| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 1, Number 3, June 2010, pages 101-110
Intraperitoneal Alpha-Radioimmunotherapy of Advanced Ovarian Cancer in Nude Mice Using Different High Specific Activities
Jorgen Elgqvista, e, Daniel Ahlbergb, Hakan Anderssona, Holger Jensenc, Bengt R Johanssond, Helena Kahua, Marita Olssonb, Sture Lindegrena
aInstitute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, Sweden
bMathematical Sciences, University of Gothenburg and Chalmers University of Technology, Sweden
cPET and Cyclotron Unit, Rigshospitalet, Copenhagen, Denmark
dElectron Microscopy Unit, Institute of Biomedicine, Sahlgrenska Academy, University of Gothenburg, Sweden
eCorresponding author: Institute of Clinical Sciences, Sahlgrenska Academy, University of Gothenburg, SE-413 45 Gothenburg, Sweden. E-mail:
Manuscript accepted for publication May 11, 2010
Short title: Alpha-RIT of Ovarian Cancer
doi: https://doi.org/10.4021/wjon2010.05.208w
| Abstract | ▴Top |
Background: The aim of this study was to investigate the therapeutic efficacy of advanced ovarian cancer in mice, using α-radioimmunotherapy with different high specific activities. The study was performed using the monoclonal antibody (mAb) MX35 F(ab′)2 labeled with the α-particle emitter 211At.
Methods: Animals were intraperitoneally inoculated with ≥1 × 107 cells of the ovarian cancer cell line NIH:OVCAR-3. Four weeks later 9 groups of animals were given 25, 50, or 400 kBq 211At-MX35 F(ab′)2 with specific activities equal to 1/80, 1/500, or 1/1200 (211At atom/number of mAbs) for every activity level respectively (n = 10 in each group). As controls, animals were given PBS or unlabeled MX35 F(ab′)2 in PBS (n = 10 in each group). Eight weeks after treatment the animals were sacrificed and the presence of macroscopic tumors was determined by meticulous ocular examination of the abdominal cavity. Cumulated activity and absorbed dose calculations on tumor cells and tumors were performed using in house developed program. Specimens for scanning electron-microscopy analysis were collected from the peritoneum at the time of dissection.
Results: Summing over the different activity levels (25, 50, and 400 kBq 211At-MX35 F(ab′)2) the number of animals with macroscopic tumors was 13, 17, and 22 (n = 30 for each group) for the specific activities equal to 1/80, 1/500, or 1/1200, respectively. Logistic-regression analysis showed a significant trend that higher specific activity means less probability for macroscopic tumors (P = 0.02).
Conclusions: Increasing the specific activity indicates a way to enhance the therapeutic outcome of advanced ovarian cancer, regarding macroscopic tumors. Further studies of the role of the specific activity are therefore justified.
Keywords: Radioimmunotherapy; Alpha; 211At; Astatine; Specific activity; Intraperitoneal; Ovarian cancer; Mice
| Introduction | ▴Top |
Ovarian cancer frequently recurs from remaining micrometastatic growth on the peritoneal surface in spite of debulking surgery and systemic chemotherapy. External abdominal radiotherapy has proven unsuccessful due to absorbed dose limitations in normal tissues. Therefore, adjuvant locoregional treatment with intraperitoneal administration of specific antibodies could be decisive in the treatment of remaining micrometastatic disease. Generally, clinical application of 211At-labeled mAbs is a promising research field and has to be investigated further [1]. In this study we used the monoclonal antibody (mAb) MX35 F(ab′)2, which recognizes the sodium dependant phosphate transport protein 2b (NaPi2b) of 90 kDa on ovarian cancer cells. The cytotoxicity was mediated by labeling the mAbs with an α-particle-emitting radionuclide, 211At. We used an animal model mimicking the clinical situation with intraperitoneal radioimmunotherapy (RIT). The intraperitoneal approach allows a high absorbed dose to non-vascularized peritoneal tumors with low myelotoxicity as the clearance rate from the peritoneal cavity to the systemic circulation is delayed [2].
Studies have been performed using RIT against ovarian cancer, mostly mAbs labeled with 90Y, 177Lu, or 131I in animals [3-14] and in humans [7, 15-20]. The β-emitting radionuclides have too long a range for the treatment of microscopic tumors [21], thus we believe it is important to continue the investigations of the efficacy of mAbs labeled with α-particle emitters when treating microscopic disease on the peritoneum. In this study, as in a series of earlier studies [22-29], we used the α-particle emitter 211At, with a half-life of 7.2 h, a mean range in tissue of 62 µm, and a mean linear energy transfer (LET) of 111 keV/µm. The half-life of this radionuclide makes it ideal for local treatment as the target cells are easily reached while the transfer of the radioimmunocomplex to the systemic circulation is delayed. The short range ensures a significant absorbed dose in microscopic tumors or even single tumor cells. The high LET, together with the high relative biological effectiveness (RBE) of the α-particles necessitating only a few hits to kill the cell, indicates that only a small number of 211At-atoms have to be targeted to each cell [2, 30, 31].
