| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 2, April 2024, pages 246-256
Association of Lung Cancer Risk With the Presence of Both Lung Nodules and Emphysema in a Lung Cancer Screening Trial
Ya Liua, Zhuo Wei Fenga, Xiao Min Liua, Hong Yuan Duana, Zhang Yan Lyua, Yu Bei Huanga, Fang Fang Songa, Feng Ju Songa, b
aDepartment of Epidemiology and Biostatistics, National Clinical Research Center for Cancer, Key Laboratory of Molecular Cancer Epidemiology of Tianjin, Key Laboratory of Cancer Prevention and Therapy of Tianjin, Tianjin’s Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin Medical University, Tianjin 300060, China
bCorresponding Author: Feng Ju Song, Department of Epidemiology and Biostatistics, National Clinical Research Center for Cancer, Key Laboratory of Molecular Cancer Epidemiology of Tianjin, Key Laboratory of Cancer Prevention and Therapy of Tianjin, Tianjin’s Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin Medical University, Tianjin 300060, China
Manuscript submitted November 30, 2023, accepted February 17, 2024, published online March 21, 2024
Short title: Lung Cancer Risk With Lung Nodules and Emphysema
doi: https://doi.org/10.14740/wjon1782
| Abstract | ▴Top |
Background: The coexistence of emphysema and lung nodules could interact with each other and then lead to potential higher lung cancer risk. The study aimed to explore the association between emphysema combined with lung nodules and lung cancer risk.
Methods: A total of 21,949 participants from the National Lung Screening Trial (NLST) who underwent low-dose computed tomography (LDCT) examination were included. Participants were categorized into four groups (NENN group (non-emphysema and non-nodules), E group (emphysema without nodules), N group (nodules without emphysema), and E + N group (nodules with emphysema)) according to whether there were lung nodules and emphysema. Multivariable Cox regression and stratified analyses were performed to estimate the association between the four groups and lung cancer risk.
Results: Among the 21,949 participants, there were 9,040 (41.2%), 5,819 (26.5%), 4,737 (21.6%), and 2,353 (10.7%) participants in the NENN group, E group, N group, and E + N group. The risk of lung cancer incidence increased in turn in NENN group, E group, N group and E + N group. Compared with NENN group, the age-adjusted hazard ratios (HRs) (95% confidence intervals (CIs)) of lung cancer incidence were 2.07 (1.69 - 2.54) for E group, 4.13 (3.47 - 5.05) for N group, and 6.26 (5.14 - 7.62) for E + N group. The association was robust to adjustment for potential confounders (1.83 (1.47 - 2.27) for E group, 3.97 (3.24 - 4.86) for N group, and 5.23 (4.28 - 6.48) for E + N group). Comparable results as the lung cancer incidence were observed for lung cancer mortality, whether in age-adjusted model (E group: 1.85 (1.39 - 2.46), N group: 2.49 (1.89 - 3.29), E + N group: 4.27 (3.21 - 5.68)) or fully adjusted model (E group: 1.56 (1.15 - 2.11), N group: 2.43 (1.81 - 3.26), E + N group: 3.39 (2.50 - 4.61)). However, the trend of all-cause mortality risk among the four groups was somewhat different from that of lung cancer risk, whether in age-adjusted model (1.37 (1.21 - 1.54) for E group, 1.06 (0.92 - 1.21) for N group, and 1.75 (1.51 - 2.02) for E + N group) or fully adjusted model (1.26 (1.10 - 1.44) for E group, 1.09 (0.94 - 1.27) for N group, and 1.52 (1.30 - 1.79) for E + N group).
Conclusion: Based on a large-scale lung cancer screening trial in the United States, this study demonstrated that either emphysema or lung nodules can increase lung cancer risk, and lung nodules combined with emphysema can further increase the lung cancer risk and all-cause mortality. The significance of these findings for lung cancer screening should be evaluated.
Keywords: Lung nodules; Emphysema; Lung cancer; Incidence; Mortality
| Introduction | ▴Top |
Lung cancer has been the most common cancer and the main cause of cancer death for many years. In 2020, it is estimated that there were 2.2 million new lung cancer cases and 1.8 million lung cancer deaths, accounting for 11.4% of all new cancer cases and 18.0% of all cancer deaths [1]. Despite the evolving systemic treatment scenario for lung cancer [2-4], resulting in a 5-year survival rate of 17.4% [5], the increasing burden of lung cancer combined with poor prognosis is a challenge to cancer prevention. In 2011, the National Lung Screening Trial (NLST) proved for the first time that low-dose computed tomography (LDCT) screening in high-risk population can reduce lung cancer mortality by 20-33% [6]. Therefore, LDCT screening has been introduced and is now recommended as a strategy for early detection of lung cancer all over the world [7].
Lung nodule is a common finding of LDCT in lung cancer screening. Although about 95% of nodules are benign, patients and clinicians are still worried about the diagnosis of lung cancer [8-11]. Recently, the Dutch-Belgian Randomized Lung Cancer Screening Trial (Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON)) reported new solid nodules discovered at two post-baseline screens of approximately 8,000 subjects [12]. Several other studies have also reported on the presence of risks associated with new nodules, including the Mayo Clinic study and the International Early Lung Cancer Action Program [13, 14]. These studies reported rates of 5-13% for the detection of new nodules per annual post-baseline screen [12-14]. Overall lung cancer risks for new nodules were generally approximately 5%.
LDCT can not only detect lung nodules in early lung cancer, but also reveal other important lung findings, such as emphysema. There are a lot of studies on emphysema as a risk factor for lung cancer [15, 16]. More and more evidence confirmed that there is a correlation between the existence of emphysema and lung cancer incidence and cancer-related mortality, regardless of smoking history and nodule size [17-23].
