However, there was peritoneal dissemination of endometrial cancer 1 year after surgery, which could be removed laparoscopically followed by adjuvant chemotherapy. Ten years after breast cancer surgery, pleural metastasis of breast cancer was diagnosed and treated by endocrine therapy. Thirteen years after breast cancer surgery, a pancreatic tumor with multiple liver masses emerged. It was difficult to diagnose whether primary or metastasis cancer by the results of the pathological analysis. Finally, we diagnosed primary pancreatic cancer with liver metastasis by clinical examination with the BRCA2-pathogenic variant. These tumors were well responded to chemotherapy and the patient survived during a follow-up period of 8 months. According to MPMNs, breast cancer patients should be followed-up carefully for the possibility of BRCA pathogenic variant and development of different primary malignant neoplasms.
Keywords: BRCA mutation; Breast cancer; Multiple primary malignant neoplasms; Pancreatic cancer
| Introduction | ▴Top |
Due to the prolonged survival of cancer patients, the diagnosis of multiple primary malignant neoplasms (MPMNs) has gradually increased with the incidence of 0.73-11.7% [1, 2] among cancer patients. Here, we report a case of a patient presenting with metachronous MPMNs of the breast, endometrium, and pancreas with a BRCA2 pathogenic variant.
| Case Report | ▴Top |
This is a case of a 42-year-old woman with hypothyroidism as comorbidity. The family history revealed that her mother had lung cancer, her paternal aunt had breast cancer, her paternal uncle had pancreatic cancer, and her maternal uncle had prostate cancer and malignant lymphoma. She presented with a left breast mass with pain. A core needle biopsy of breast mass showed invasive ductal carcinoma (IDC). After several examinations, such as computed tomography (CT) and magnetic resonance imaging (MRI), she was diagnosed with 13 × 13 mm breast cancer (cT1N0M0) and underwent breast-conserving surgery with sentinel lymph node biopsy. Pathological analysis showed 12 × 10 mm of IDC with nuclear grade 1, estrogen receptor (ER)-positive, progesterone receptor (PgR)-positive, and human epidermal growth factor receptor 2 (HER2)-negative (pT1N0M0, stage I) (Fig. 1a). She received adjuvant radiotherapy (50 Gy) and endocrine therapy (tamoxifen). At the age of 45 years, the follow-up CT scan revealed a tumor in the uterus (Fig. 2a). She was diagnosed with endometrial cancer and underwent a laparoscopic modified radical hysterectomy with bilateral oophorectomy with pelvic lymph node dissection. Pathological analysis showed localized endometrioid adenocarcinoma, exophytic type, ly0, v0, margin (-), grade 1 (pT1aN0M0) (Fig. 1b). Then she received six cycles of adjuvant chemotherapy, combined with paclitaxel and carboplatin. One year after, the follow-up CT scan revealed multiple intraabdominal masses (Fig. 2b). As peritoneal dissemination from breast or endometrial cancer was suspected, laparoscopic peritoneal dissemination resection was performed. Pathological analysis of peritoneal tumor confirmed the dissemination of endometrioid adenocarcinoma (Fig. 1c). Ten years after breast cancer surgery, mass lesions were noticed in her thoracic cavity on a follow-up CT scan (Fig. 3a). The positron emission tomography-computed tomography (PET-CT) scan showed an accumulation of maximum standardized uptake value (SUVmax) = 2.5 (Fig. 3b). Video-assisted thoracoscopic surgery was performed to identify the primary lesion of this tumor. The pathological analysis revealed the tumor positive for ER, PgR, and GATA binding protein 3 (GATA-3), which led to the diagnosis of metastasis from breast cancer (Fig. 3c).