As indicated in earlier studies the specific activity could be a decisive parameter deciding whether a treatment will be successful or not [25, 28]. The advantages with administering a radioimmunocomplex with a high specific activity we believe are: (i) the ability to treat low antigen expressing tumor and/or tumors having restricted diffusion; and (ii) the ability to treat more patients per unit radioactivity. A new radiolabeling technique enables us now to substantially achieve higher specific activities [32]. Therefore, the aim of the study was to investigate the therapeutic efficacy of α-RIT of ovarian cancer in nude mice using different high specific activities. The study is the first one investigating the role of increasing the specific activity during RIT. The study was performed using the mAb MX35 F(ab′)2 labeled with the α-particle emitter 211At.
A phase I study on women, with recurrent epithelial ovarian cancer after second-line chemotherapy treated with 211At-MX35 F(ab′)2, was published in 2009 [33]. Since we now are planning for a phase II study, intended as a “boost” treatment for women having had cytoreductive surgery and first-line chemotherapy, it is of outmost importance to investigate different aspects of the therapeutic efficacy, among them the role of higher specific activities.
| Materials and Methods | ▴Top |
Radionuclide
211At was produced by the 209Bi(α, 2n)211At reaction in a cyclotron (Scanditronix MC32 at the Positron Emission Tomography and Cyclotron Unit, Rigshospitalet, Copenhagen, Denmark) by irradiating a 209Bi target with 28-MeV α-particles. The 211At was isolated using a dry-distillation procedure [34].
Monoclonal Antibodies
MX35 is a murine IgG1-class mAb, developed and characterized at the Memorial Sloan-Kettering Cancer Center (MSKCC), New York, USA. MX35 is directed towards the sodium dependant phosphate transport protein 2b (NaPi2b) of 90 kDa on OVCAR-3 cells [35] and is expressed strongly and homogeneously on 90% of human epithelial ovarian cancers [36]. A batch of MX35 F(ab′)2, produced by Strategic BioSolutions (Newark, USA) for clinical use, was provided by MSKCC.
Antibody Conjugation and Radiolabeling
The MX35 F(ab′)2 antibody was labeled via the intermediate labeling reagent N-succinimidyl-3-(trimethylstannyl)benzoate (m-MeATE) [32]. A stock solution of m-MeATE was prepared by dissolving the 50 mg batch from the supplier Toronto Research Chemicals Inc. (North York, Canada) in 1 mL of chloroform. From the stock solution was taken 2 µL (100 µg, 0.26 µmole) and the chloroform was evaporated. The reagent was redissolved in 20 µL of dimethylsulphoxide (DMSO). To 1 mg of antibody at a concentration of 2.87 mg/mL in 0.2 M carbonate buffer pH 8.5 was added 4 µL of the m-MeATE/DMSO preparation under vigorous agitation. The reaction was allowed to proceed for 30 min at room temperature under gentle agitation. The resulting ε-lysyl-3-(trimethylstannyl)benzamide-MX35 F(ab′)2 immunoconjugate was then isolated from low un-reacted molecular species by size exclusion chromatography on a NAP-5 column (GE-Healthcare, Sweden) using 0.2 M acetate buffer (pH 5.5) as mobile phase.
The produced immunoconjugate was labeled with 211At and prepared at three levels of specific radioactivity. To a dry residue of 211At (241 MBq) was added 20 µL of N-iodosuccinimide (NIS), 50 µg of MX35 F(ab′)2-lysyl-3-(trimethylstannyl)benzoate at a concentration of 0.25 mg/ml in 0.2 M acetate buffer pH 5.5. After 1 min reaction time 3 µL of NIS (20 µg/mL) in methanol/1% HAc was added to iodosubstitute any remaining stannyl groups on the antibody. After an additional 1 min reaction time the reaction was stopped with an access of sodium metasulfite. The antibody fraction was finely isolated from low molecular weight species by size exclusion chromatography on a NAP-5 column. After labeling nine activity injection solutions were prepared from the labeled stock preparation, 25, 50, and 400 kBq at a specific activity of 1920, 500 or 120 MBq/mg, corresponding to 1/80, 1/500, and 1/1200 211At atoms/mAbs, respectively.
Cell Line
The cell line OVCAR-3 (NIH:OVCAR-3: National Institutes of Health ovarian cancer cell line 3, USA) was used [37]. The cell line was obtained from the American Type Culture Collection, Rockville, MD, USA. The cells were cultured in T-75 culture flasks at 37°C in a humidified atmosphere of 95% O2/5% CO2 with RPMI-1640 cell culture medium supplemented with 10% fetal calf serum, 1% L-glutamine and 1% penicillin-streptomycin.