Some studies on lung cancer screening show that 48-58% of patients with emphysema also have lung nodules [24], and the coexistence of emphysema and lung nodules will affect carcinogenesis, which may lead to higher lung cancer mortality because of increased sensitivity to biological damage [25]. There is also evidence that almost 80% of patients screened by LDCT have solitary lung nodules coexisting with chronic obstructive pulmonary disease (COPD) or emphysema, which is related to the unfavorable outcome [26-28].
In view of the above, several studies have explored the relationship between the solitary existence of lung nodules or emphysema and lung cancer risk. But there is no independent study on the risk of emphysema combined with pulmonary nodules. The purpose of this study was to explore the association of lung cancer risk with the presence of both lung nodules and emphysema by using the first round of LDCT screening data in NLST database.
| Materials and Methods | ▴Top |
Institutional Review Board approval
Consent to access data from the NLST was obtained from the National Cancer Institute’s Cancer Data Access System through a data transfer agreement among us, the authors, and the National Cancer Institute (NCI). Nonidentifiable patient data were used in this study for secondary data analysis, and its use was approved by the institutional review boards of our institutions.
Ethical statement
This human study was approved by NCI and their local institutional review board. Each participating center’s institutional review board approved the protocols and all participants provided written informed consent. The trial registration number (on ClinicalTrials.gov) of NLST is NCT00047385.
Ethical compliance with human/animal study
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Source of population
The designs of the NLST cancer screening trials have been previously published. Briefly, from 2002 to 2004, the NLST cancer screening trial randomized 53,452 smokers aged 55 - 74 years with at least 30 pack-years of smoking history and at most 15 years smoking cessation history to receive three annual LDCT screening (the intervention arm) or posterior-anterior chest X-ray screening (the control arm) in 1:1 ratio [6].
Selection of participants
In the NLST, we first excluded 26,730 participants in the control group who received chest X-ray screening, and 26,722 participants in the intervention group who received LDCT screening were initially included in this study. After further excluding the participants who did not meet the enrollment conditions, did not have LDCT in the first round (T0) or had no information about the results of LDCT, a total of 21,949 participants were finally included in this study (Fig. 1). According to the LDCT results of the first round (T0) on whether there is emphysema, the participants were divided into two groups (emphysema group and non-emphysema group), and further according to the presence or absence of lung nodules, the participants were divided into four groups: NENN group (non-emphysema and non-nodules), E group (emphysema without nodules), N group (nodules without emphysema), and E + N group (nodules with emphysema).
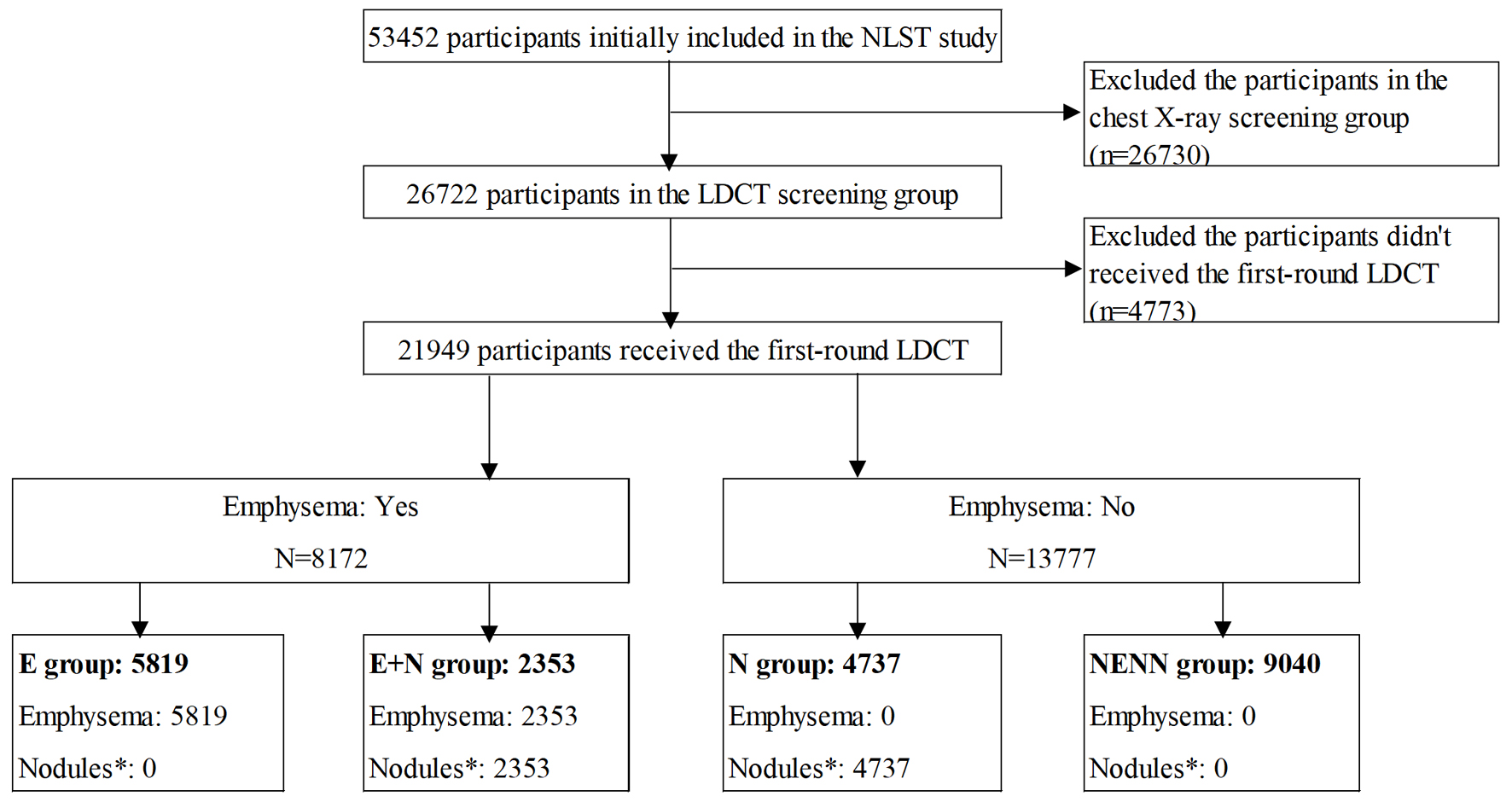 Click for large image | Figure 1. Flow chart of participants’ selection. NENN group: non-emphysema and non-nodules; E group: emphysema without nodules; N group: nodules without emphysema; E + N group: nodules with emphysema. |
Information of baseline variables
After informed consent, a baseline questionnaire was provided to all participants in the NLST to collect the information about cancer-related demographics and potential risk factors, such as demographics, smoking history, family history of cancer, medical history, etc. In the end, these potential confounders were included in this analysis based on previous literature and availability of data in the NLST: age (55 - 59, 60 - 64, 65 - 69, 70 - 74 years), sex (female, male), race/ethnicity (non-Hispanic White, others), education level (< senior high school, senior high school, college and above), marital status (married/living as married, widowed/divorced/separated, never married), smoking status (former smoking, current smoking), smoking pack-year (30 - 59, 60 - 89, ≥ 90 pack-year), body mass index (BMI) (< 25, 25 - 29.9, ≥ 30 kg/m2) and family history of lung cancer (no, yes). BMI is calculated by dividing the weight in kilograms by the square of the height in meters (kg/m2).