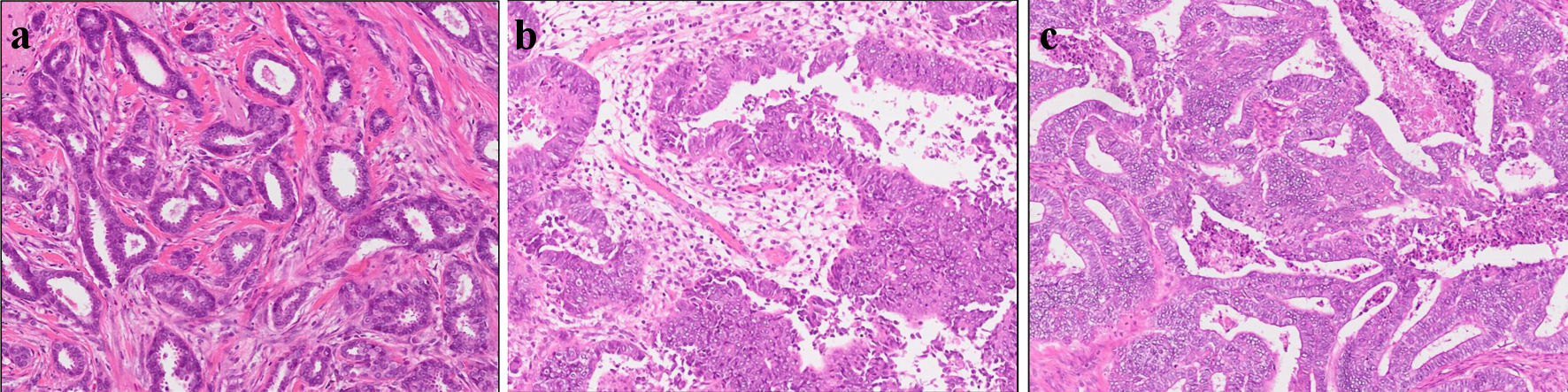 Click for large image | Figure 1. Histopathological results. Pathological analysis of surgical tissue from (a) breast, (b) uterus, and (c) abdominal tumor. |
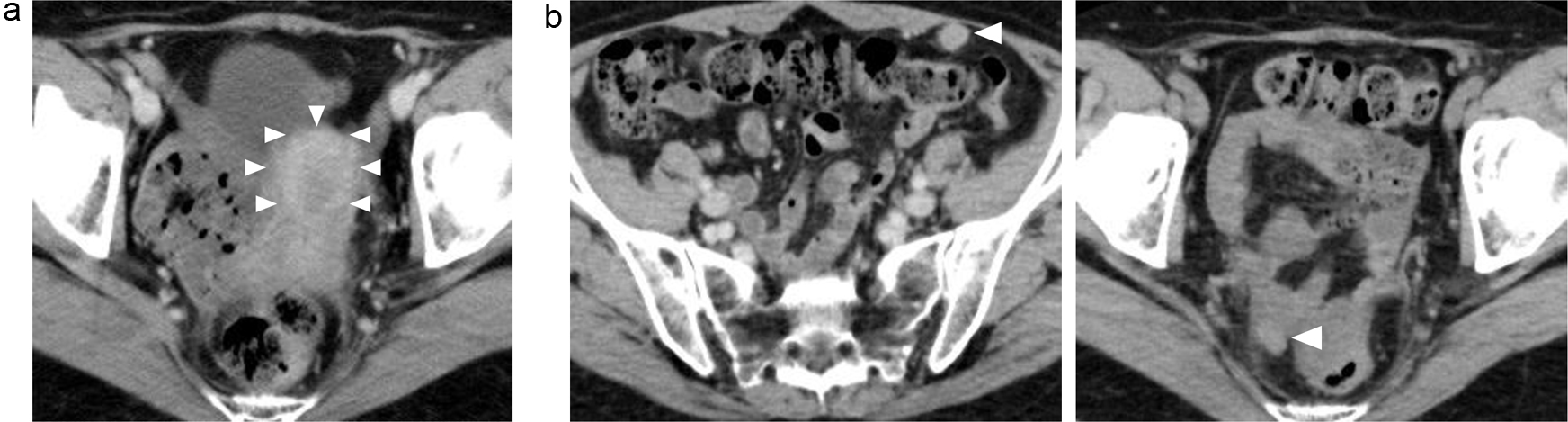 Click for large image | Figure 2. (a, b) Abdominal CT images revealing the multiple abdominal mass (arrows). CT: computed tomography. |
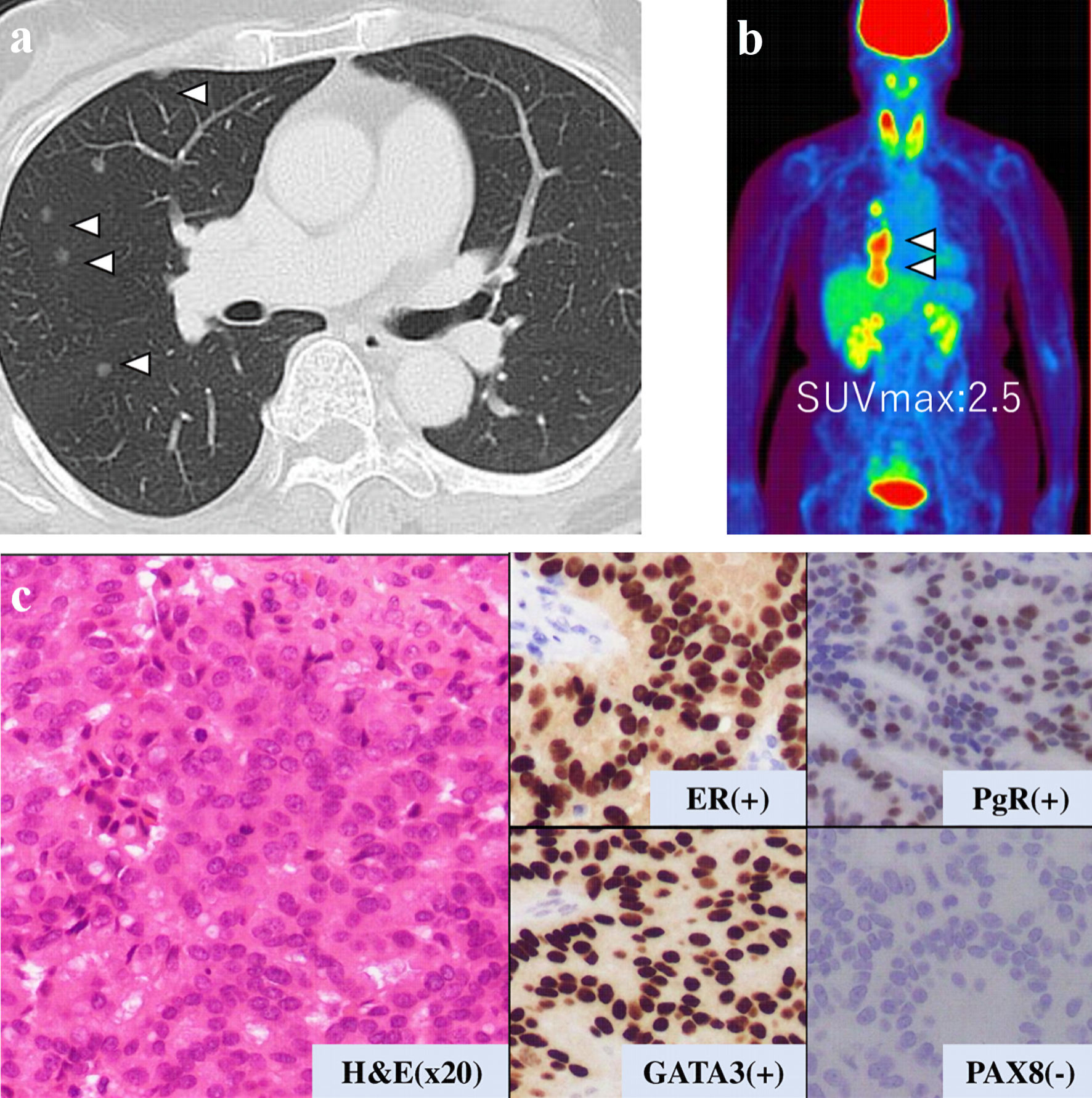 Click for large image | Figure 3. Examination results for mass in her thoracic cavity by (a) CT, (b) PET-CT (SUVmax = 2.5), and (c) pathological analysis. ER: estrogen receptor; PgR: progesterone receptor; H&E: hematoxylin and eosin stain; CT: computed tomography; PET-CT: positron emission tomography-computed tomography; SUVmax: maximum standardized uptake value. |
The patient then received an aromatase inhibitor after the surgery. Three years later, a pancreatic and hepatic mass was noticed (Fig. 4a, b). Although tumor markers of pancreatic cancer (Duke pancreatic monoclonal antigen type 2 (DUPAN-2) and s-pancreas antigen-1 (SPAN-1), carbohydrate