Immunoreactivity of Antibodies
For determination of the immunoreactive fraction, a single-cell suspension of NIH:OVCAR-3 cells was prepared at a concentration of 5 × 106 cells/mL. The cells were serially diluted, 1 : 2, and a constant amount, 5 ng, of 211At-MX35 F(ab′)2 was added to each dilution. The 211At-MX35 F(ab′)2 was reacted with the cells for 3 h at room temperature during gentle agitation. After incubation and repeated washing, the bound fraction of each dilution was determined by measuring the activity of the cells. Double inverse plots were derived from the data and the immunoreactive fraction calculated [38]. Nonspecific binding of the astatinated MX35 F(ab′)2 was examined by saturating the antigens on the OVCAR-3 cells with an excess of unlabeled MX35 F(ab′)2.
Cumulated Activity and Absorbed Dose
A previously developed dynamic compartment model, which enables the computation of the cumulated activity on a tumor cell, was used [25]. Two compartments were defined: one representing the injected volume and the number of mAbs in the abdominal cavity, and one representing the number of mAbs bound to the antigenic sites of one tumor cell (Fig. 1). The initial value in the first compartment was NmAb-that is, the total number of intraperitoneally injected mAbs at t = 0. The transition of mAbs from the first compartment to the second compartment was determined using the previously in vitro determined kon-that is, the rate at which mAbs are bound to the antigenic sites on the cell surface per unit time for a specific antigen concentration. The effect of the gradual saturation of the antigenic sites with time was considered by introducing a gradual reduction in the number of free sites from Bmax to zero into the calculations. According to the conditions during the determination of the kinetic and equilibrium constants, the dissociation rate constant, koff, was negligible and therefore set to zero. During the period of binding and irradiation, a constant concentration of mAbs in the abdominal fluid was assumed. For determination of the cumulated activity on a cell, originating from cell surface-bound mAbs, the number of 211At atoms on the cell surface at different times (NAt-mAb) was calculated using the different values of the specific activity, Asp (1/80, 1/500, or 1/1200 211At atom/mAbs).
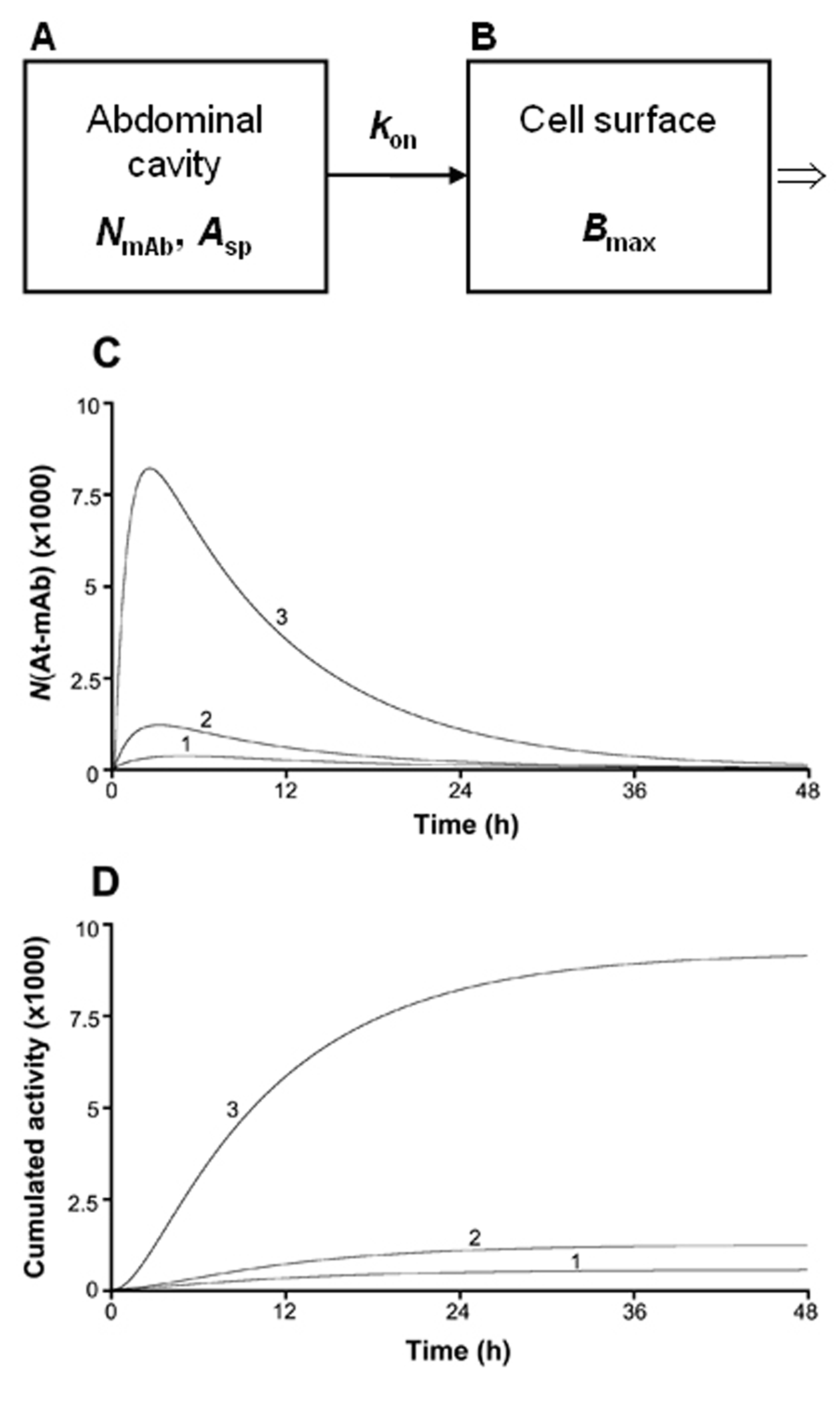 Click for large image | Figure 1. The computational model used for investigating of how the cumulated activity on a tumor cell surface varies for different specific activities (Asp). NmAb in compartment A is the total number of mAbs injected in the abdominal cavity. kon is the rate at which the mAbs are bound to the antigenic sites on the cell surface per unit time for a specific antigen concentration, and Bmax in compartment B is the total number of antigens present on one tumor cell. Bmax was in the above calculations set to 106, a value previously determined in vitro for the OVCAR-3 cells. NAt-mAb is the number of 211At atoms situated on the tumor cell surface at certain times after injection. Curve 1, 2, and 3 in panel C and D corresponds to a specific activity equal to 1/1200, 1/500, and 1/80 211At atom/mAbs, respectively. |