Nodules characteristics
We defined lung nodules as participants with nodules ≥ 4 mm, otherwise as having no lung nodules. According to whether the participants were diagnosed as emphysema in the first round (T0), we divided the participants diagnosed as lung nodules into two groups: N group (nodules without emphysema), and E + N group (nodules with emphysema). The characteristics of lung nodules include size (4 - 6, 7 - 10, 11 - 20, 21 - 30, > 30 mm), localization (right upper, right middle, right lower, left upper, lingula, right lower), and component (soft tissue, ground glass, or mixed). If participants had multiple nodules, we would analyze the largest nodule as the main nodule.
Ascertainment of endpoints
The primary endpoint events of this study were lung cancer incidence and mortality. The NLST confirmed diagnosis of lung cancer through medical record abstraction (MRA), which was triggered by annual or semi-annual study update form, positive computed tomography (CT) or chest X-ray screening exam, direct report by relatives or physicians, and supplemented by NDI Plus searches. Active follow-up data were collected on cancer diagnoses and deaths that occurred through December 31, 2009. Extended follow-up data were collected for deaths through December 31, 2015. Furthermore, in this study, the primary outcomes were censored at the date of the lung cancer diagnosis, death, loss of follow-up, or end of the follow-up period, whichever came first.
Statistical analysis
The main purpose of this study was to determine the association of lung cancer risk with the presence of both lung nodules and emphysema. According to the diagnosis of emphysema and lung nodules based on the LDCT results of the first round (T0), we defined four groups in advance (NENN group (non-emphysema and non-nodules), E group (emphysema without nodules), N group (nodules without emphysema), and E + N group (nodules with emphysema)) to evaluate the association between lung nodules combined with emphysema and lung cancer risk. The NENN group was used as the reference category. Descriptive analysis (variance and Chi-square test) was conducted to evaluate the distribution of baseline characteristics in different groups and the distribution of lung nodules in participants with or without emphysema. Log-rank test was originally used to compare the lung cancer incidence, lung cancer mortality and all-cause mortality of four groups. Multivariate Cox proportional hazard regression model was used to analyze the associations between the four groups on lung cancer incidence, lung cancer mortality and all-cause mortality. These associations were measured by hazard ratio (HR) and 95% confidence interval (CI). We constructed two models, namely, the age-adjusted model (model 1) only adjusted to age, and the fully adjusted model (model 2) adjusted to age, gender, race/ethnicity, education level, marital status, smoking status, smoking pack-year, BMI and family history of lung cancer.
We conducted subgroup analysis according to covariables (age, sex, smoking status, smoking pack-year, and family history of lung cancer) to identify the potential subgroups significantly related to the lung cancer risk among the four groups and confirm the robustness of the results.
All statistical analyses were performed using SPSS 23.0 software and R version 3.4.3. P value < 0.05 was considered statistically significant.
| Results | ▴Top |
Baseline characteristics of the participants according to study group
A total of 21,949 participants were included in this analysis (Fig. 1). There were 9,040 (41.2%) participants in the NENN group, 5,819 (26.5%) participants in the E group, 4,737 (21.6%) participants in the N group, and 2,353 (10.7%) participants in the E + N group (Table 1). The average age of the participants was 61.66 (6.04) years old, 12,953 (59.0%) were male and 10,703 (48.8%) were current smokers. Of the 21,949 participants, 7,090 (32.3%) had lung nodules and 8,172 (37.2%) had emphysema.
 Click to view | Table 1. Baseline Characteristics of the Study Group |
Characteristics of nodules, overall and stratified by with and without emphysema
As shown in Table 2, of the 7,090 participants with lung nodules, 4,737 (66.8%) participants did not have emphysema and 2,353 (33.2%) participants had emphysema. Compared with the participants without emphysema, the participants with emphysema had larger nodules (7 - 10 mm: 29.4% vs. 31.1%, 11 - 20 mm: 12.7% vs. 14.4%, 21 - 30 mm: 2.4% vs. 3.5%, > 30 mm: 1.4% vs. 1.9%, P < 0.001). Participants with emphysema had a higher proportion of nodules in the upper lung than those without emphysema (right upper: 24.0% vs. 25.3%, left upper: 13.5% vs. 16.2%, P = 0.013). Participants with emphysema were more likely to have spiculated nodules than those without emphysema (9.8% vs. 17.1%, P < 0.001). Most participants with nodules had soft tissue nodules (73.1%, 5,128/7,090), followed by ground glass nodules (15.4%, 1,093/7,090) and partial solid nodules (5.5%, 389/7,090). This remained true when participants were divided into those with and without emphysema.
