| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 15, Number 3, June 2024, pages 382-393
The Potential Role of Virus Infection in the Progression of Thyroid Cancer
Yong Ke Wua, c, Tian Tian Jianga, c, Yuan Hao Sua, Lin Meib, Ting Kai Suna, Yun Hao Lia, Zhi Dong Wanga, d, Yuan Yuan Jib, d
aDepartment of General Surgery, The Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an, China
bScientific Research Center and Precision Medical Institute, The Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an, China
cThe two authors contributed equally to this work.
dCorresponding Author: Zhi Dong Wang, Department of General Surgery, The Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an 710004, China; Yuan Yuan Ji, Scientific Research Center and Precision Medical Institute, The Second Affiliated Hospital, Xi’an Jiaotong University, Xi’an 710004, China
Manuscript submitted January 29, 2024, accepted March 16, 2024, published online April 15, 2024
Short title: Virus Infection in the Progression of TC
doi: https://doi.org/10.14740/wjon1830
- Abstract
- Introduction
- EBV and TC
- HCV and TC
- HIV and TC
- SARS-CoV-2 and TC
- Advanced Therapy
- Prospective
- Conclusions
- References
| Abstract | ▴Top |
Multiple factors have engaged in the progression of thyroid cancer (TC). Recent studies have shown that viral infection can be a critical factor in the pathogenesis of TC. Viruses, such as Epstein-Barr virus (EBV), hepatitis C virus (HCV), human immunodeficiency virus (HIV), and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), may play an essential role in the occurrence, development, and even prognosis in TC. This review mainly explored the potential role of viral infection in the progress of TC. The possible mechanisms could be recognizing the host cell, binding to the receptors, affecting oncogenes levels, releasing viral products to shape a beneficial environment, interacting with immune cells to induce immune evasion, and altering the pituitary-thyroid axis. Thus, comprehensive knowledge may provide insights into finding molecular targets for diagnosing and treating virus-related TC.
Keywords: Thyroid cancer; Virus diseases; Epstein-Barr virus infections; Hepacivirus; HIV; SARS-CoV-2; Therapeutics
| Introduction | ▴Top |
Thyroid cancer (TC) is the most common malignant tumor of the endocrine system worldwide. Global Cancer Statistics 2020 estimated that there were 586,000 TC cases, ranking ninth in cancer incidence worldwide [1]. The latest study reported the nationwide cancer incidence and mortality in China in 2022, and the results showed that an estimated number of 466,100 TC cases (including 124,900 males and 341,200 females) occurred in 2022, which is the third most common cancer in 2022, and the number of TC deaths were 11,600 (4,300 males and 7,200 females) [2]. Most thyroid nodules are benign, low-risk, or malignant [3]. Over 90% of TC include papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), and rare cancers are medullary thyroid cancer (MTC) and anaplastic thyroid cancer (ATC) [4, 5]. Currently, diagnosis of pathology by fine-needle aspiration (FNA) is classified into six general categories in The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) 2017 [6]. And each category’s risk of malignancy (ROM) is different [6]. Undoubtedly, indeterminate cytology was highlighted as a subdivision needing urgently addressed [7-9]. Subdivision of intermediate suspicion is crucial for effective FNA and the size of the fine needle, which could decrease the implied ROM [10, 11].
The etiology of TC lesions involves various factors, such as genetic mutations, environmental factors, ionizing radiation, and iodine-related factors [12]. Some recent studies have reported that TC pathogenesis was associated with present viral infections, such as Epstein-Barr virus (EBV) DNA and erythrovirus B19 (EVB19) DNA, which have been detected in thyroid tumors by quantitative real-time polymerase chain reaction (qPCR) [13-15]. Moreover, some patients with TC were detected among hepatitis C virus (HCV)+ [16] or human immunodeficiency virus (HIV)+ patients [17], which could have a past viral infection history. Furthermore, a meta-analysis has investigated that the prevalence of viral infections in TC was 37% (95% confidence interval (CI): 22% - 55%), and a significant association was identified between viral infections and TC risk (log (odds ratio (OR)): 1.51, 95% CI: 0.68 - 2.39) [18].
There is evidence [19, 20] that viruses can contribute to carcinogenesis in man by direct and indirect mechanisms. In one case, the virus can induce the expression of specific oncogenic proteins and then play a direct role in cell transformation; alternatively, the mutation is associated indirectly with the virus-induced chronic infection and inflammation through activating signaling pathways and cytokine secretion related to tumor occurrence, stimulating cell proliferation and inhibiting apoptosis. The virus infection may induce a series of immune-inflammatory responses and attack the normal gene metabolism in the cell [21]. EBV, HCV, HIV, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) play a vital role in the occurrence, development, and even prognosis of TC.
In this review, we focused on reports dealing with the function of the virus on TC and its involved mechanisms. Moreover, advanced anti-virus therapy on TC is discussed compared to standard treatments. Antiviral therapy may also become an emerging treatment method for TC.
| EBV and TC | ▴Top |
EBV remains the most common persistent, asymptomatic viral infection in humans; about 95% of adults worldwide are infected with EBV [22]. Multiple EBV infections are the causative agent of infectious mononucleosis and are closely associated with several lymphomas and epithelial malignancies, including Hodgkin lymphoma (HL), Burkitt’s lymphoma, natural killer (NK)/T-cell lymphoma, nasopharyngeal cancer, and gastric cancer. EBV infection causes 200,000 cases of malignant tumors of epithelium and B cells and 140,000 deaths annually [23].
EBV has been identified as one of the most critical risk factors in epithelium cancers, such as PTC [13]. The expression of EBV in thyroid gland neoplasms has been detected [24, 25]. In the past few years, a study conducted among Iranian patients with PTC investigated the relationship between EBV and PTC, and the results showed that 27 out of 41 individuals were Epstein-Barr virus nuclear antigen 1 (EBNA1) positive by PCR [26]. Another study has come to a similar conclusion that EBA DNA was detected in 29 (29/57) patients with PTC by PCR from Iran [27]. Almeida et al have found EBV DNA sequences in 29 (29/183) thyroid tissue samples from Brazilians and observed that viral load was higher in tumors than in normal tissues (P < 0.01) [13]. Moghoofei et al have proved that EBV infection may play a role in promoting the development of TC, where EBV DNA was detected in 71.9% of TC patients (41/57) by PCR and increasing expression of latent membrane protein (LMP)-1A/LMP-2A were positively correlated with tumor stage progression [14]. Almeida et al have found that EBV DNA viral load was higher than their corresponding normal tissues by PCR (P < 0.05), and EBV-encoded RNA (EBER) expression mainly appeared in malignant samples [13]. On the contrary, Bychkov et al have found that EBV detection was negative in 20 TC samples from Thailand analyzed by EBER in situ hybridization (ISH) [28]. Another cohort study from Guangdong found that none of the 384 thyroid samples, including PTC, FTC, MTC, nodular goiters, and follicular adenomas, showed EBV positive by ISH [25]. Stamatiou et al have demonstrated that gene sequences of EBV had a comparable incidence both in postoperative nodular and adjacent normal thyroid tissues. Thus, their findings cannot determine the precise role of EBV [29]. At present, the detection of EBV in TC remains contradictory.
In conclusion, despite differences in detection techniques, regional ethnicities, and histological types, a range of positive results were found, which warrants consideration of the relationship between EBV and TC. Thus, we need to discuss the possible mechanism of EBV and TC regarding host cells, latent infection, and tumor microenvironment (TME) (Fig. 1).