The specific energy imparted to cell nuclei situated at different depths in tumors was calculated using a Monte-Carlo program presented in an earlier paper [26]. The calculations in this study were performed for: 3 tumor sizes; 30, 45, and 95 µm radius, 3 activity levels; 25, 50, and 400 kBq 211At MX35 F(ab′)2, 3 specific activity levels; 1/80, 1/500, and 1/1200 211At atom/number of mAbs, and assuming a diffusion depth of 30 µm into the tumors, previously shown to be the activity distribution most consistent with the therapeutic results [27].
Animals
We used 110 female, nude Balb/c nu/nu mice (Charles River Laboratories International Inc., Wilmington, MA, USA) in this study. The animals were housed at 22°C and 50%-60% humidity with a light/dark cycle of 12 h. They were given autoclaved standard pellets and water ad libitum. All the experiments were approved by the Ethics Committee of the University of Gothenburg.
In vivo Procedures and Study Groups
At the age of 5 weeks all mice were intraperitoneally inoculated with ≥1 × 107 OVCAR-3 cells suspended in 0.2 mL saline. Four weeks after cell inoculation the animals were divided into 11 groups. The animals in groups 1-9 were intraperitoneally injected with 25, 50, or 400 kBq 211At-MX35 F(ab′)2 in 1 mL saline using specific activities equal to 1/80, 1/500, or 1/1200 (211At atom/mAbs) for each activity level, respectively (n = 10 in each group). As controls (group 10 and 11), animals were treated with PBS or unlabeled MX35 F(ab′)2 in PBS (n = 10 in each group). The animals were weighed weekly. Eight weeks after the intraperitoneal treatment, i.e. 12 wk after cell inoculation, all animals were sacrificed by cervical dislocation and dissected. The abdominal cavity was opened and the presence of ascites, and micro- and macroscopic tumors was determined as “yes” or “no”. Animals dissected and judged were blinded from knowledge from exposure conditions.
Scanning Electron Microscopy
Specimens for ultrastructural analysis were obtained from mice anesthetized with Metofane (Mallinckrodt Veterinay Inc.) at the time of dissection 12 weeks after the cell inoculation. The thoracic cavity was exposed and the heart root was clamped to arrest blood flow, after which an intraperitoneal injection (5 mL) of a mixture of 2.5% glutaraldehyde, 2% paraformaldehyde, and 0.01% sodium azide in 0.05 mol/L sodium cacodylate (pH 7.2) was given. After 10 min of primary fixation, the abdominal cavity was exposed and specimens, including peritoneal lining and jejunum (including mesenteries), were harvested. Specimens were further fixed overnight in the aldehyde mixture. After rinsing in 0.15 mol/L cacodylate, specimens for scanning electron microscopy (SEM) were subjected to an OTOTO postfixation procedure [39]. Dehydration followed in a series of ethanol, finally replaced by two changes of hexamethyldisalazane, which was allowed to evaporate under a fume hood. The dried specimens were mounted on aluminum stubs and were examined in a Zeiss 982 Gemini field emission scanning electron microscope after coating with palladium in an Emitech 550 sputter coater. Digital images were collected at a resolution of 1024 × 1024 pixels.