 Click to view | Table 2. Characteristics of Nodules, Overall and Stratified by With and Without Emphysema |
Kaplan-Meier survival curves of cumulative lung cancer incidence, lung cancer mortality, and all-cause mortality
Throughout follow-up, the cumulative incidence of lung cancer and cumulative mortality of lung cancer in the four groups had the same trend. The cumulative incidence of lung cancer (Fig. 2a) and the cumulative mortality of lung cancer (Fig. 2b) were the highest in the E + N group, followed by the N group, then the E group and the lowest in the NENN group. All-cause cumulative mortality was the highest in the E + N group, followed by the E group, then N group and the lowest in the NENN group (Fig. 2c).
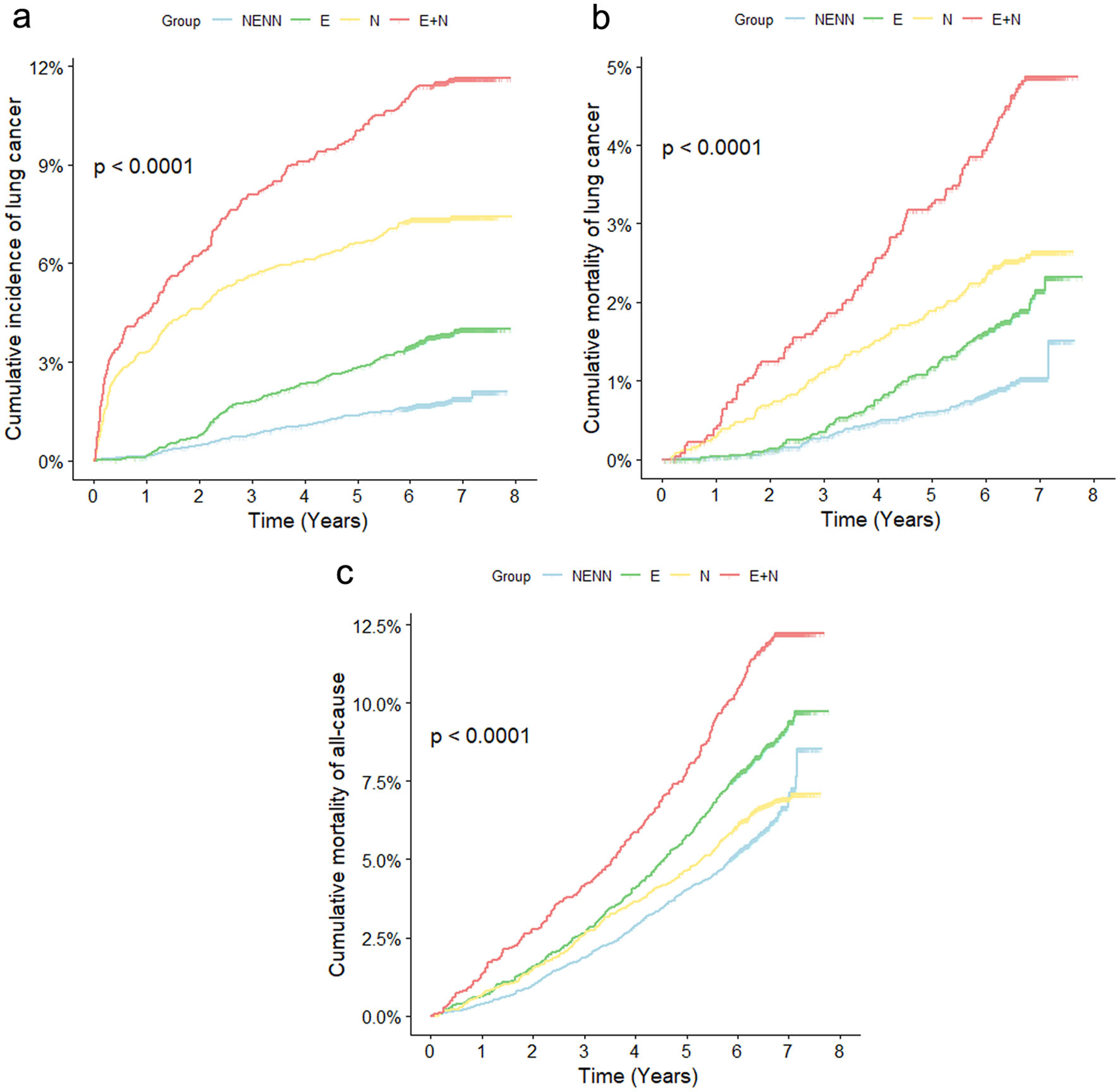 Click for large image | Figure 2. Kaplan-Meier survival curves of lung cancer incidence (a), lung cancer mortality (b) and all-cause mortality (c). NENN group: non-emphysema and non-nodules; E group: emphysema without nodules; N group: nodules without emphysema; E + N group: nodules with emphysema. |
Forest plot of the adjusted HRs for the association between lung nodules combined with emphysema and lung cancer incidence, lung cancer mortality, and all-cause mortality
HR from the Cox proportional hazard regression model was used to evaluate the relationship between the incidence of lung cancer, mortality of lung cancer and all-cause mortality (Fig. 3). We provided two models: age-adjusted model (model 1) and fully adjusted model (model 2). It was found that the risk of lung cancer incidence increased in turn in NENN group, E group, N group and E + N group (Fig. 3). In the age-adjusted model (model 1), compared with NENN group, the HRs (95% CIs) of lung cancer incidence were 2.07 (1.69 - 2.54) for E group, 4.13 (3.47 - 5.05) for N group, and 6.26 (5.14 - 7.62) for E + N group. The association was robust to adjustment for the age, sex, race, marital status, education level, BMI, smoking status, smoking package years and family history of lung cancer. In the fully adjusted model (model 2), compared with NENN group, adjusted HRs (95%CIs) of lung cancer incidence were 1.83 (1.47 - 2.27) for E group, 3.97 (3.24 - 4.86) for N group, and 5.23 (4.28 - 6.48) for E + N group.