antigen 19-9 (CA19-9)) were highly elevated, the other tumor markers (carcinoembryonic antigen (CEA), CA15-3) were slightly elevated (DUPAN-2: 530 U/mL, SPAN-1 490 U/mL, CA19-9: 2,492 U/mL, CEA: 16.6 ng/mL, and CA15-3: 30.3 U/mL). Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) was then performed and revealed adenocarcinoma with ER-negative, PgR-positive (partially), GATA-3-positive, paired box 8 (PAX8)-negative, and gross cystic disease fluid protein 15 (GCDFP15)-negative (Fig. 5).
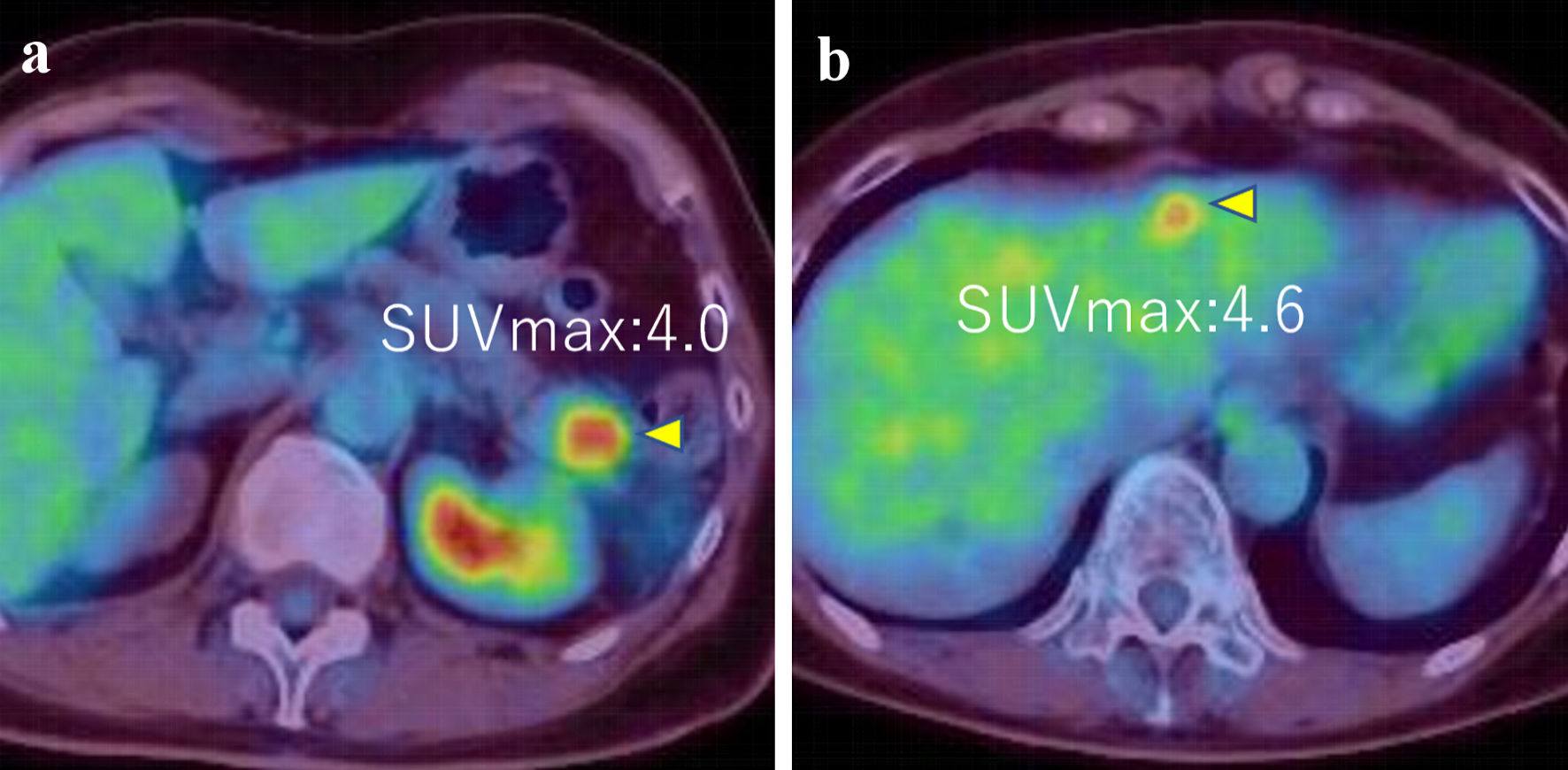 Click for large image | Figure 4. (a, b) PET-CT images showed tumors in pancreas and liver (SUVmax = 4.0 and 4.6, respectively) (arrows). PET-CT: positron emission tomography-computed tomography; SUVmax: maximum standardized uptake value. |
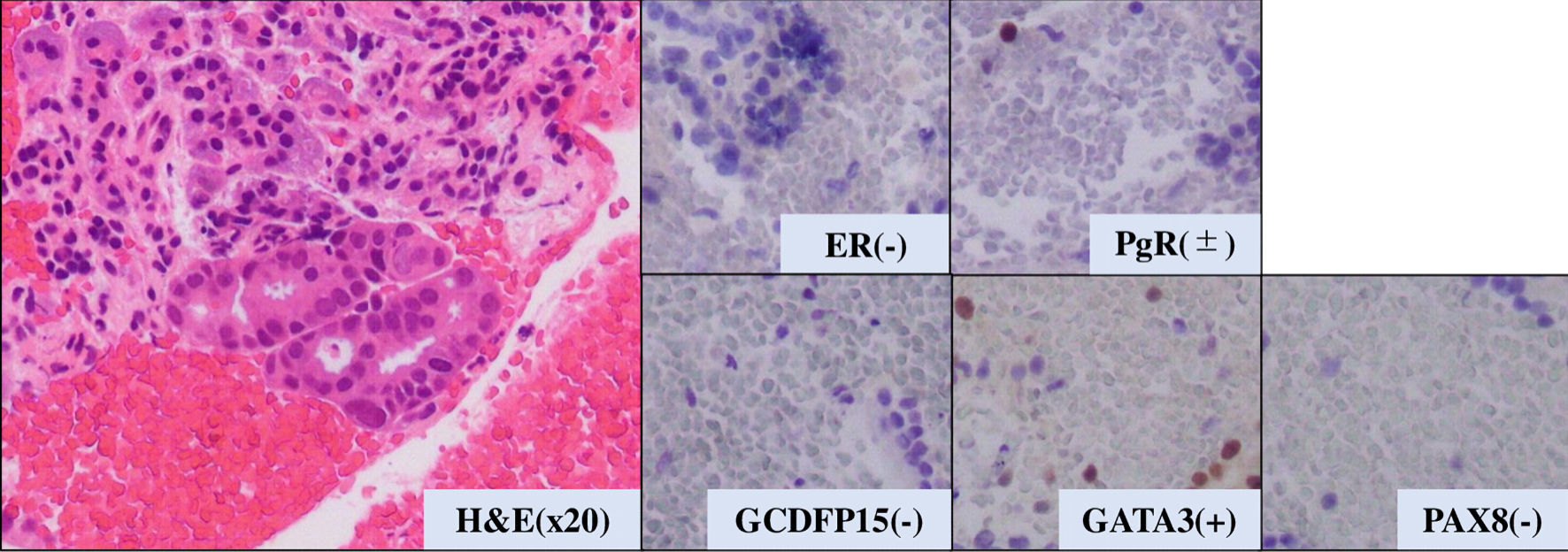 Click for large image | Figure 5. Pathological analysis of pancreatic tumor tissue. H&E: hematoxylin and eosin stain. ER: estrogen receptor; PgR: progesterone receptor; H&E: hematoxylin and eosin stain; GATA-3: GATA binding protein 3; PAX8: paired box 8; GCDFP15: gross cystic disease fluid protein 15. |
These results could not diagnose the pancreatic tumor as a metastatic tumor from breast cancer or primary pancreatic cancer. A BRCA gene analysis (BRCAnalysis®) was performed to investigate the possibility of primary pancreatic cancer. The result showed the germline BRCA2 mutation (p.Lys2162Asnfs*5). DUPAN-2, SPAN-1, and CA19-9 levels were rapidly elevated while CEA and CA15-3 were stable (DUPAN-2: 1,200 U/mL, and SPAN-1: 1,300 U/mL, CA19-9: 6,640 U/mL, CEA: 18.5 ng/mL, and CA15-3: 30.3 U/mL) during 1 month period. Based on these results, we diagnosed primary pancreatic cancer with liver metastasis and started chemotherapy with gemcitabine and nab-paclitaxel as pancreatic cancer treatment. One month later tumor markers of pancreatic cancer were markedly decreased (CA19-9: 1,592 U/mL, DUPAN-2: 1,000 U/mL, and SPAN-1: 350 U/mL) (Fig. 6), although CA15-3 level has no change (28.6 U/mL). The patient has received systemic chemotherapy and is alive at 8 months after diagnosis of pancreatic cancer.