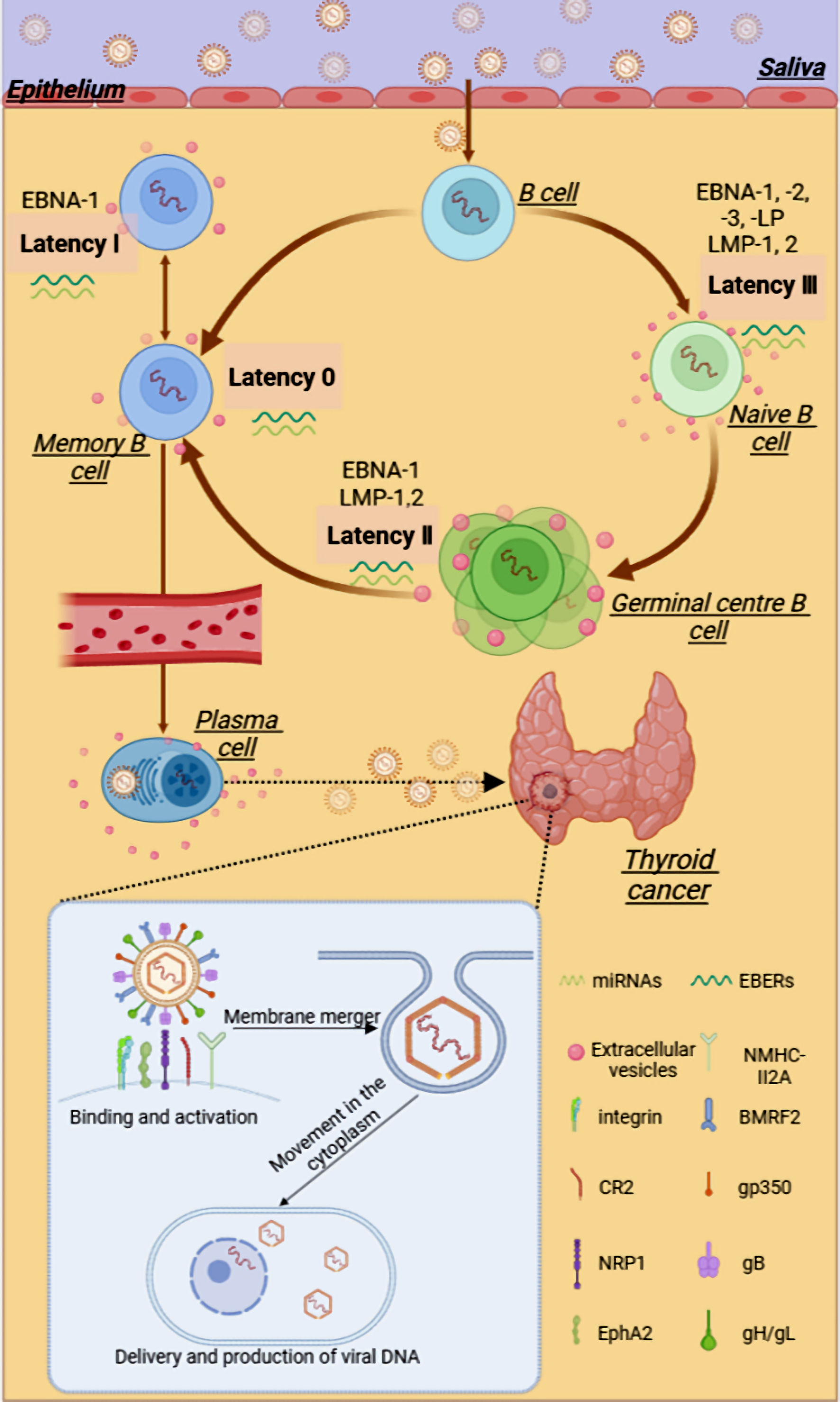 Click for large image | Figure 1. The possible relationship between EBV and thyroid cancer. EBV can be transmitted through saliva, passing through the oropharyngeal epithelium and tonsils, infecting lymphoid B cells, and subsequently infecting the initial B cells. After a complex series of latent infections, EBV may enter thyroid tissue through the blood circulation or lymphatic system. EBV can package various viral products, such as viral proteins and RNAs, into the extracellular vesicles to aid in the evasion of the host immune system and promote tumor progression within the TME. In the presence of multiple viral glycoproteins, EBV binds and activates receptors on cells, invading and proliferating within thyroid cells. gB: glycoprotein B; gH: glycoprotein H; gL: glycoprotein L; NRP1: neuropilin 1; EphA2: ephrin receptor A2; EBV: Epstein-Barr virus; EBNA1: Epstein-Barr virus nuclear antigen 1; EBER: EBV-encoded RNA; CR2 or CD21: complement receptor type 2; LMP: latent membrane protein; miRNA: microRNA. |
EBV life cycle and latent infection
Latent infection is a part of the lifecycle of EBV, where EBV persists and replicates within the host cells. According to recent reports, when the balance between the virus and the host immune system is disrupted, the latent EBV gene can promote tumor development, inhibit cell apoptosis, and suppress the recognition of infected cells by the host immune cells [23, 30]. In the process of primary infection, EBV-infected naive B cells lead to a latency growth program, in which cells could express all the latent genes, including EBNAs, LMPs, EBV-encoded small RNAs (EBERs), and microRNAs (miRNAs) [30]. Primary infection with EBV is followed by asymptomatic persistence of the virus in memory B cells, where EBV gene expression is probably completely silenced [31].
The functions of the respective EBV gene products give the virus its oncogenic abilities. These latent EBV proteins are necessary and sufficient for tumor formation. The power of EBV-driven tumor formation is reduced without pre-established latency III persistence, soluble EBV protein, and EBV miRNA expression [30, 32]. Studies have suggested that LMP-2A, associated with metastatic properties in tumors, can induce cell transformation through the phosphoinositide 3-kinase/protein kinase B (PI3K/AKT) pathway [33]. As a result, EBV proteins exert effects on some cellular proteins involved in the anoikic inhibition process and cause TC development.
In summary, the viral genes and their expression products during latent infection may be related to the formation and development of several tumors. There is increasing evidence focusing on the mechanism of EBV maintaining infection and survival of host cells. However, the relationship between EBV and TC is still unknown, and it is worth establishing the exact role of EBV in TC based on comprehensive knowledge. Whether there are more unknown EBV latency proteins to assist the infection process should be attached more attention in the future.
EBV and host cells
The two primary host cells that EBV virus targets are B cells and epithelial cells [22, 34]. EBV encodes various envelope glycoproteins, which help the virus complete the attachment and entry of host cells [24]. Compared with the mechanism of EBV entering the B cells, EBV entering into target epidermal cells could be roughly divided into five stages: 1) Binding: EBV binds to target cells using variable host cell surface receptors and multiple viral envelope glycoproteins; 2) Activation: binding of EBV virions can induce signaling pathway activation; 3) Membrane fusion: direct membrane fusion or fusion with the endosomal membrane; 4) Movement in the cytoplasm: the viral capsid is then transported in the cytosol to the nuclear periphery; 5) Reproduction: nuclear delivery of viral DNA and productive infection [34].
Connolly et al have reported that the glycoprotein 350/220 (gp350/220) complex, although not essential, could enhance EBV infection efficiency in epithelial cells by binding to cellular receptor complement receptor type 2 (CR2 or cluster determinant (CD)21) [35]. In addition, BMRF2 plays a vital role in tethering EBV to attach to β1 or α5β1 integrins-expressing epithelial cells [36]. These glycoproteins on EBV bind to the above cellular receptors, then the virus fuses with the cell membrane, and subsequently, the EBV genetic material enters the cytoplasm and begins a range of biological behaviors. Based on the research above, we may counteract the virus entry into epithelial cells to cause infection and latency by targeting the viral glycoprotein and reducing the expression of the receptor on epithelial cells.
EBV and TME
Oncogenic viruses could shape a beneficial pro-tumoral milieu to promote the development and progression of malignancies. The EBV-infected malignant cells shape and establish an immunosuppressive TME by communicating the cellular components, altering the molecular factors, and releasing extracellular vesicles (EVs) [37]. The local secretion of proinflammatory and immunosuppressive cytokines and chemokines is induced by EBV-infected cells. These aberrant soluble components also affect the function of immune cells and thus shape a TME where EBV-infected cells can proliferate, escape from apoptosis, and survive host antitumor defense [37]. Some pathogens that lead to persistent infections and cause inflammation strongly correlate with tumor development. In recent years, inflammation can be considered a significant hallmark of tumor development [38].
Further experiments indicate that PTC tissues have a higher proportion of tumor-promoting immune cells and a lower proportion of antitumor immune cells [39]. The study of Moghoofei et al suggests that the expression levels of inflammatory factors, including interleukin (IL)-6 and nuclear factor kappa-B (NF-κB), were statistically higher in the EBV-positive group than in the EBV-negative group [14]. Interestingly, there was an association between increased expression levels of some EBV genes (LMP-1 and LMP-2A) and increased levels of expression in some inflammatory cytokines in patients with breast and thyroid cancers. The inflammatory cytokines such as IL-1, IL-6, tumor necrosis factor-α (TNF-α), and transforming growth factor-β (TGF-β) could induce cancer cell proliferation and tumoral invasion through activation of NF-κB [14, 40]. Other research showed a specific correlation between some cytokines and EBV infection. The cytokine interferon (IFN)-γ, released by immune cells, such as T cells and dendritic cells (DCs), which can be recruited by EBV-infected cells, plays a central role in the resistance of the host to EBV infection via direct antiviral effects as well as modulation of the immune response. The basic leucine zipper nuclear factor 1 (BLZF1) encoded by EBV-infected cells seems to inhibit the function of the IFN-γ signaling pathway by downregulating the IFN-γ receptor and the downstream effector of IFN-γ [37]. And IL-10, a potent immunosuppressive cytokine, can induce Tregs and inhibit T helper 1 (Th1) cells and cytotoxic T lymphocytes (CTLs) [41]. In EBV-infected cells, LMP2A can induce the expression of IL-10, which also plays an immunosuppressive role in EBV-associated malignancies [27]. The interplay between EBV-infected cells’ viral proteins and immunosuppressive cytokines contributes to establishing a beneficial environment for tumor growth and development. However, further studies are required to investigate the role of EBV infection in TC.