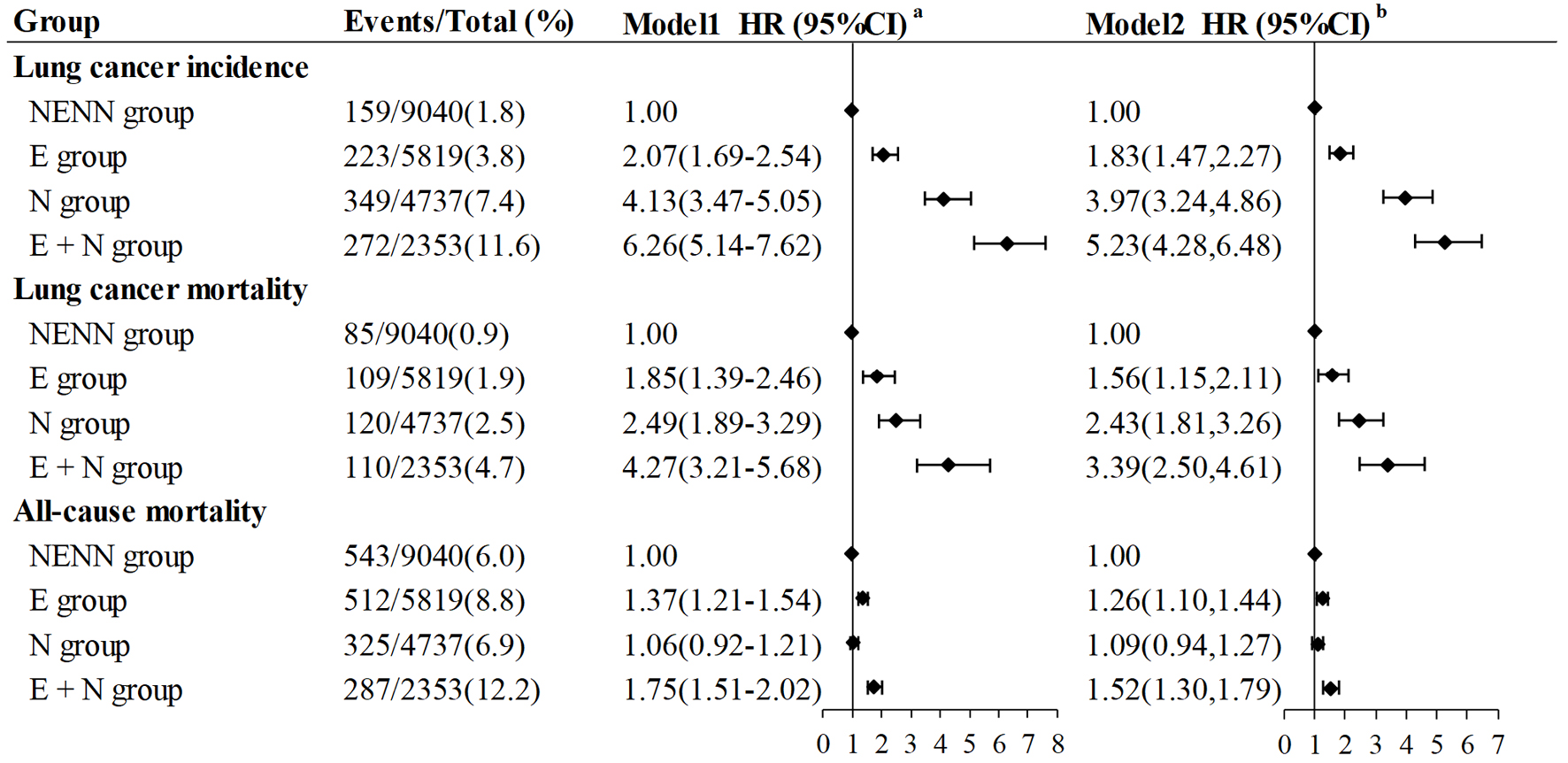 Click for large image | Figure 3. Forest plot of associations of four groups participants with lung cancer incidence, lung cancer mortality and all-cause mortality. Model 1a: adjusted for age. Model 2b: adjusted for age, gender, race/ethnicity, education level, marital status, smoking status, smoking pack-year, BMI and family history of lung cancer. NENN group: non-emphysema and non-nodules; E group: emphysema without nodules; N group: nodules without emphysema; E + N group: nodules with emphysema; BMI: body mass index. |
Comparable results as the lung cancer incidence were observed for lung cancer mortality (Fig. 3). Compared with NENN group, the risk of lung cancer mortality increased in turn in E group (age-adjusted HR (model 1): 1.85 (1.39 - 2.46), fully adjusted HR (model 2): 1.56 (1.15 - 2.11)), N group (age-adjusted HR (model 1): 2.49 (1.89 - 3.29), fully adjusted HR (model 2): 2.43 (1.81 - 3.26)) and E + N group (age-adjusted HR (model 1): 4.27 (3.21 - 5.68), fully adjusted HR (model 2): 3.39 (2.50 - 4.61)), whether in age-adjusted model (model 1) or fully adjusted model (model 2).
However, the trend of all-cause mortality risk among the four groups was somewhat different from that of lung cancer risk (Fig. 3). Compared with NENN group, E + N group (age-adjusted HR (model 1): 1.75 (1.51 - 2.02), fully adjusted HR (model 2): 1.52 (1.30 - 1.79)) had the highest risk of all-cause mortality, followed by E group (age-adjusted HR (model 1): 1.37 (1.21 - 1.54), fully adjusted HR (model 2): 1.26 (1.10 - 1.44)) and N group (age-adjusted HR (model 1): 1.06 (0.92 - 1.21), fully adjusted HR (model 2): 1.09 (0.94 - 1.27)). The association between lung nodules (N group) and all-cause mortality was not significant and had no statistical significance.