Statistical Analysis
Logistic-regression models were set up in the R software (www.r-project.org) and used to evaluate the effects of treatment (kBq) and specific activity (211At atom/mAbs) on the probability of an animal remaining free from macroscopic tumors at the time of dissection. A model including specific activity, treatment and an interaction between treatment and specific activity did not, however, result in a significant improvement in explaining the probability of macroscopic tumors, than when only specific activity was included as explanatory variable (P > 0.77). Hence, the final model for the logistic regression included only the specific activity as explanatory variable.
| Results | ▴Top |
The radiochemical yield following labeling of the MX35 F(ab′)2 was 80% resulting in a product with an activity concentration of 213 MBq/mL and a specific activity of 1920 MBq/mg corresponding to an antibody to 211At ratio of 84 : 1. The immunoreactivive fraction was excellent (95%) indicating retained biological function despite the very high specific activity. The therapeutic efficacy was estimated by determining the presence of macroscopic tumors by meticulous ocular examination of the abdominal cavity at the time of dissection. Summing over the different activity levels (25, 50, and 400 kBq 211At-MX35 F(ab′)2) the number of animals with macroscopic tumors were 13 (n = 30), 17 (n = 30), and 22 (n = 30) when treated with 211At-MX35 F(ab′)2 using specific activities equal to 1/80, 1/500, and 1/1200 211At atoms/mAbs, respectively. Treatment with PBS (n = 10) or unlabeled MX35 F(ab′)2 in PBS (n = 10) resulted in only 3 out of 10 animals in each group being free from macroscopic tumors (Table 1). The logistic regression analysis showed a significant trend that higher specific activity means less probability for macroscopic tumors (P = 0.02) (Fig. 2). No significant difference in the probability for macroscopic tumors could be detected between the groups with different amount of activity (P > 0.05). Because the batch of animals we received for this study seemed to be more immunosuppressed than usual, the disease became very advanced and the presence of ascites and microscopic tumors were higher (100%) than expected at the time of dissection, irrespective of treatment regimen, and could therefore not lay any ground for estimating any differences in the therapeutic efficacy.