EBV and tumor EVs
EVs are a significant component in facilitating intercellular communications in the TME. EBV can package various viral products, such as viral proteins and RNAs, into the EVs to aid in the evasion of the host immune system and promote tumor progression within the TME [42]. LMP1 is found to regulate programmed cell death ligand 1 (PD-L1) and increase the packaging of PD-L1 into exosomes [43]. However, further research is urgently needed to explore whether EVs can serve as potential specific therapeutic targets for EBV-related malignant tumors or be used for early diagnosis of latency infection, for example, how to inhibit the release of tumor EVs or suppress their binding to receptor cells. The TME is an indispensable interaction environment with the surrounding tissues in the biological behavior of the tumor. The mechanism of EBV in the TC TME is still not precise. Understanding the TME can help one better understand EBV’s tumorigenic mechanism, study the exact aspects of EBV life cycle diagnosis, and seek effective antiviral therapy targets. It is hoped that antiviral therapy will be a promising avenue in treating TC in the future.
EBV can infect epithelial cells and cause cytopathic effects (CPE) [44]. The tumorigenic transformation of epithelial cells by EBV is a long process and involves intricate interplay involving genetic alterations and transcription factors of host cells, stromal inflammation, and tumorigenic actions of lytic and latent EBV genes [45]. EBV-encoded genes, LMP-1, LMP-2A, EBER-1, EBER-2, and so on, were shown to be expressed at higher levels in thyroid tumor tissues than in healthy controls. EBV gene products can differently affect the expression levels of TC oncogenes, which can lead to the progression of TC. EBV may play an essential role in changing the immune microenvironment of PTC. EBV and coding genes can induce immune cells in the environment to produce immunosuppressive cytokines, affect immune checkpoints, and affect tumor cell progression through inflammation. Some inflammatory factors have been confirmed to be associated with thyroid tumor stage [14]. At present, it has been proved that EBV infection is closely related to tumors derived from multiple epithelial cells, especially focusing on nasopharyngeal carcinoma, gastric cancer, and lung cancer. However, reports on the association between EBV and TC are relatively limited. Whether EBV promotes thyroid tumorigenesis remains controversial, which is attributed to different methods applied and populations. Hence, retrospective clinical research and testing are needed to verify whether EBV plays a role in the metastasis of TC.
| HCV and TC | ▴Top |
HCV infection is a global health concern and a significant risk factor for cirrhosis, hepatocellular carcinoma (HCC), hepatic decompensation, and liver transplantation [46]. Chronic hepatitis C can also cause extrahepatic diseases and manifestations, in particular autoimmune disorders, such as mixed cryoglobulinemia (MC), Sjogren’s syndrome, and endocrinological diseases, including autoimmune thyroid disorders (AITD) [47]. Related reports involve an increased incidence of thyroid disorders in patients with HCV infection [48], and HCV infection may play a contributory role in thyroid tumorigenesis and progression [49]. In this review, we will explore the relationship between HCV and TC and how HCV affects the development and progression of thyroid tumors.
Several studies have reported the association between HCV infection and the risk of TC. However, the results were inconsistent. A meta-analysis was conducted to assess the impact of HCV infection on TC risk with a total of 751,551 individuals and 367 cases with TC. The results showed that there was no significant association between HCV infection and TC risk (summary risk ratio (RR): 2.09, 95% CI: 0.78 - 5.64, P = 0.145; I2 = 81.2%), but HCV infection was positively correlated with the risk of TC after adjusting the heterogeneity (summary RR: 2.86, 95% CI: 1.63 - 5.03, P = 0.003; I2 = 24.9%) [50]. On the contrary, another meta-analysis has demonstrated that patients with HCV infection have a greater risk of TC (summary OR: 16.36, 95% CI: 4.65 - 57.62, P < 0.001) [49]. The association between HCV infection and TC risk will need further confirmation, which may be attributed to a few cohort studies, high heterogeneity, and other factors (such as gender, age, and pathology classification, which may affect the incidence of TC). HCV is the cause of various autoimmune diseases, including hypothyroidism and autoimmune thyroid diseases, while autoimmune thyroiditis is a risk factor for TC [50-52]. As a result, research on the association between HCV infection and thyroid diseases has attracted more attention. Autoimmune thyroid involvement and hypothyroidism were more frequent in patients with chronic hepatitis C than in the comparison groups [53]. HCV infection can increase the risk of autoimmune thyroiditis (AITD) by causing immune system dysfunction, especially in those receiving IFN treatment, while AITD is a risk factor for TC [49]. Thus, viruses can activate both innate and adaptive immunity and may act as a trigger of Hashimoto’s thyroiditis (HT). The most crucial candidate viruses for AITD include HCV [54].
Meanwhile, it was noted that the interaction of HCV core protein with gC1qR could induce macrophages to secrete C-X-C motif chemotactic factor 10 (CXCL10) through the NF-κB signaling pathway [55]. Chronic HCV infection may upregulate CXCL10 expression and secretion in infected thyrocytes recruiting Th1 lymphocytes, which may be responsible for enhanced IFN-γ and TNF-α production, inducing a further CXCL10 secretion by a variety of cells and creating an amplification feedback loop which initiates and perpetuates the immune cascade, and results in the appearance of AITDs in genetically predisposed subjects (Fig. 2) [56, 57]. We can study the mechanism of chemokines and cytokines in the pathogenesis of autoimmune diseases, and further experiments need to explore whether CXCL10 can be used as a new therapeutic target for thyroid autoimmune disease.
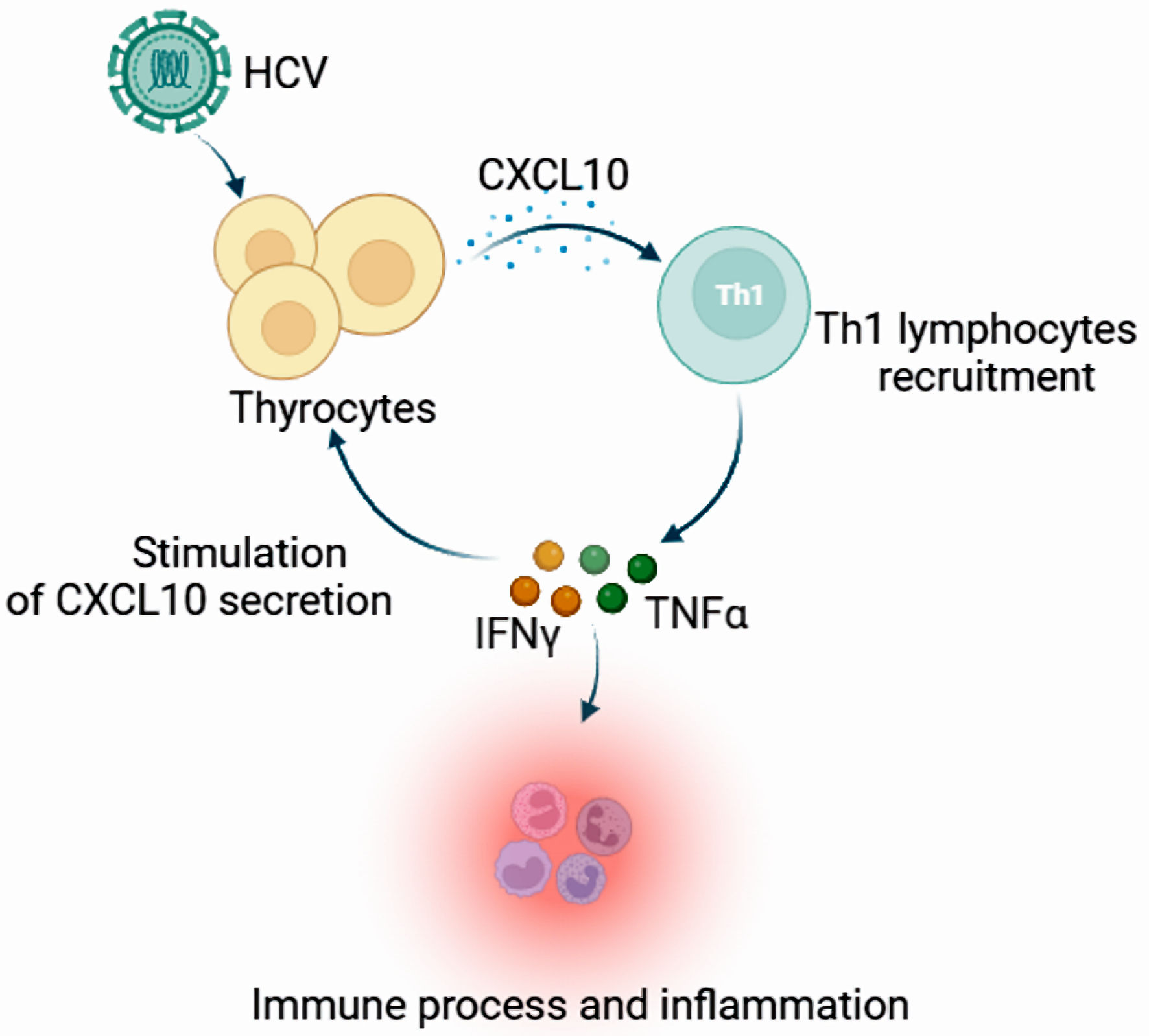 Click for large image | Figure 2. The expression and function of CXCL10 in the appearance of autoimmune thyroid disorders. Chronic HCV infection may upregulate CXCL10 expression and secretion in infected thyrocytes, recruiting Th1 lymphocytes that may be responsible for enhanced IFN-γ and TNF-α production, inducing a further CXCL10 secretion by a variety of cells and creating an amplification feedback loop which initiates and perpetuates the immune cascade, and results in the appearance of autoimmune thyroid disorders in genetically predisposed subjects. CXCL10: C-X-C motif chemotactic factor 10; HCV: hepatitis C virus; IFN-γ: interferon-γ; Th1 cells: T helper 1 cells; TNF-α: tumor necrosis factor-α. |
Thyroiditis with inflammatory and immune infiltration is a risk factor for thyroid tumors [58]. A study has shown that thyroid autoantibodies were observed in 83% of HCV patients with PTC and 31% of HCV patients without TC, suggesting people may suffer from an increased risk of TC after HCV infection [16]. However, HCV is an RNA virus that cannot be integrated into the host genome, and its oncogenic potential must be exerted through an indirect mechanism [59]. Therefore, we can hypothesize that autoimmune thyroid disease mediated by chronic HCV infection may lead to thyroid carcinogenesis.