Subgroup analysis
Supplementary Materials 1 and 2 (www.wjon.org) reveal the subgroup analysis of the relationship between the incidence of lung cancer and the mortality of lung cancer in the four groups, respectively. We found that the trend of lung cancer risk among the four groups remained unchanged after stratification to age, sex, smoking status, smoking package year, and family history of lung cancer. That is to say, compared with NENN group, it was found that the risk of lung cancer in E group, N group and E + N group increased in turn, whether it is age-adjusted model or fully adjusted model. Subgroup analysis of all-cause mortality relationship of the four groups is shown in Supplementary Material 3 (www.wjon.org). The result of the highest all-cause mortality rate in the E + N group is reliable.
| Discussion | ▴Top |
In this study, we used the lung cancer screening LDCT data of NLST in the United States to focus on the association of lung cancer risk with the presence of both lung nodules and emphysema. This study revealed that the size, location and margins shape of lung nodules were different between participants with emphysema and those without emphysema. Among participants with lung nodules, participants with emphysema have larger lung nodules, and have a higher proportion of locating in the upper lobe of the lung and spiculated margins. What’s more, compared with NENN group, lung cancer risk in the E group, N group and E + N group increased gradually. Lung cancer risk in NENN group was the lowest and that in E + N group is the highest, even if stratified according to age, sex, smoking status, smoking package year, and family history of lung cancer. However, the trend of all-cause mortality risk was different.
Many previous studies showed that lung nodules with the above characteristics are more likely to be malignant nodules or developing into lung cancer [29-34]. Gomez-Saez et al found that cancer risk was associated with smoking habit, nodule size and spiculated edge which was nearly significant and there was a linear relationship between nodule size and risk of lung cancer [35]. The recent Fleischer guidelines mention that nodules located in the (right) upper lobe of lung have been identified as independent risk factors for lung cancer, and have been added to the definition of “high-risk” nodules, even in low-risk individuals [34]. In a systematic literature review by Wahidi et al, irregular, spiculated, and lobulated margin were found to be predictive of malignancy [36]. Hence, our research results show that participants with lung nodules coexisting with emphysema have a higher lung cancer risk because of the more malignant nodule characteristics.
We found that there is a correlation between the existence of emphysema and the incidence and mortality of lung cancer, independent of age, sex, smoking history and lung nodules. Consistent with our findings, a systematic review and meta-analysis [37] revealed that emphysema detected visually at CT is associated with significantly increased odds of lung cancer. However, not all studies report a positive correlation between emphysema and lung cancer. Maldonado et al did not confirm the influence of emphysema on the diagnostic frequency of malignant nodules [38].
The role of lung nodules as a risk factor for lung cancer has been widely studied [39-41]. Beyond that, our study also found that the existence of lung nodules combined with emphysema can further increase the lung cancer risk. Similarly, a study mentioned that solitary lung nodules may have a greater probability of malignancy in the presence of emphysema [29].
As mentioned above, emphysema and lung nodules do increase the lung cancer risk. Interestingly, though the group with lung nodules (N group) has a higher lung cancer risk than the group with emphysema (E group), there is no such trend in all-cause mortality risk. Suffering from emphysema can increase the risk of all-cause mortality, and lung nodules have no obvious significance for all-cause mortality. Similarly, Lee’s study [42] also revealed that emphysema was associated with long-term all-cause mortality; suspicious nodules were not identified as an independent risk factor for all-cause mortality in the multivariable Cox proportional hazards regression analysis. The mechanism that emphysema and lung nodules have different effects on lung cancer risk and all-cause mortality risk needs more research to explore.
Our study explored association of lung cancer risk with the presence of both lung nodules and emphysema, which provided a more solid foundation for the traditional risk prediction method of lung cancer. Currently, there are also more and more emerging methods to predict the lung cancer risk. Radiomic features, calculated based on LDCT images, and clinical data are frequently used for lung cancer screening and risk prediction, and some models [43, 44] have been independently validated. Developing alternative methods based on deep learning to perform feature extraction similar to radiology is also the main research focus of many teams. However, the interpretability of deep learning models should be taken into account during model development, and further research should be conducted in this field. In addition, biomolecular markers are also a hot method to predict the risk of lung cancer. At present, promising candidate molecules include autoantibodies, microRNAs, circulating tumor DNA and so on [45]. The emergence of genome-wide association studies (GWAS) may provide powerful evidence of genetic susceptibility genes for the lung cancer, and these genes may be included in the lung cancer risk prediction model [46]. Although no molecular biomarker has been routinely used for lung cancer screening, the existing candidate methods have great potential and represent a very promising method for early lung cancer screening in the future. In the future, if more related research can be done in the above field, we believe that this will provide more new insights for the occurrence and development mechanism and risk prediction of lung cancer.
Additionally, several limitations of this study should also be considered. First of all, the subtypes and severity of emphysema are not measured and classified in our data. Secondly, we have not explored the mechanism of the result that lung nodules have a higher lung cancer risk than emphysema. Thirdly, we did not explore the etiological relationship between emphysema and lung nodules. Fourth, the participants in this study are heavy smokers, and the generalizability of our results to populations outside the NLST eligibility criteria is indeterminate. Fifth, this study enrolled mainly (> 90%) Caucasian patients, so the results may not be applicable to all populations at risk for lung cancer. Finally, at present, radiomics and deep learning algorithms are significantly improving the sensitivity of predicting lung cancer risk from lung nodule imaging, but the radiographic descriptions of the lung nodules are also fairly limited in this study. Though they have limitations in population promotion, they are currently recommended high-risk screening groups, and the results of this study have targeted guiding significance for screening.