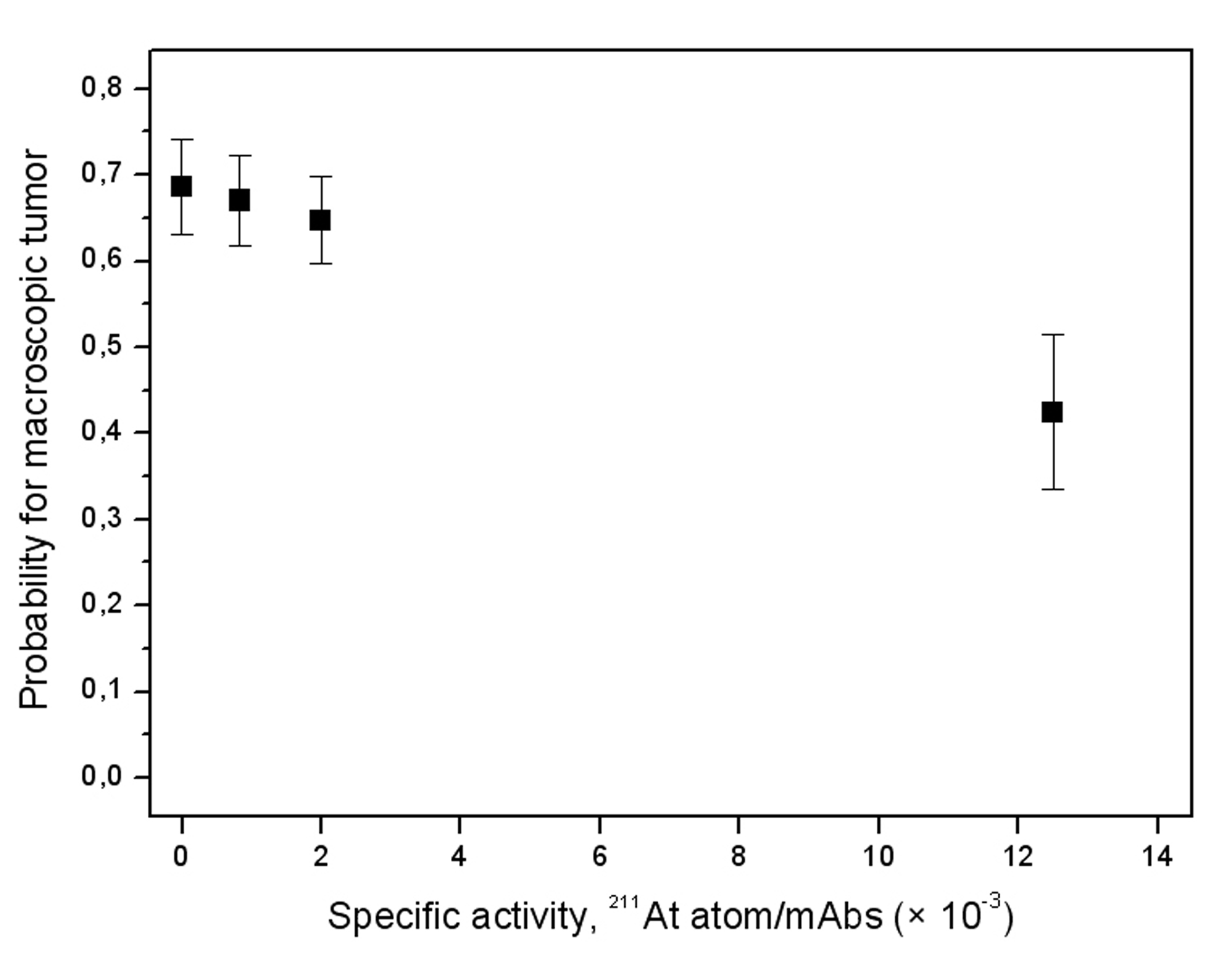 Click for large image | Figure 2. Probability for macroscopic tumor as a function of the specific activity (± SEM). Controls were given PBS (n = 10) or unlabeled MX35 F(ab′)2 in PBS (n = 10). Each specific activity level (n = 30) includes all 3 treatment levels (25, 50, and 400 kBq MX35 F(ab′)2). |
 Click to view | Table 1. Study Groups and Number of Mice with Macroscopic Tumors |
The specific energy values shown in Figure 3 ranged from 7 Gy (25 kBq 211At MX35 F(ab′)2, rtumor = 95 µm, and 1/1200 211At atom/mAbs) to 1185 Gy (400 kBq 211At MX35 F(ab′)2, rtumor = 95 µm, and 1/80 211At atom/mAbs). For the assumed largest tumor (rtumor = 95 µm) the specific energy to a cell nucleus situated in the core of the tumor increased from 22 to 334 Gy for 400 kBq 211At MX35 F(ab′)2 when using a specific activity of 1/80 instead of 1/1200, significantly increasing the probability for a cell kill. The successive decrease of the specific energy to cell nucleus situated closer to the core of the tumor is explained by the limited path length of the emitted α-particles.
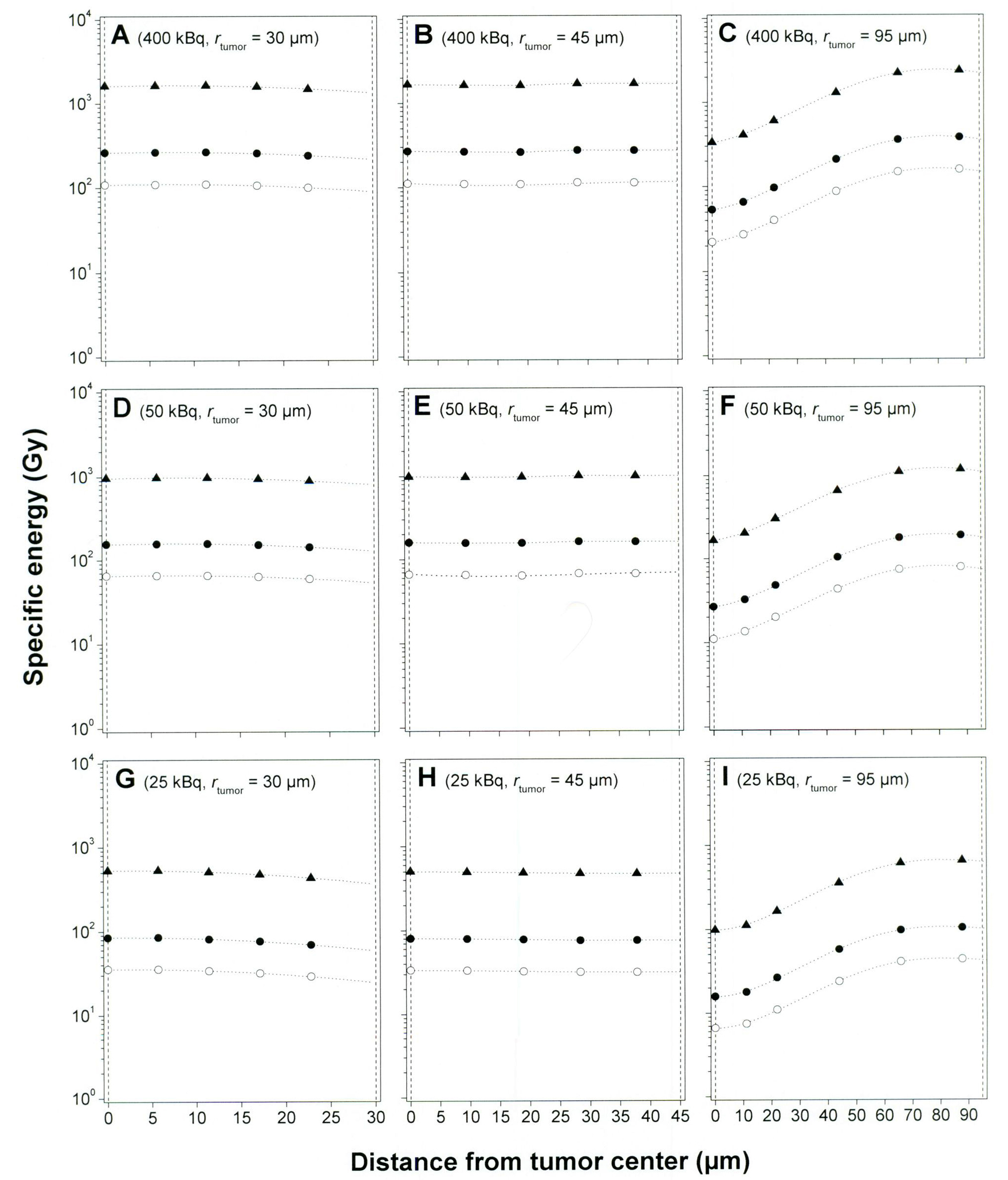 Click for large image | Figure 3. Specific energy imparted to a cell nucleus situated at different distances from the tumor center. The calculations were performed for 3 tumor sizes: 30, 45, and 95 µm radius; 3 activity levels: 25, 50, and 400 kBq 211At MX35 F(ab′)2; and 3 specific activity levels: 1/80 (▲), 1/500 (•), and 1/1200 (○) 211At atom/mAbs. A diffusion depth of 30 µm into each tumor was assumed for the radioactivity in all calculations. The vertical dashed lines in each panel represent the tumor center and surface, respectively. |
The findings on the peritoneal biopsies at the time of dissection revealed both larger tumor cell clusters of several millimetres in diameter as well as clusters consisting of only a few tumor cells. The tumors were sometimes only loosely adhered to the peritoneum, but were sometimes firmly anchored penetrating under the mesothelial cell layer (Fig. 4).