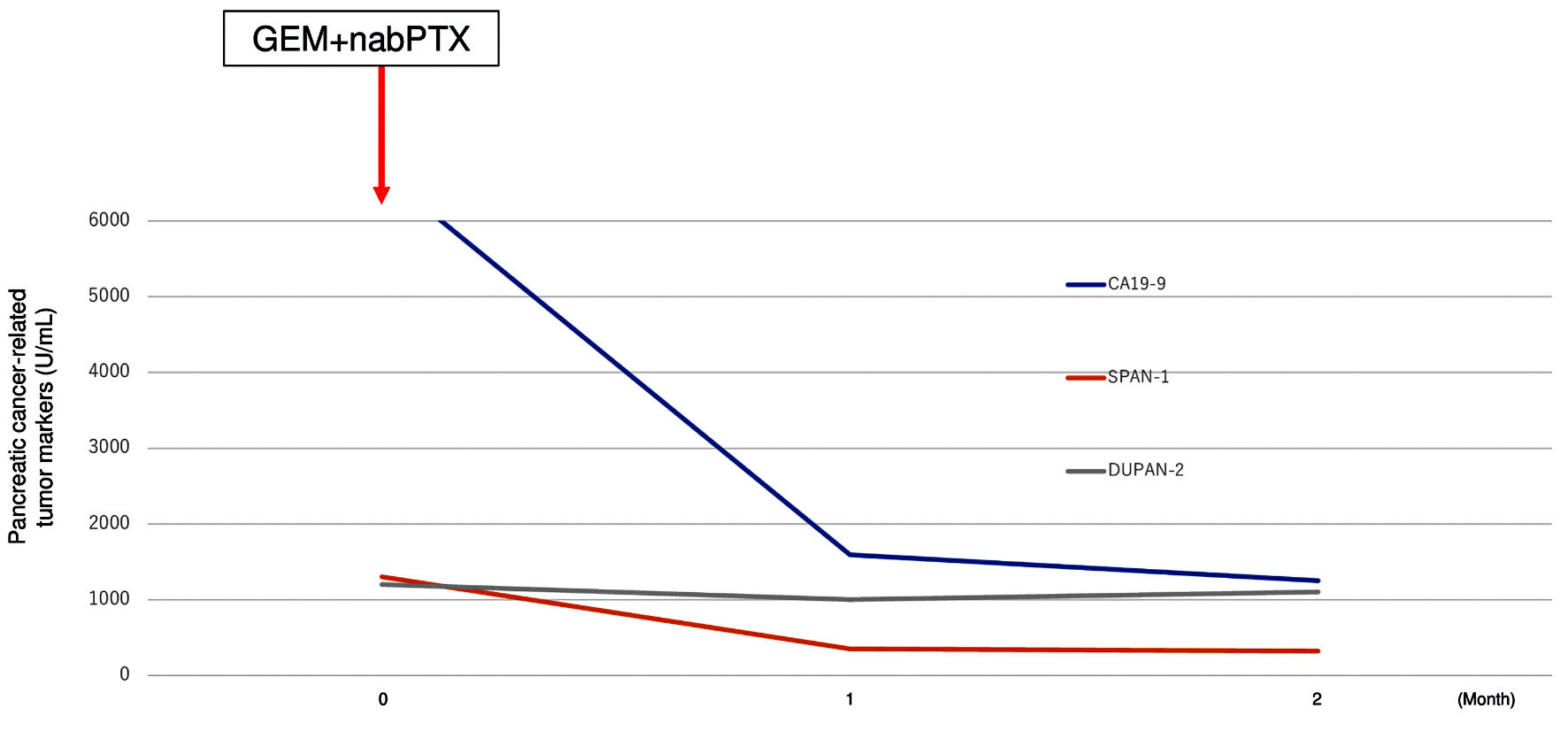 Click for large image | Figure 6. Transition graph of tumor markers (CA19-9, SPAN-1, and DUPAN-2). GEM+nabPTX: gemcitabine with nab-paclitaxel; DUPAN-2: Duke pancreatic monoclonal antigen type 2; SPAN-1: s-pancreas antigen-1; CA19-9: carbohydrate antigen 19-9. |
| Discussion | ▴Top |
Since the diagnosis and therapeutic outcomes of malignant tumors have improved due to the advancement of medical technology in recent years, patient prognosis has been improved in several cancers. On the other hand, with long-term survival, the development of multiple cancers has also increased [3]. Since MPMNs were reported by Billroth et al in 1889, the phenomenon of MPMNs has been defined by several researchers [4]. It was generally defined as the coexistence of at least two unrelated primary malignancies in a single patient. In 1932, Warren et al classified MPMNs as metachronous (interval of more than 6 months) and synchronous (interval of less than 6 months between primary malignancies) [5].