In summary, there is still a lack of definite conclusion suggesting that HCV infection is significantly associated with an increased risk of TC, which may be due to insufficient samples, heterogeneity, and lack of pathological classification of TC. At present, the mechanism of how HCV affects TC is not well understood, mainly including immunity system and autoimmunity disorders. However, it is still a promising research topic because the awareness of the association between HCV and TC could play a vital role in the early diagnosis and improvement of prognosis in HCV patients. In the future, more well-designed research is needed to further identify the relationship between HCV and TC.
| HIV and TC | ▴Top |
HIV can lead to acquired immune deficiency syndrome (AIDS) and susceptibility to opportunistic infections and certain malignant tumors [60]. Chronic diseases, including cancer, have emerged as health problems in HIV-infected people in middle-income and high-income countries [61]. HIV-induced immune deficiency was the most common risk factor for developing malignancies [62]. HIV infection can cause many types of tumors. Among them, three types of tumors are directly related to AIDS such as non-Hodgkin lymphoma (NHL), cervical premalignant lesions, and Kaposi sarcoma (KS), which are defined as AIDS-defining cancers (ADCs). Non-AIDS-defining cancers (NADCs) included lung cancer, HCC, breast cancer, colorectal cancer (CRC), prostate cancer, HL, laryngeal cancer, and anal cancer [63, 64].
ADCs are strongly associated with immune suppression induced by HIV infection [65]. The incidence rate of NADCs has been rising, including TC, and NADCs are an increasingly important reason for the global increase in the incidence and mortality of people living with HIV (PLHIV) [66]. This trend is expected to continue to rise [67]. However, little is known about the impact of HIV infection on TC. Herein, we will explore the impact of HIV on the occurrence and development of TC.
It has been shown that HIV infection has an impact on the clinical pathological characteristics of PTC. HIV infection was a risk factor for larger tumors, more severe extrathyroidal extension (ETE), more lymph node metastasis, and more distant metastasis. HIV infection could promote proliferation and make PTC more aggressive [68]. A retrospective cohort study aimed to assess the prevalence of TC in a large cohort of HIV-infected patients showed that 11 cases were diagnosed with TC among 6,343 HIV-infected patients, including seven PTC cases, two MTC cases, one FTC, and one patient not recorded. Interestingly, compared with ordinary people, MTC appears to be more common in PLHIV [69]. Thus, there may be potential mechanisms between HIV infection and TC.
Immune surveillance deficiency
HIV-induced immune deficiency was the most common risk factor for developing malignancies [70]. HIV infection targets CD4 T cells, and immune responses progressively decrease, which allows cancer cells to escape immune surveillance and grow rapidly. Therefore, HIV infection could promote PTC proliferation, and HIV infection also makes PTC more aggressive, leading to capsular invasion, ETE, lymph node metastasis, and distant metastasis [68].
Secondary infection
At this point, it is difficult for the infected person with immune deficiency to provide specific immunity. Chronic inflammation caused by immunosuppression and viral persistence may also be involved in tumor formation and progression. For example, some viruses, such as human papillomavirus, cytomegalovirus, EBV, and herpes virus, promote tumorigenesis and the development of PTC [71], mainly due to HIV-induced immune deficiency, which reduces the ability to resist the infection and proliferation of carcinogenic pathogens in PLHIV.
Pituitary-thyroid hormone axis or direct effects on the thyroid
Studies have reported that hypothyroidism and mildly elevated thyroid-stimulating hormone (TSH) levels are more frequently observed in the HIV-positive population and that thyroid dysfunction is joint in newly diagnosed HIV-positive patients [68]. TSH is released from the anterior pituitary gland and is positively regulated by TSH-releasing hormones and negatively fed back by thyroid hormones triiodothyronine (T3) and thyroxine (T4) [72]. HIV infection frequently results in early and protracted disturbances of hypothalamus/pituitary and thyroid dysfunction, which could change TSH concentrations in serum [73]. Higher TSH proliferates PTC growth, most likely mediated by TSH receptors on tumor cells [68]. HIV may affect normal thyroid physiology by altering CD4 and CD8 [74]. Alternatively, HIV affects thyroid hormone metabolism by interacting with peripheral T3 receptors, activating the state of thyroid follicular cells [75]. However, these mechanisms still need to be confirmed.
Side effects of HIV drugs
Highly active antiretroviral therapy (HAART) has dramatically changed the overall survival rate of people living with HIV. HAART suppresses viral replication, restores immunity, and reduces mortality. However, HAART may alter the thyroid gland’s physiological functions and clinicopathological features through drug interactions or effects on the immune system. Some types of thyroid abnormalities are the result of treatment and are not caused by HIV infection [76]. The study shows that thyroid dysfunction was more common in HAART-naive HIV seropositive subjects than in the general population, with subclinical hypothyroidism emerging as the most common abnormality [77]. HAART may disrupt the thyroid hormone axis and change the thyroid-related cytokines, which affect the progression of thyroid disease and even tumors [68]. Although these mechanisms are currently unclear, evidence is needed to support them.
In summary, HIV infection is a risk factor for tumors. The nationwide cross-sectional ONCOVIH study suggested that compared with the general population, the RR of cancer in the HIV-infected population was 3.5 (age- and sex-standardized, 95% CI: 3.4 - 3.7) in France [78]. AIDS increases the susceptibility of PLHIV to opportunistic infections and cancer. With the widespread use of HAART, the survival cycle of AIDS patients has been extended, and the prevalence and mortality rate of NADCs have also increased. In this review, we briefly introduce information related to HIV infection and thyroid tumors and explore the possible mechanisms underlying HIV infection and TC. Furthermore, HIV and thyroid tumors are studied to understand the relationship between HIV and TC better and to discover relevant mechanistic pathways or specific targets for interventions to enhance the survival and quality of life of patients with HIV-NADC.
| SARS-CoV-2 and TC | ▴Top |
In December 2019, the recent coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is placing health systems in serious challenges worldwide. The main features of COVID-19 include fever, fatigue, headache, and other flu-like symptoms [79]. Currently, most treatments are symptomatic and supportive. However, treatments are of limited effectiveness. Notably, with the increased understanding of the SARS-CoV-2 and global vaccination efforts, the incidence and mortality of COVID-19 have declined. However, with most people worldwide infected or even repeatedly infected, the complications and sequelae of COVID-19 are increasingly receiving global attention. Compared with the catarrh of the upper respiratory tract, patients typically present with nasal congestion, rhinorrhea, sore throat, cough, general malaise, and low-grade fever [80]. It is more likely to suffer from extreme fatigue, muscle soreness, and dyspnea after infection with COVID-19, which led to the formulation of the novel term “post-COVID-19 syndrome” [81].