Conclusion
Based on a large-scale lung cancer screening trial in the United States, this study demonstrated that either emphysema or lung nodules can increase lung cancer risk, and lung nodules combined with emphysema can further increase the lung cancer risk and all-cause mortality. Compared with NENN group, lung cancer risk in the E group, N group and E + N group increased gradually. In addition, among participants with lung nodules, participants with emphysema have more malignant characteristics of lung nodules than those without emphysema. The significance of these findings for lung cancer screening should be evaluated.
| Supplementary Material | ▴Top |
Suppl 1. Subgroup Analysis Between Four Groups Participants and Lung Cancer Incidence.
Suppl 2. Subgroup Analysis Between Four Groups Participants and Lung Cancer Mortality.
Suppl 3. Subgroup Analysis Between Four Groups Participants and All-Cause Mortality.
Acknowledgments
This research was conducted using the NLST resource under application number 982. We thank the study participants, the investigators, and the NCI for data collected in the NLST trials. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by the above organizations.
Financial Disclosure
This work was supported by the Chinese National Key Research and Development Project (No. 2021YFC2500400) and National Natural Science Foundation of China (81974439).
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Informed Consent
Each participating center’s institutional review board approved the protocols and all participants provided written informed consent.
Author Contributions
Ya Liu: conceptualization, methodology, data curation, formal analysis, investigation, writing - original draft, writing - review and editing. Zhuo Wei Feng: data curation, investigation, writing - review and editing. Xiao Min Liu: data curation, investigation, writing - review and editing. Hong Yuan Duan: data curation, investigation, writing - review and editing. Zhang Yan Lyu: data curation, writing - review and editing. Yu Bei Huang: data curation, investigation, writing - review and editing, supervision. Fang Fang Song: data curation, writing - review and editing. Feng Ju Song: conceptualization, data curation, investigation, writing - review and editing, supervision, funding acquisition.
Data Availability
The data are available on application to the NLST trial (https://cdas.cancer.gov/nlst/).
Abbreviations
BMI: body mass index; CI: confidence interval; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; LDCT: low-dose computed tomography; NLST: National Lung Screening Trial
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Lamberti G, Andrini E, Sisi M, Rizzo A, Parisi C, Di Federico A, Gelsomino F, et al. Beyond EGFR, ALK and ROS1: Current evidence and future perspectives on newly targetable oncogenic drivers in lung adenocarcinoma. Crit Rev Oncol Hematol. 2020;156:103119.
doi pubmed - Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L, Marchetti A, et al. The impact of gender on the efficacy of immune checkpoint inhibitors in cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170:103596.
doi pubmed - Rizzo A, Cusmai A, Giovannelli F, Acquafredda S, Rinaldi L, Misino A, Montagna ES, et al. Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: a systematic review and meta-analysis. Cancers (Basel). 2022;14(6):1404.
doi pubmed pmc - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - National Lung Screening Trial Research T, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
doi pubmed pmc - Rampinelli C, De Marco P, Origgi D, Maisonneuve P, Casiraghi M, Veronesi G, Spaggiari L, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ. 2017;356:j347.
doi pubmed pmc - Gould MK, Tang T, Liu IL, Lee J, Zheng C, Danforth KN, Kosco AE, et al. Recent trends in the identification of incidental pulmonary nodules. Am J Respir Crit Care Med. 2015;192(10):1208-1214.
doi pubmed - Farjah F, Monsell SE, Gould MK, Smith-Bindman R, Banegas MP, Heagerty PJ, Keast EM, et al. Association of the intensity of diagnostic evaluation with outcomes in incidentally detected lung nodules. JAMA Intern Med. 2021;181(4):480-489.
doi pubmed pmc - Slatore CG, Golden SE, Ganzini L, Wiener RS, Au DH. Distress and patient-centered communication among veterans with incidental (not screen-detected) pulmonary nodules. A cohort study. Ann Am Thorac Soc. 2015;12(2):184-192.
doi pubmed pmc - Wiener RS, Gould MK, Woloshin S, Schwartz LM, Clark JA. What do you mean, a spot?: A qualitative analysis of patients' reactions to discussions with their physicians about pulmonary nodules. Chest. 2013;143(3):672-677.
doi pubmed pmc - de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JJ, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382(6):503-513.
doi pubmed - International Early Lung Cancer Action Program I, Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763-1771.
doi pubmed - Swensen SJ, Jett JR, Sloan JA, Midthun DE, Hartman TE, Sykes AM, Aughenbaugh GL, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med. 2002;165(4):508-513.
doi pubmed - de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, Pueyo JC, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132(6):1932-1938.
doi pubmed - Koshiol J, Rotunno M, Consonni D, Pesatori AC, De Matteis S, Goldstein AM, Chaturvedi AK, et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS One. 2009;4(10):e7380.
doi pubmed pmc - Mehal JM, Holman RC, Steiner CA, Bartholomew ML, Singleton RJ. Epidemiology of asthma hospitalizations among American Indian and Alaska Native people and the general United States population. Chest. 2014;146(3):624-632.
doi pubmed - Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, Silvestri GA, et al. Targeting of low-dose CT screening according to the risk of lung-cancer death. N Engl J Med. 2013;369(3):245-254.
doi pubmed pmc - Maisonneuve P, Bagnardi V, Bellomi M, Spaggiari L, Pelosi G, Rampinelli C, Bertolotti R, et al. Lung cancer risk prediction to select smokers for screening CT—a model based on the Italian COSMOS trial. Cancer Prev Res (Phila). 2011;4(11):1778-1789.
doi pubmed - Kinsey CM, San Jose Estepar R, Wei Y, Washko GR, Christiani DC. Regional emphysema of a non-small cell tumor is associated with larger tumors and decreased survival rates. Ann Am Thorac Soc. 2015;12(8):1197-1205.
doi pubmed pmc - Bishawi M, Moore W, Bilfinger T. Severity of emphysema predicts location of lung cancer and 5-y survival of patients with stage I non-small cell lung cancer. J Surg Res. 2013;184(1):1-5.