In this literature review, we collected 37 reports of MPMNs cases with three or more primary cancers, which included breast cancer as one of the primary cancers, including our case. We excluded case reports with a diagnosed association with genetic syndromes other than, hereditary breast and ovarian cancer syndrome, such as Li-Fraumeni syndrome, Lynch syndrome, Cowden syndrome, CDH1-associated breast cancer, Peutz-Jeghers syndrome, and PALB2-associated breast cancer. To our knowledge, this is the first time to report the incidence of a combination of primary breast cancer, endometrial cancer, and pancreatic primary cancer in the reports of MPMNs. Nineteen cases (51%) were metachronous, 15 cases (41%) were synchronous, and three cases (8%) were both metachronous and synchronous (Fig. 7a). Breast cancer was the initial diagnosis in 69% of cases (Fig. 7b). The other primary cancers that configured MPMNs were colorectal cancer (17%), lung cancer (14%), urinary cancer (13%), head and neck cancer (10%), and gynecological cancer (9%) (Fig. 7c). However, these results may not represent the entirety of MPMNs because the rare cases were only reported. Nyqvist et al reported that other previously diagnosed primary malignancies before breast cancer comprised gynecological cancer (27%), malignant melanoma (25%), and gastrointestinal carcinoma (18%) [6]. They also reported that 0.8% were patients with three or more multiple primary cancers that included breast cancer.
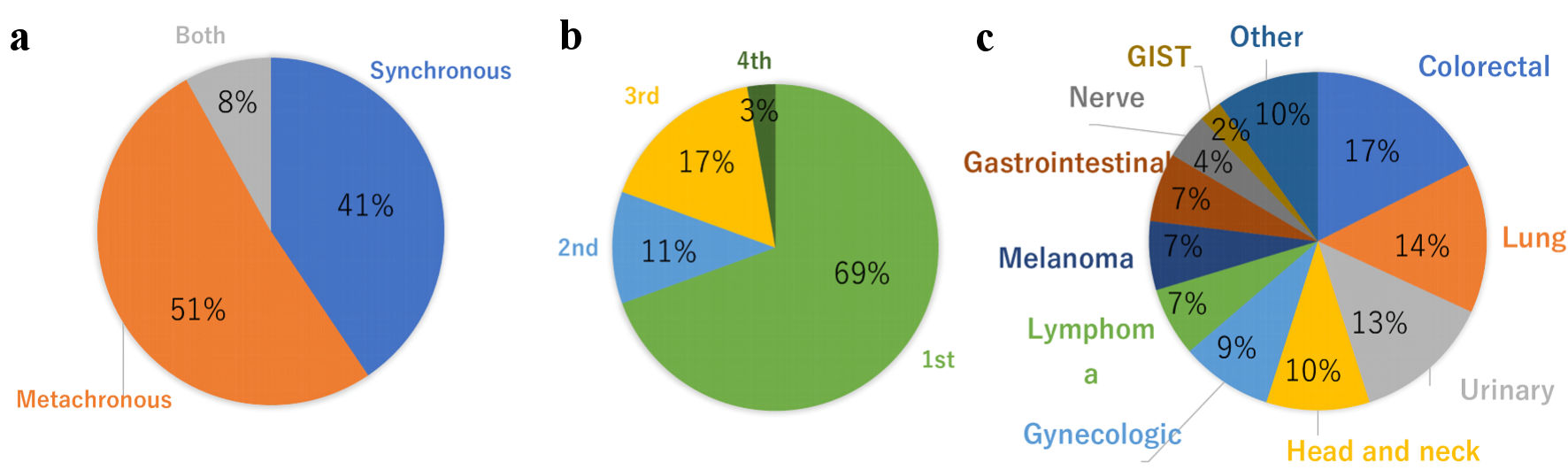 Click for large image | Figure 7. Distribution of MPMNs with breast cancer patients. (a) Synchronous vs. metachronous. (b) Breast cancer onset timing. (c) Comorbid cancer. MPMNs: multiple primary malignant neoplasms; GIST: gastrointestinal stromal tumor. |
Warren et al proposed three criteria for the diagnosis of MPMNs including: 1) Each tumor must present a definite clinical and histological picture of malignancy; 2) Each tumor must be histologically distinct; and 3) The probability that one was a metastatic lesion from the other must be excluded [5]. In our case, the diagnosis of the pancreatic tumor was particularly problematic. Immunohistochemical analysis of the pancreatic biopsy showed that the neoplastic cells were positive for GATA-3 and PgR, while ER, GCDFP-15, PAX8 were negative. GATA-3 is positive in 74-90% of breast cancer, 37% of pancreatic cancer, and 7% of endometrial cancer [7-9]. ER was positive in primary breast tumor and negative in pancreatic tumor in our case. However, ER expression could alter in metastatic site from primary site [10-12], and it could not exclude that pancreatic tumor was metastatic lesion from breast cancer on this basis alone. Therefore, to definitively differentiate between metastases from breast cancer and primary pancreatic cancer, we considered multidisciplinary aspects including the clinical features, tumor markers, and BRCA analysis in our case.