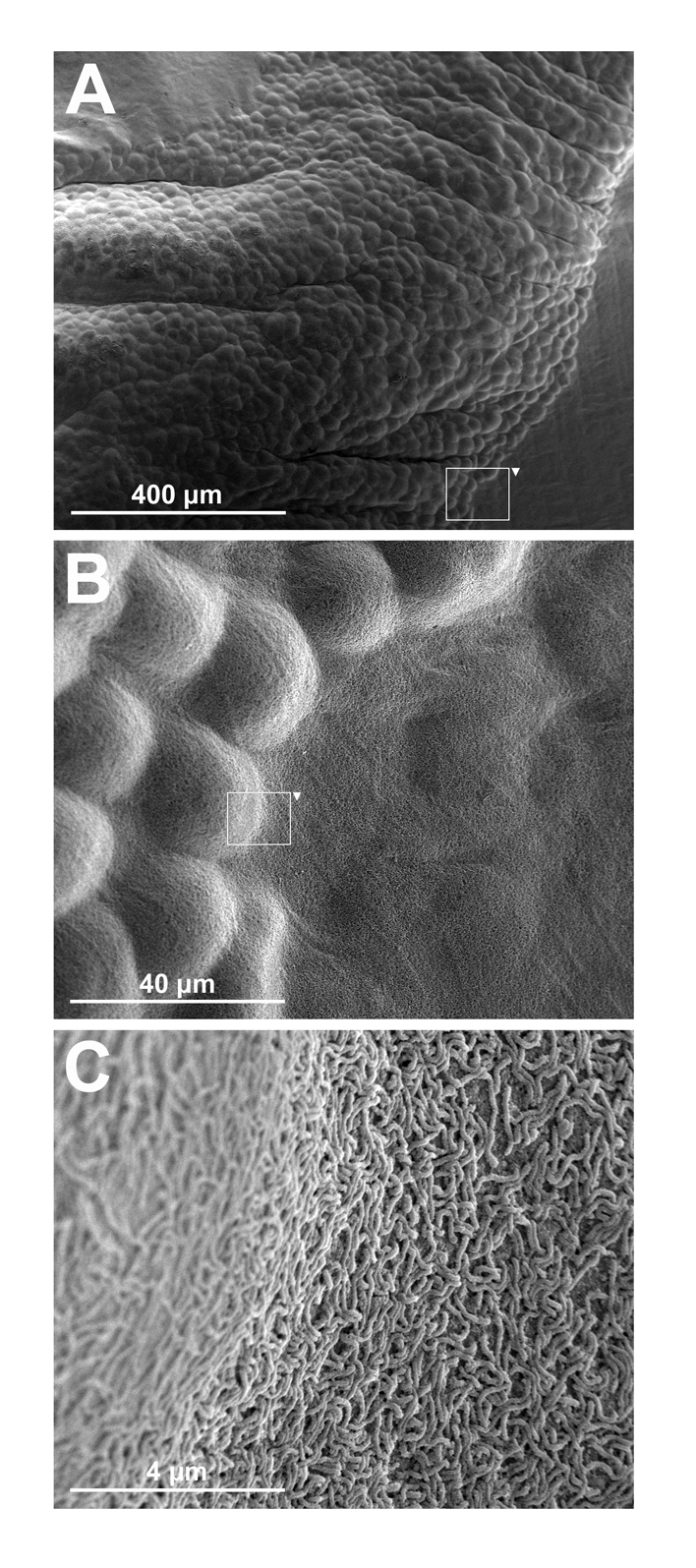 Click for large image | Figure 4. Scanning electron-microscopy images of macroscopic tumors on the peritoneum in nude mice at the time of dissection. Panel A shows a part of a macroscopic tumor several millimeters in length visible to the naked eye. Panel B shows an enlargement of a segment shown in panel A illustrating how the tumor cells disseminate under the mesothelial cell layer of the peritoneum. Panel C shows an enlargement of a segment shown in panel B, illustrating the microvilli covered transition from tumor to healthy tissue. |
| Discussion | ▴Top |
We have previously investigated the therapeutic efficacy of the intact specific IgG1 mAbs MOv18 and MX35 as well as fragmented mAbs (MX35 F(ab′)2 and non-specific Rituximab F(ab′)2) in the treatment of nude mice in an ovarian cancer model [22-27]. Those studies showed good therapeutic efficacy when injecting 400 kBq 211At-MX35 3 wk after cell inoculation, as well as the increasing importance of using a specific mAb compared to a non-specific mAb when treating larger tumors. In the interval of 400-1200 kBq the therapeutic efficacy was not correlated with the administered activity. This could be explained by the saturation of the antigenic sites, which-according to the dynamic compartmental model used in both those and this study-occurs within a few hours after the injection, resulting in a similar absorbed dose for the 3 activity levels of 400, 800, and 1200 kBq. The rationale for choosing the fragmented mAb instead of the whole IgG is due to 4 facts: (i) The fragmented mAb was the only clinical grade version of the mAb available at the time of the study; (ii) We have received an approval by the Swedish Medical Products Agency for completing a phase I study with this fragmented mAb [33]; (iii) We believe that the diffusion into tumors using the fragmented mAb is higher than compared to whole IgG; and (iv) We believe that the immunogenicity of the fragmented mAb is lower than the whole IgG, reducing the risk for HAMA response, especially if repeated treatments are considered in the future.
As shown in one of our earlier studies there will be tumors with maximum radii of 95 µm when injecting the radioimmunocomplex 4 wk after tumor cell inoculation [27]. This means that the core of the largest tumors will not be irradiated if the activity is only situated on the surface of the tumors. However, if we have a diffusion of the radioimmunocomplex of 30 µm in the tumors the core will be irradiated. A highest activity of 400 kBq was chosen in this study based on two facts; (i) it is well within the toxic limit [2]; (ii) further increase in the activity does not improve the therapeutic efficacy. In those earlier studies the specific activity had been 1/1200 211At atom/mAbs.