The causative pathogen of COVID-19, SARS-CoV-2, is a single-stranded RNA b-coronavirus 2 with a length of 29.9 kb. SARS-CoV-2 infection involves two crucial steps: the initial recognition of the receptor angiotensin-converting enzyme 2 (ACE2) via S protein, and then the effective fusion of the cell membrane via transmembrane protease serine 2 (TMPRSS2). ACE2 and TMPRSS2 are essentially involved in SARS-CoV-2 internalization into host cells, which plays a relevant role in the pathogenesis of COVID-19 in several species, including humans [82]. Both ACE2 and TMPRSS22 are highly expressed in the thyroid [83]. ACE2 and TMPRSS2 expression levels are high in the thyroid gland and more than in the lungs. These endocrine tissues may be susceptible to viral attack. SARS-CoV-2 RNA has been detected in the thyroid, implying that SARS-CoV-2 can infect the thyroid and alter the pituitary-thyroid axis [84]. SARS-CoV-2 infection has been reported to induce thyroid disease, including subacute thyroiditis, HT, thyrotoxicosis with thyroid dysfunction, and severe low triiodothyronine syndrome [85]. SARS-CoV-2 appears to influence thyroid function through multiple mechanisms. Due to high ACE expression in the thyroid, the critical molecular complex SARS-CoV-2 is used to infect the host cells. The thyroid may be a target for coronavirus infection [86]. In addition, SARS-CoV-2 may induce an aggressive inflammatory response and cytokine storm, causing damage to the airway and thyroid [87].
Previous findings [88-90] are the collection of expert opinions and recommendations on the new strategies of care of thyroid patients in the face of COVID-19 transmission risk and health care surge capacity, but there are few studies on the pathophysiology of thyroid tumors by SARS-CoV-2. As a result, the long-term impact of SARS-CoV-2 on thyroid function and structure will be observed.
There is an attractive secret between SARS-CoV-2, post-COVID-19 syndrome, and the occurrence and progress of thyroid tumors, which deserves the effort we put into studying it. In summary, significant achievements have been achieved in understanding the pathophysiology of the thyroid dysfunction caused by SARS-CoV-2 infection, which affects the diagnosis and treatment of TC. More molecular and clinical research is still needed to help us better understand the impact of COVID-19 on thyroid tumor development and malignant behavior. The potential mechanisms between HIV/SARS-CoV-2 and TC are shown in Table 1.
 Click to view | Table 1. The Potential Mechanisms Between HIV/SARS-COV-2 and TC |
| Advanced Therapy | ▴Top |
Some antiviral drugs may also process anticancer properties through specific targeted sites or signaling pathways. Ribavirin, an antiviral medication, has been identified as an inhibitor of eukaryotic initiation factor 4E (eIF4E) [91]. Shen et al demonstrates that ribavirin acts on TC cells by inhibiting eIF4E/β-catenin signaling, suggesting that ribavirin has the potential to be repurposed for TC treatment and highlighting the therapeutic value of inhibiting eIF4E-β-catenin in TC [92]. Other research showed that cidofovir (CDV) can reduce follicular thyroid carcinoma cell viability and apoptosis induction in a virus-independent manner. CDV may have therapeutic potential as an antineoplastic agent; in particular, considering its mode of action, CDV, in combination with radiation therapy and chemotherapeutics, may be expected to result in synergistic antitumor activity [93]. The HIV protease inhibitor nelfinavir (NFV) inhibits PI3K/AKT and mitogen-activated protein kinases/extracellular signal-regulated kinase (MAPK/ERK) signaling pathways, emerging targets in TCs. Jensen et al showed that NFV inhibits proliferation and induces DNA damage in TC cell lines [94] and expression of NFV molecular targets in metastatic MTC [95], suggesting that NFV has the potential to become a new TC therapeutic agent.
Exploring the potential characteristics of antiviral drugs is a significant theme in drug development and utilization. Studying the molecular pathogenesis of thyroid tumors can also increase the number of anticancer drugs. Antiviral medicaments are the ones where several observations provide a stimulus to research further, which possibly supplements the treatment of TC and can combine with other treatment methods to improve patient prognosis.
| Prospective | ▴Top |
With the deepening of the research, more evidence suggests that viruses participate in the occurrence and development of TC. Viruses may emerge as a trigger, a risk factor, an inhibitory factor, or a carcinogen in the progression of TC. The definite link between the virus and TC lies in the mechanism of infection and survival of host cells at the modular levels. Therefore, comprehensive knowledge may provide insights into finding molecular targets for diagnosing and treating virus-related TC.
Several limitations in this study need to be considered. Firstly, the mechanisms of viral carcinogenesis are not completely clear. In addition, antiviral therapy is still under exploration, and further clinical trials are needed to verify its efficacy and safety.
| Conclusions | ▴Top |
The incidence of TC has increased over the past several decades with the wide and sensitive application of ultrasonography worldwide. It has also been reported that there was a possible association between viral infection and the occurrence of TC. Viruses, including EBV, HCV, HIV, and SARS-CoV-2, play a significant role in the progression of TC by recognizing the host cell, binding to the receptors, affecting oncogenes levels, and releasing viral products to shape a beneficial environment, interacting with immune cells to induce immune evasion and altering the pituitary-thyroid axis, which can maintain continuous infection in host cells. However, the exact mechanisms still require more molecular and clinical research. Antiviral therapies have demonstrated promising antiviral effects on TC, which may provide more options for patients with TC. Therefore, further research should be performed on the relationship between virus and TC, which can potentially affect the diagnosis, treatment, and management of TC (Fig. 3).
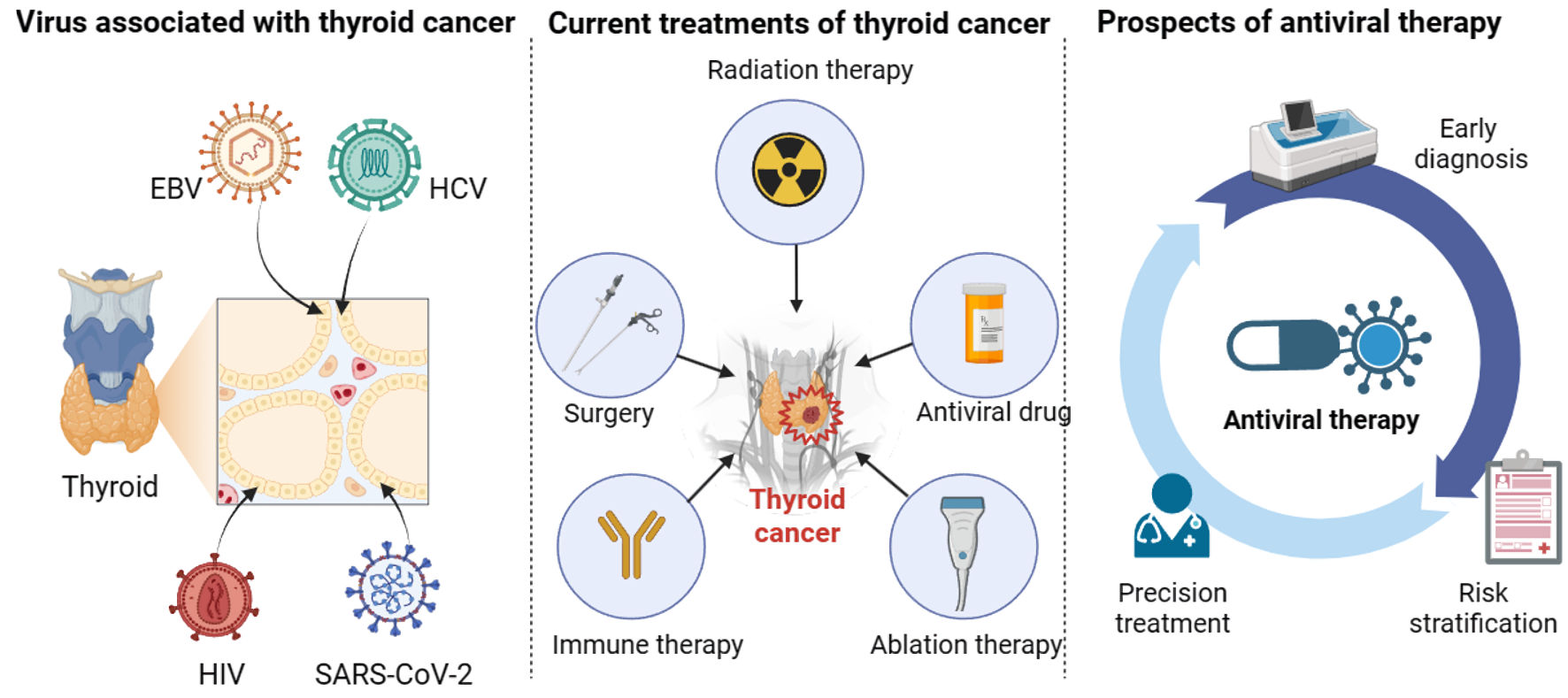 Click for large image | Figure 3. The relationship between viruses and thyroid cancer. Viruses, including EBV, HCV, HIV, and SARS-CoV-2, play an essential role in the progression of thyroid cancer. In addition to surgery, radiation therapy, ablation therapy, and immune therapy, antiviral therapy can facilitate the treatment and management of thyroid cancer patients in terms of early diagnosis, risk stratification, and precision treatment. EBV: Epstein-Barr virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2. |
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by the Natural Science Basic Research Program of Shaanxi (2022JZ-60) and the Exploration Research Foundation of the Second Affiliated Hospital of Xi’an Jiaotong University (2020YJ(ZYTS) 607 and 608).