MPMNs could be caused by a variety of endogenous, exogenous, genetic, and therapeutic factors [13, 14]. It is also possible that a common etiology may play a pivotal role in the development of different malignancies, in which the common early genetic events occur at different time points and exhibit a long latency period [15]. Genome-wide association studies have identified 72 loci associated with breast cancer susceptibility, 17 of which are associated with MPMNs [16]. Since our patient has a family history of several cancers, the possibility of the involvement of genetic factors was high. BRCA pathogenic variant in women confers a high risk for breast cancer and ovarian cancer. Both BRCA1 and BRCA2 are involved in pathways that are important for DNA damage recognition, double-strand break repair, checkpoint control, transcription regulation, and chromatin remodeling. These functions are essential for all cell types. Mutation of these genes will lead to the initiation and proliferation of cancer cells [17]. Within minutes of DNA damage, the BRCA1 gene product is recruited to the sites of double-strand DNA breaks and initiates repair by modifying the local chromatin structure, thereby allowing other DNA repair proteins access to the damaged site. The BRCA2 protein is part of the homologous recombination DNA repair complex. These complex repairs double-strand breaks by homologous recombination through interaction with RAD51, a key component of the double-strand break repair pathway. In the absence of BRCA2, critical events in the initiation of homologous recombination are impaired; repair and replication errors accrue with each cell cycle [18].
BRCA2 pathogenic variant has been reported to the association with a high risk of not only breast and ovarian cancer but also prostate and pancreatic cancer. For pancreatic cancer, most research showed an increased risk of developing pancreatic cancer in individuals with BRCA pathogenic variants or individuals at high risk of having a BRCA pathogenic variant. The relative risk of pancreatic cancer for germline BRCA2 and BRCA1 pathogenic variants was 3.5 - 10 and 2.26, respectively, and the cumulative incidence was 2-7% and 1-3%, respectively [19].
Our case is consistent with the features of existing reports of luminal type breast cancer and pancreatic cancers occurring in women with BRCA2 pathogenic variants. As for endometrial cancer, De Jonge et al [20] pointed out endometrial cancer risk in women with germline BRCA1 or BRCA2 mutations in a multicenter cohort study. They studied 6,000 germlines BRCA1/2 mutation carriers and 8,451 non-BRCA1/2 mutation carriers and observed over 22 years and found a significant 2 - 3 folds increased risk of endometrial cancer in BRCA1 or BRCA2 mutation carriers. They also reported that it is causally related to germline BRCA1/2 mutation because the increased risk cannot be fully explained by a history of hormone therapy treatment. Tamoxifen could increase the risk of endometrial cancer in postmenopausal patients, but not in premenopausal patients [21].
In our case, breast cancer and endometrial cancer could be diagnosed by pathological analyses. However, it was difficult to make a definitive diagnosis of whether the pancreatic tumor was a primary or metastasis from pathological analysis alone. Therefore, our patient was diagnosed with primary pancreatic cancer by clinical features and the response to treatment of pancreatic cancer. In case of difficulty in diagnosis by the site of the tumor lesion and pathological analysis, the appropriate diagnosis and precise treatment could be possibly decided through multidisciplinary discussions.
The problems in treating MPMNs are the priority of treatment and the extent to which cure is sought. Factors that determine the order of treatment include the progress speed and status of each cancer, the invasiveness of the surgery, and the patient’s general condition. In cases where simultaneous treatment is difficult, the treatment of cancer that determines the prognosis should be given the highest priority. In our case, since the patient’s general condition was good when breast and endometrial cancers developed, surgery could be performed. However, at the time of the onset of pancreatic cancer, the patient had multiple metastases of breast cancer and endometrium cancer, therefore; systemic therapy rather than local therapy was chosen as a treatment.
In the future, the incidence of cancer is expected to increase further with the aging society. New screening tools are needed for the early detection of second and third primary tumors in the risk groups for the development of MPMNs. Research is also needed in developing the proper treatment and tools to follow-up these patients. It is expected that more genetic research will be conducted in the future.