doi pubmed - Sanchez-Salcedo P, Berto J, de-Torres JP, Campo A, Alcaide AB, Bastarrika G, Pueyo JC, et al. Lung cancer screening: fourteen year experience of the Pamplona early detection program (P-IELCAP). Arch Bronconeumol. 2015;51(4):169-176.
doi pubmed - Ueda K, Jinbo M, Li TS, Yagi T, Suga K, Hamano K. Computed tomography-diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin Cancer Res. 2006;12(22):6730-6736.
doi pubmed - Diver WR, Jacobs EJ, Gapstur SM. Secondhand smoke exposure in childhood and adulthood in relation to adult mortality among never smokers. Am J Prev Med. 2018;55(3):345-352.
doi pubmed - Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med. 2007;176(3):285-290.
doi pubmed - Chae KJ, Jin GY, Goo JM, Chung MJ. Interstitial lung abnormalities: what radiologists should know. Korean J Radiol. 2021;22(3):454-463.
doi pubmed pmc - Hino T, Lee KS, Han J, Hata A, Ishigami K, Hatabu H. Spectrum of pulmonary fibrosis from interstitial lung abnormality to usual interstitial pneumonia: importance of identification and quantification of traction bronchiectasis in patient management. Korean J Radiol. 2021;22(5):811-828.
doi pubmed pmc - Chae KJ, Chung MJ, Jin GY, Song YJ, An AR, Choi H, Goo JM. Radiologic-pathologic correlation of interstitial lung abnormalities and predictors for progression and survival. Eur Radiol. 2022;32(4):2713-2723.
doi pubmed - Liu Y, Wang H, Li Q, McGettigan MJ, Balagurunathan Y, Garcia AL, Thompson ZJ, et al. Radiologic features of small pulmonary nodules and lung cancer risk in the national lung screening trial: a nested case-control study. Radiology. 2018;286(1):298-306.
doi pubmed pmc - Winer-Muram HT. The solitary pulmonary nodule. Radiology. 2006;239(1):34-49.
doi pubmed - Horeweg N, van Rosmalen J, Heuvelmans MA, van der Aalst CM, Vliegenthart R, Scholten ET, ten Haaf K, et al. Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. Lancet Oncol. 2014;15(12):1332-1341.
doi pubmed - Horeweg N, van der Aalst CM, Thunnissen E, Nackaerts K, Weenink C, Groen HJ, Lammers JW, et al. Characteristics of lung cancers detected by computer tomography screening in the randomized NELSON trial. Am J Respir Crit Care Med. 2013;187(8):848-854.
doi pubmed - de Hoop B, van Ginneken B, Gietema H, Prokop M. Pulmonary perifissural nodules on CT scans: rapid growth is not a predictor of malignancy. Radiology. 2012;265(2):611-616.
doi pubmed - MacMahon H, Naidich DP, Goo JM, Lee KS, Leung ANC, Mayo JR, Mehta AC, et al. Guidelines for management of incidental pulmonary nodules detected on ct images: from the fleischner society 2017. Radiology. 2017;284(1):228-243.
doi pubmed - Gomez-Saez N, Hernandez-Aguado I, Vilar J, Gonzalez-Alvarez I, Lorente MF, Domingo ML, Valero MP, et al. Lung cancer risk and cancer-specific mortality in subjects undergoing routine imaging test when stratified with and without identified lung nodule on imaging study. Eur Radiol. 2015;25(12):3518-3527.
doi pubmed - Wahidi MM, Govert JA, Goudar RK, Gould MK, McCrory DC, American College of Chest P. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):94S-107S.
doi pubmed - Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77(1):58-63.
doi pubmed - Maldonado F, Bartholmai BJ, Swensen SJ, Midthun DE, Decker PA, Jett JR. Are airflow obstruction and radiographic evidence of emphysema risk factors for lung cancer? A nested case-control study using quantitative emphysema analysis. Chest. 2010;138(6):1295-1302.
doi pubmed - Xiao H, Shi Z, Zou Y, Xu K, Yu X, Wen L, Liu Y, et al. One-off low-dose CT screening of positive nodules in lung cancer: A prospective community-based cohort study. Lung Cancer. 2023;177:1-10.
doi pubmed - Pinsky PF, Gierada DS, Nath PH, Munden R. Lung cancer risk associated with new solid nodules in the national lung screening trial. AJR Am J Roentgenol. 2017;209(5):1009-1014.
doi pubmed - Walter JE, Heuvelmans MA, de Jong PA, Vliegenthart R, van Ooijen PMA, Peters RB, Ten Haaf K, et al. Occurrence and lung cancer probability of new solid nodules at incidence screening with low-dose CT: analysis of data from the randomised, controlled NELSON trial. Lancet Oncol. 2016;17(7):907-916.
doi pubmed - Lee JE, Jeong WG, Lee HJ, Kim YH, Chae KJ, Jeong YJ. Relationship between incidental abnormalities on screening thoracic computed tomography and mortality: a long-term follow-up analysis. Korean J Radiol. 2022;23(10):998-1008.
doi pubmed pmc - McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, Yasufuku K, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med. 2013;369(10):910-919.
doi pubmed pmc - Gould MK, Ananth L, Barnett PG, Veterans Affairs SCSG. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest. 2007;131(2):383-388.
doi pubmed pmc - Seijo LM, Peled N, Ajona D, Boeri M, Field JK, Sozzi G, Pio R, et al. Biomarkers in Lung Cancer Screening: Achievements, Promises, and Challenges. J Thorac Oncol. 2019;14(3):343-357.
doi pubmed pmc - Ten Haaf K, Jeon J, Tammemagi MC, Han SS, Kong CY, Plevritis SK, Feuer EJ, et al. Risk prediction models for selection of lung cancer screening candidates: A retrospective validation study. PLoS Med. 2017;14(4):e1002277.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