Conflict of Interest
The authors declare that they have no known conflict of interest or personal relationships that could have appeared to influence the work reported in this paper.
Author Contributions
Yong Ke Wu: writing - original draft, visualization. Tian Tian Jiang: writing - original draft, visualization. Yuan Hao Su: review and editing. Lin Mei: review and editing. Ting Kai Sun: review and editing. Yun Hao Li: review and editing. Zhi Dong Wang: conceptualization, funding acquisition, project administration, supervision, writing - review and editing. Yuan Yuan Ji: conceptualization, funding acquisition, project administration, supervision, writing - review and editing.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
TC: thyroid cancer; EBV: Epstein-Barr virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; PTC: papillary thyroid cancer; FTC: follicular thyroid cancer; MTC: medullary thyroid cancer; ATC: anaplastic thyroid cancer; TBSRTC: The Bethesda System for Reporting Thyroid Cytopathology; ROM: risk of malignancy; EVB19: erythrovirus B19; NK cell: natural killer cell; EBNA1: Epstein-Barr virus nuclear antigen 1; PCR: polymerase chain reaction; qPCR: quantitative real-time PCR; EBER: EBV-encoded RNA; ISH: in situ hybridization; gp350/220: glycoprotein 350/220; CR2 or CD21: complement receptor type 2; LMP: latent membrane protein; miRNA: microRNA; TME: tumor microenvironment; IL-1: interleukin-1; TNF-α: tumor necrosis factor-α; TGF-β: transforming growth factor-β; NF-κB: nuclear factor kappa-B; DCs: dendritic cells; BLZF1: basic leucine zipper nuclear factor 1; IFN-γ: interferon-γ; Th1 cells: T helper 1 cells; CTLs: cytotoxic T lymphocytes; EVs: extracellular vesicles; PD-L1: programmed cell death ligand 1; CPE: cytopathic effect; HCC: hepatocellular carcinoma; MC: mixed cryoglobulinemia; AITD: autoimmune thyroid disorders; HT: Hashimoto’s thyroiditis; CHC: chronic hepatitis C; CXCL10: C-X-C motif chemotactic factor 10; AIDS: acquired immune deficiency syndrome; CD: cluster determinant; HAART: highly active antiretroviral therapy; NHL: non-Hodgkin lymphoma; KS: Kaposi sarcoma; ADCs: AIDS-defining cancers; NADCs: non-AIDS-defining cancers; PLHIV: people living with HIV; ETE: extrathyroidal extension; TSH: thyroid-stimulating hormone; T3: triiodothyronine; T4: thyroxine; COVID-19: coronavirus disease 2019; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ACE2: angiotensin-converting enzyme 2; TMPRSS2: transmembrane protease serine 2; FNA: fine-needle aspiration; PD-1: programmed cell death protein 1; CTLA4: cytotoxic T lymphocyte antigen 4; eIF4E: eukaryotic initiation factor 4E; CDV: cidofovir; NFV: nelfinavir; PI3K: phosphoinositide 3-kinase; AKT: protein kinase B; MAPK: mitogen-activated protein kinases; ERK: extracellular signal-regulated kinase; gB: glycoprotein B; gH: glycoprotein H; gL: glycoprotein L; NRP1: neuropilin 1; EphA2: ephrin receptor A2
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Han B, Zheng R, Zeng H, Wang S, Sun K, Chen R, Li L, et al. Cancer incidence and mortality in China, 2022. Journal of the National Cancer Center. 2024
- Alexander EK, Doherty GM, Barletta JA. Management of thyroid nodules. Lancet Diabetes Endocrinol. 2022;10(7):540-548.
doi pubmed - Wong R, Farrell SG, Grossmann M. Thyroid nodules: diagnosis and management. Med J Aust. 2018;209(2):92-98.
doi pubmed - Kobaly K, Kim CS, Mandel SJ. Contemporary management of thyroid nodules. Annu Rev Med. 2022;73:517-528.
doi pubmed - Cibas ES, Ali SZ. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid. 2017;27(11):1341-1346.
doi pubmed - Sengul I, Sengul D. Blurred lines for management of thyroid nodules in the era of atypia of undetermined significance/ follicular lesion of undetermined significance: novel subdivisions of categories IIIA and IIIB in a possible forthcoming The Bethesda System for Reporting Thyroid Cytopathology, 3rd edition; amending versus unnecessary? Rev Assoc Med Bras (1992). 2021;67(10):1385-1386.
doi pubmed - Sengul I, Sengul D. The 2023 Bethesda system for reporting thyroid cytopathology: novi sub sole, subdivision is no more debatable, in thyroidology. Rev Assoc Med Bras (1992). 2023;69(12):e20231124.
doi pubmed pmc - Sengul I, Sengul D. Comment on: "Evaluating treatment options in managing thyroid nodules with indeterminate cytology of TBSRTC in thyroidology: addendum aut non?". Rev Assoc Med Bras (1992). 2022;68(7):973-974.
doi pubmed pmc - Sengul I, Sengul D. Apropos of quality for fine-needle aspiration cytology of thyroid nodules with 22-, 23-, 25-, even 27-gauge needles and indeterminate cytology in thyroidology: an aide memory. Rev Assoc Med Bras (1992). 2022;68(8):987-988.
doi pubmed pmc - Sengul D, Sengul I. Subdivision of intermediate suspicion, the 2021 K-TIRADS, and category III, indeterminate cytology, the 2017 TBSRTC, 2nd edition, in thyroidology: let bygones be bygones? Ultrasonography. 2023;42(4):600-601.
doi pubmed pmc - Marcello MA, Malandrino P, Almeida JF, Martins MB, Cunha LL, Bufalo NE, Pellegriti G, et al. The influence of the environment on the development of thyroid tumors: a new appraisal. Endocr Relat Cancer. 2014;21(5):T235-254.
doi pubmed - Almeida JFM, Campos AH, Marcello MA, Bufalo NE, Rossi CL, Amaral LHP, Marques AB, et al. Investigation on the association between thyroid tumorigeneses and herpesviruses. J Endocrinol Invest. 2017;40(8):823-829.
doi pubmed - Moghoofei M, Mostafaei S, Nesaei A, Etemadi A, Sadri Nahand J, Mirzaei H, Rashidi B, et al. Epstein-Barr virus and thyroid cancer: The role of viral expressed proteins. J Cell Physiol. 2019;234(4):3790-3799.
doi pubmed - Etemadi A, Mostafaei S, Yari K, Ghasemi A, Minaei Chenar H, Moghoofei M. Detection and a possible link between parvovirus B19 and thyroid cancer. Tumour Biol. 2017;39(6):1010428317703634.
doi pubmed - Antonelli A, Ferri C, Fallahi P, Pampana A, Ferrari SM, Barani L, Marchi S, et al. Thyroid cancer in HCV-related chronic hepatitis patients: a case-control study. Thyroid. 2007;17(5):447-451.
doi pubmed - Wang F, Xiang P, Zhao H, Gao G, Yang D, Xiao J, Han N, et al. A retrospective study of distribution of HIV associated malignancies among inpatients from 2007 to 2020 in China. Sci Rep. 2021;11(1):24353.
doi pubmed pmc - Mostafaei S, Keshavarz M, Sadri Nahand J, Farhadi Hassankiadeh R, Moradinazar M, Nouri M, Babaei F, et al. Viral infections and risk of thyroid cancer: A systematic review and empirical bayesian meta-analysis. Pathol Res Pract. 2020;216(4):152855.