Conclusions
We report a rare case of MPMNs and discuss the nature, pathogenesis, genetic factor, and therapeutic points of the MPMNs. As life expectancy increases, the number of MPMNs is expected to increase further, and accordingly, early diagnosis and treatment, including gene search for MPMNs, will increasingly become an important issue. Since BRCA pathogenic variant has been reported to link with several types of cancers and at a younger age, it should be recognized that such patients have the potential to develop MPMNs, especially in young patients. Communication with other professions is also important to provide precise treatment at each time.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no competing interest.
Informed Consent
Consent was obtained.
Author Contributions
MO researched the literature and wrote the manuscript. TK, SY, and SF contributed to being involved in the pathological diagnosis of the patient. AY, AK, NK, YI, and IE contributed to the manuscript review. All authors read and approved the final manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
DCIS: ductal carcinoma in situ; ER: estrogen receptor; HER2: human epidermal growth factor receptor 2; IDC: invasive ductal carcinoma; MPMNs: multiple primary malignant neoplasms; PgR: progesterone receptor
| References | ▴Top |
- Lv M, Zhang X, Shen Y, Wang F, Yang J, Wang B, Chen Z, et al. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine (Baltimore). 2017;96(17):e6799.
doi pubmed pmc - Liu Z, Liu C, Guo W, Li S, Bai O. Clinical analysis of 152 cases of multiple primary malignant tumors in 15,398 patients with malignant tumors. PLoS One. 2015;10(5):e0125754.
doi pubmed pmc - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Billing PS, Miller DL, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg. 2001;122(3):548-553.
doi pubmed - Warren S. Multiple primary malignant tumors: a survey of the literature and a statistic study. American Journal of Cancer. 1932;16:1358-1414.
- Nyqvist J, Parris TZ, Helou K, Sarenmalm EK, Einbeigi Z, Karlsson P, Nasic S, et al. Previously diagnosed multiple primary malignancies in patients with breast carcinoma in Western Sweden between 2007 and 2018. Breast Cancer Res Treat. 2020;184(1):221-228.
doi pubmed pmc - Yang M, Nonaka D. A study of immunohistochemical differential expression in pulmonary and mammary carcinomas. Mod Pathol. 2010;23(5):654-661.
doi pubmed - Clark BZ, Beriwal S, Dabbs DJ, Bhargava R. Semiquantitative GATA-3 immunoreactivity in breast, bladder, gynecologic tract, and other cytokeratin 7-positive carcinomas. Am J Clin Pathol. 2014;142(1):64-71.
doi pubmed - Liu H, Shi J, Wilkerson ML, Lin F. Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol. 2012;138(1):57-64.
doi pubmed - Kotecha R, Tonse R, Rubens M, McDermott MW, Odia Y, Appel H, Mehta MP. Systematic review and meta-analysis of breast cancer brain metastasis and primary tumor receptor expression discordance. Neurooncol Adv. 2021;3(1):vdab010.
doi pubmed pmc - Gong Y, Han EY, Guo M, Pusztai L, Sneige N. Stability of estrogen receptor status in breast carcinoma: a comparison between primary and metastatic tumors with regard to disease course and intervening systemic therapy. Cancer. 2011;117(4):705-713.
doi pubmed - Thompson AM, Jordan LB, Quinlan P, Anderson E, Skene A, Dewar JA, Purdie CA, et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Res. 2010;12(6):R92.
doi pubmed pmc - Yamamoto S, Yoshimura K, Ri S, Fujita S, Akasu T, Moriya Y. The risk of multiple primary malignancies with colorectal carcinoma. Dis Colon Rectum. 2006;49(10 Suppl):S30-36.
doi pubmed - De Luca A, Frusone F, Vergine M, Cocchiara R, La Torre G, Ballesio L, Monti M, et al. Breast cancer and multiple primary malignant tumors: case report and review of the literature. In Vivo. 2019;33(4):1313-1324.
doi pubmed pmc - Spratt JS, Meyer JS, Spratt JA. Rates of growth of human neoplasms: Part II. J Surg Oncol. 1996;61(1):68-83.
doi pubmed - Ghoussaini M, Pharoah PDP, Easton DF. Inherited genetic susceptibility to breast cancer: the beginning of the end or the end of the beginning? Am J Pathol. 2013;183(4):1038-1051.
doi pubmed - Friedenson B. BRCA1 and BRCA2 pathways and the risk of cancers other than breast or ovarian. MedGenMed. 2005;7(2):60.
pubmed pmc - Tutt A, Ashworth A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol Med. 2002;8(12):571-576.
doi pubmed - Wong W, Raufi AG, Safyan RA, Bates SE, Manji GA. BRCA mutations in pancreas cancer: spectrum, current management, challenges and future prospects. Cancer Manag Res. 2020;12:2731-2742.
doi pubmed pmc - de Jonge MM, de Kroon CD, Jenner DJ, Oosting J, de Hullu JA, Mourits MJE, Gomez Garcia EB, et al. Endometrial Cancer Risk in Women With Germline BRCA1 or BRCA2 Mutations: Multicenter Cohort Study. J Natl Cancer Inst. 2021;113(9):1203-1211.
doi pubmed pmc - Early Breast Cancer Trialists' Collaborative Group, Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771-784.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