doi pubmed - Pierangeli A, Antonelli G, Gentile G. Immunodeficiency-associated viral oncogenesis. Clin Microbiol Infect. 2015;21(11):975-983.
doi pubmed - Read SA, Douglas MW. Virus induced inflammation and cancer development. Cancer Lett. 2014;345(2):174-181.
doi pubmed - Kitsou K, Iliopoulou M, Spoulou V, Lagiou P, Magiorkinis G. Viral causality of human cancer and potential roles of human endogenous retroviruses in the Multi-Omics era: an evolutionary epidemiology review. Front Oncol. 2021;11:687631.
doi pubmed pmc - Yu H, Robertson ES. Epstein-Barr virus history and pathogenesis. Viruses. 2023;15(3):714.
doi pubmed pmc - Chakravorty S, Afzali B, Kazemian M. EBV-associated diseases: current therapeutics and emerging technologies. Front Immunol. 2022;13:1059133.
doi pubmed pmc - Almeida JFM, Peres KC, Teixeira ES, Teodoro L, Bo IFD, Ward LS. Epstein-Barr virus and thyroid cancer. Crit Rev Oncog. 2019;24(4):369-377.
doi pubmed - Yu ST, Ge JN, Li RC, Wei ZG, Sun BH, Jiang YM, Luo JY, et al. Is Epstein-Barr virus infection associated with thyroid tumorigenesis? A Southern China cohort study. Front Oncol. 2019;9:312.
doi pubmed pmc - Homayouni M, Mohammad Arabzadeh SA, Nili F, Razi F, Amoli MM. Evaluation of the presence of Epstein-Barr virus (EBV) in Iranian patients with thyroid papillary carcinoma. Pathol Res Pract. 2017;213(7):854-856.
doi pubmed - Incrocci R, Barse L, Stone A, Vagvala S, Montesano M, Subramaniam V, Swanson-Mungerson M. Epstein-Barr Virus Latent Membrane Protein 2A (LMP2A) enhances IL-10 production through the activation of Bruton's tyrosine kinase and STAT3. Virology. 2017;500:96-102.
doi pubmed pmc - Bychkov A, Keelawat S. Epstein-Barr virus and thyroid cancer: the controversy remains. J Endocrinol Invest. 2017;40(8):891-892.
doi pubmed - Stamatiou D, Derdas SP, Symvoulakis EK, Sakorafas GH, Zoras O, Spandidos DA. Investigation of BK virus, Epstein-Barr virus and human papillomavirus sequences in postoperative thyroid gland specimens. Int J Biol Markers. 2015;30(1):e104-110.
doi pubmed - Munz C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat Rev Microbiol. 2019;17(11):691-700.
doi pubmed - Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350(13):1328-1337.
doi pubmed - Buschle A, Hammerschmidt W. Epigenetic lifestyle of Epstein-Barr virus. Semin Immunopathol. 2020;42(2):131-142.
doi pubmed pmc - Scholle F, Bendt KM, Raab-Traub N. Epstein-Barr virus LMP2A transforms epithelial cells, inhibits cell differentiation, and activates Akt. J Virol. 2000;74(22):10681-10689.
doi pubmed pmc - Chen J, Longnecker R. Epithelial cell infection by Epstein-Barr virus. FEMS Microbiol Rev. 2019;43(6):674-683.
doi pubmed pmc - Connolly SA, Jackson JO, Jardetzky TS, Longnecker R. Fusing structure and function: a structural view of the herpesvirus entry machinery. Nat Rev Microbiol. 2011;9(5):369-381.
doi pubmed pmc - Xiao J, Palefsky JM, Herrera R, Berline J, Tugizov SM. EBV BMRF-2 facilitates cell-to-cell spread of virus within polarized oral epithelial cells. Virology. 2009;388(2):335-343.
doi pubmed pmc - Zheng X, Huang Y, Li K, Luo R, Cai M, Yun J. Immunosuppressive tumor microenvironment and immunotherapy of Epstein-Barr virus-associated malignancies. Viruses. 2022;14(5):1017.
doi pubmed pmc - Khodabandehlou N, Mostafaei S, Etemadi A, Ghasemi A, Payandeh M, Hadifar S, Norooznezhad AH, et al. Human papilloma virus and breast cancer: the role of inflammation and viral expressed proteins. BMC Cancer. 2019;19(1):61.
doi pubmed pmc - Xie Z, Li X, He Y, Wu S, Wang S, Sun J, He Y, et al. Immune Cell Confrontation in the Papillary Thyroid Carcinoma Microenvironment. Front Endocrinol (Lausanne). 2020;11:570604.
doi pubmed pmc - Mostafaei S, Kazemnejad A, Norooznezhad AH, Mahaki B, Moghoofei M. Simultaneous effects of viral factors of human papilloma virus and epstein-barr virus on progression of breast and thyroid cancers: application of structural equation modeling. Asian Pac J Cancer Prev. 2020;21(5):1431-1439.
doi pubmed pmc - Ren Y, Yang J, Li M, Huang N, Chen Y, Wu X, Wang X, et al. Viral IL-10 promotes cell proliferation and cell cycle progression via JAK2/STAT3 signaling pathway in nasopharyngeal carcinoma cells. Biotechnol Appl Biochem. 2020;67(6):929-938.
doi pubmed - Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H. Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells. J Virol. 2013;87(18):10334-10347.
doi pubmed pmc - Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382-386.
doi pubmed pmc - Almeida JFM, Proenca-Modena JL, Bufalo NE, Peres KC, de Souza Teixeira E, Teodoro L, Beck RM, et al. Epstein-Barr virus induces morphological and molecular changes in thyroid neoplastic cells. Endocrine. 2020;69(2):321-330.
doi pubmed - Tsao SW, Tsang CM, Pang PS, Zhang G, Chen H, Lo KW. The biology of EBV infection in human epithelial cells. Semin Cancer Biol. 2012;22(2):137-143.
doi pubmed - Liu CH, Kao JH. Acute hepatitis C virus infection: clinical update and remaining challenges. Clin Mol Hepatol. 2023;29(3):623-642.
doi pubmed pmc - Adamson LA, Fowler LJ, Ewald AS, Clare-Salzler MJ, Hobbs JA. Infection and persistence of erythrovirus B19 in benign and cancerous thyroid tissues. J Med Virol. 2014;86(9):1614-1620.
doi pubmed - Montella M, Pezzullo L, Crispo A, Izzo F, Amore A, Marone U, Tamburini M, et al. Risk of thyroid cancer and high prevalence of hepatitis C virus. Oncol Rep. 2003;10(1):133-136.
pubmed - Wang P, Jing Z, Liu C, Xu M, Wang P, Wang X, Yin Y, et al. Hepatitis C virus infection and risk of thyroid cancer: A systematic review and meta-analysis. Arab J Gastroenterol. 2017;18(1):1-5.
doi pubmed - Wang H, Liu Y, Zhao Y. The association of hepatitis C virus infection and thyroid disease: A systematic review and meta-analysis. Int J Biol Markers. 2021;36(4):3-9.
doi pubmed - Molleston JP, Mellman W, Narkewicz MR, Balistreri WF, Gonzalez-Peralta RP, Jonas MM, Lobritto SJ, et al. Autoantibodies and autoimmune disease during treatment of children with chronic hepatitis C. J Pediatr Gastroenterol Nutr. 2013;56(3):304-310.
doi pubmed pmc - Obermayer-Straub P, Manns MP. Hepatitis C and D, retroviruses and autoimmune manifestations. J Autoimmun. 2001;16(3):275-285.
doi pubmed - Ferri C, Colaci M, Fallahi P, Ferrari SM, Antonelli A, Giuggioli D. Thyroid involvement in hepatitis C virus-infected patients with/without mixed cryoglobulinemia. Front Endocrinol (Lausanne). 2017;8:159.
doi pubmed pmc - Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5.
doi pubmed pmc - Song X, Gao X, Wang Y, Raja R, Zhang Y, Yang S, Li M, et al. HCV core protein induces chemokine CCL2 and CXCL10 expression through NF-kappaB signaling pathway in macrophages. Front Immunol. 2021;12:654998.
doi pubmed pmc - Antonelli A, Ferrari SM, Ruffilli I, Fallahi P. Cytokines and HCV-related autoimmune disorders. Immunol Res. 2014;60(2-3):311-319.
doi pubmed - Fallahi P, Ferrari SM, Politti U, Giuggioli D, Ferri C, Antonelli A. Autoimmune and neoplastic thyroid diseases associated with hepatitis C chronic infection. Int J Endocrinol. 2014;2014:935131.
doi pubmed pmc - Boi F, Pani F, Mariotti S. Thyroid autoimmunity and thyroid cancer: review focused on cytological studies. Eur Thyroid J. 2017;6(4):178-186.
doi pubmed pmc - Muhanna N, Amer J, Salhab A, Sichel JY, Safadi R. The Immune Interplay between Thyroid Papillary Carcinoma and Hepatic Fibrosis. PLoS One. 2015;10(7):e0132463.
doi pubmed pmc - McLaren PJ, Fellay J. HIV-1 and human genetic variation. Nat Rev Genet. 2021;22(10):645-657.
doi pubmed pmc - Park B, Ahn KH, Choi Y, Kim JH, Seong H, Kim YJ, Choi JY, et al. Cancer incidence among adults with HIV in a population-based cohort in Korea. JAMA Netw Open. 2022;5(8):e2224897.
doi pubmed pmc - Shmakova A, Germini D, Vassetzky Y. HIV-1, HAART and cancer: a complex relationship. Int J Cancer. 2020;146(10):2666-2679.
doi pubmed - Ceccarelli M, Venanzi Rullo E, Marino MA, d'Aleo F, Pellicano GF, D'Andrea F, Marino A, et al. Non-AIDS defining cancers: a comprehensive update on diagnosis and management. Eur Rev Med Pharmacol Sci. 2020;24(7):3849-3875.
doi pubmed - Mitsuyasu RT. Non-AIDS-defining cancers. Top Antivir Med. 2014;22(3):660-665.
pubmed pmc - Ji Y, Lu H. Malignancies in HIV-infected and AIDS patients. Adv Exp Med Biol. 2017;1018:167-179.
doi pubmed - Chiao EY, Coghill A, Kizub D, Fink V, Ndlovu N, Mazul A, Sigel K. The effect of non-AIDS-defining cancers on people living with HIV. Lancet Oncol. 2021;22(6):e240-e253.
doi pubmed pmc - Yuan T, Hu Y, Zhou X, Yang L, Wang H, Li L, Wang J, et al. Incidence and mortality of non-AIDS-defining cancers among people living with HIV: A systematic review and meta-analysis. EClinicalMedicine. 2022;52:101613.
doi pubmed pmc - Liu J, Wu D, Zhu J, Dong S. Clinicopathological features of papillary thyroid carcinoma in HIV-infected patients. Front Oncol. 2023;13:1071923.
doi pubmed pmc - Properzi M, Della Giustina T, Mentasti S, Castelli F, Chiesa A, Gregori N, Quiros-Roldan E. Low prevalence of symptomatic thyroid diseases and thyroid cancers in HIV-infected patients. Sci Rep. 2019;9(1):19459.
doi pubmed pmc - Proulx J, Ghaly M, Park IW, Borgmann K. HIV-1-mediated acceleration of oncovirus-related non-AIDS-defining cancers. Biomedicines. 2022;10(4):768.
doi pubmed pmc - Pereira LMS, Franca EDS, Costa IB, Lima IT, Freire ABC, Ramos FLP, Monteiro TAF, et al. Epstein-Barr Virus (EBV) genotypes associated with the immunopathological profile of people living with HIV-1: immunological aspects of primary EBV infection. Viruses. 2022;14(2):168.
doi pubmed pmc - Xu B, Gu SY, Zhou NM, Jiang JJ. Association between thyroid stimulating hormone levels and papillary thyroid cancer risk: A meta-analysis. Open Life Sci. 2023;18(1):20220671.
doi pubmed pmc - Collazos J, Ibarra S, Mayo J. Thyroid hormones in HIV-infected patients in the highly active antiretroviral therapy era: evidence of an interrelation between the thyroid axis and the immune system. AIDS. 2003;17(5):763-765.
doi pubmed - Emokpae MA, Akinnuoye IM. Asymptomatic thyroid dysfunction in human immunodeficiency virus-1-infected subjects. J Lab Physicians. 2018;10(2):130-134.
doi pubmed pmc - Hsia SC, Wang H, Shi YB. Involvement of chromatin and histone acetylation in the regulation of HIV-LTR by thyroid hormone receptor. Cell Res. 2001;11(1):8-16.
doi pubmed - Weetman AP. Thyroid abnormalities. Endocrinol Metab Clin North Am. 2014;43(3):781-790.
doi pubmed - Ugwueze CV, Young EE, Unachukwu CN, Onyenekwe BM, Nwatu CB, Okafor CI, Ezeude CM, et al. The prevalence and pattern of thyroid dysfunction in HAART-naive HIV patients in Enugu, Nigeria: a cross-sectional comparative study. West Afr J Med. 2021;38(12):1200-1205.
pubmed - Lanoy E, Spano JP, Bonnet F, Guiguet M, Boue F, Cadranel J, Carcelain G, et al. The spectrum of malignancies in HIV-infected patients in 2006 in France: the ONCOVIH study. Int J Cancer. 2011;129(2):467-475.
doi pubmed - Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506.
doi pubmed pmc - DeGeorge KC, Ring DJ, Dalrymple SN. Treatment of the common cold. Am Fam Physician. 2019;100(5):281-289.
pubmed - Dotan A, David P, Arnheim D, Shoenfeld Y. The autonomic aspects of the post-COVID19 syndrome. Autoimmun Rev. 2022;21(5):103071.
doi pubmed pmc - Lam SD, Bordin N, Waman VP, Scholes HM, Ashford P, Sen N, van Dorp L, et al. SARS-CoV-2 spike protein predicted to form complexes with host receptor protein orthologues from a broad range of mammals. Sci Rep. 2020;10(1):16471.
doi pubmed pmc - Qu N, Hui Z, Shen Z, Kan C, Hou N, Sun X, Han F. Thyroid cancer and COVID-19: prospects for therapeutic approaches and drug development. Front Endocrinol (Lausanne). 2022;13:873027.
doi pubmed pmc - Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45.
doi pubmed pmc - Ruggeri RM, Campenni A, Deandreis D, Siracusa M, Tozzoli R, Petranovic Ovcaricek P, Giovanella L. SARS-COV-2-related immune-inflammatory thyroid disorders: facts and perspectives. Expert Rev Clin Immunol. 2021;17(7):737-759.
doi pubmed pmc - Boaventura P, Macedo S, Ribeiro F, Jaconiano S, Soares P. Post-COVID-19 Condition: Where Are We Now? Life (Basel). 2022;12(4):517.
doi pubmed pmc - Trimboli P, Cappelli C, Croce L, Scappaticcio L, Chiovato L, Rotondi M. COVID-19-associated subacute thyroiditis: evidence-based data from a systematic review. Front Endocrinol (Lausanne). 2021;12:707726.
doi pubmed pmc - Giannoula E, Iakovou I, Giovanella L, Vrachimis A. Updated clinical management guidance during the COVID-19 pandemic: thyroid nodules and cancer. Eur J Endocrinol. 2022;186(4):G1-G7.
doi pubmed pmc - Smulever A, Abelleira E, Bueno F, Pitoia F. Thyroid cancer in the Era of COVID-19. Endocrine. 2020;70(1):1-5.
doi pubmed pmc - Chablani SV, Sabra MM. Thyroid cancer and telemedicine during the COVID-19 pandemic. J Endocr Soc. 2021;5(6):bvab059.
doi pubmed pmc - Xi C, Wang L, Yu J, Ye H, Cao L, Gong Z. Inhibition of eukaryotic translation initiation factor 4E is effective against chemo-resistance in colon and cervical cancer. Biochem Biophys Res Commun. 2018;503(4):2286-2292.
doi pubmed - Shen X, Zhu Y, Xiao Z, Dai X, Liu D, Li L, Xiao B. Antiviral drug ribavirin targets thyroid cancer cells by inhibiting the eIF4E-beta-catenin axis. Am J Med Sci. 2017;354(2):182-189.
doi pubmed - Catalani S, Palma F, Battistelli S, Nuvoli B, Galati R, Benedetti S. Reduced cell viability and apoptosis induction in human thyroid carcinoma and mesothelioma cells exposed to cidofovir. Toxicol In Vitro. 2017;41:49-55.
doi pubmed - Jensen K, Bikas A, Patel A, Kushchayeva Y, Costello J, McDaniel D, Burman K, et al. Nelfinavir inhibits proliferation and induces DNA damage in thyroid cancer cells. Endocr Relat Cancer. 2017;24(3):147-156.
doi pubmed - Kushchayeva Y, Jensen K, Recupero A, Costello J, Patel A, Klubo-Gwiezdzinska J, Boyle L, et al. The HIV protease inhibitor nelfinavir down-regulates RET signaling and induces apoptosis in medullary thyroid cancer cells. J Clin Endocrinol Metab. 2014;99(5):E734-745.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