As we just recently have published a phase I study on women with recurrent ovarian cancer, and now prepare to initiate a phase II study, we find it of outmost importance to investigate the role of higher specific activities [33]. The results from the phase I study, indicated no adverse effects of the treatment, using intraperitoneally injected 211At labeled MX35 F(ab′)2. This is very encouraging and urges us to initiate the phase II study now under planning. The choice of the 3 levels of specific activity in this study (i.e. 1/80, 1/500, and 1/1200) was based on the fact that: (i) 1/80 was for the moment the highest achievable specific activity; (ii) 1/1200 has up until recently been the highest specific activity achievable; and (iii) 1/500 was chosen as an intermediate value between 1/80 and 1/1200.
The advantages with administering a radioimmunocomplex with a high specific activity could be that: (i) able to treat low antigen expressing tumor and/or tumors having restricted diffusion; and (ii) able to treat more patients per unit radioactivity. The number of antigens on NIH:OVCAR-3 cells, Bmax, determined in vitro are 106. What Bmax will be for the patients in for example the forthcoming phase II study is not known, but it will most probably vary. Therefore, it is important to administer a radioimmunocomplex with as high specific activity as possible. Since the availability of 211At still is limited it is important to use the accessible amount of radioactivity as effectively as possible, implying the use of a high specific activity lowering the necessary total amount of administered radioactivity.
If there are insurmountable problems using only α-particle emitters when trying to treat a disseminated disease where both micro- and macrotumors are present, and for which there exists an unmanageable diffusion problem for the effector molecules into the tumors, and for which a pre-targeting system does not work satisfactory, the use of a cocktail of both α-particle and electron-emitters could be the solution. In our situation with ovarian cancer the combination of an intraperitoneal as well as an intravenous administration route could also be relevant, if there are larger vascularized extraperitoneally tumors present at the time of treatment.
As already mentioned in the Results the batch of animals we received for this study seemed to be more immunosuppressed than usual. The disease therefore became very advanced and the presence of ascites and microscopic tumors were much higher (100%) than compared to earlier studies at the time of dissection, and could therefore not lay any ground for estimating any differences in the therapeutic efficacy. However, we find the results of the study of outmost importance, indicating the role of the specific activity even when the disease is advanced, and believe it therefore very important to publish these results. A series of studies are now planned to further investigate the role of the specific activity for various levels of the specific activity, amounts of injected activity, and degree of advanced disease.
In conclusion, summing over the different activity levels (25, 50, and 400 kBq 211At-MX35 F(ab′)2) the number of animals with macroscopic tumors was 13, 17, and 22 (n = 30 for each group) for the specific activities equal to 1/80, 1/500, or 1/1200, respectively. Logistic-regression analysis showed a significant trend that higher specific activity imply a decreased probability for macroscopic tumors at the time of dissection (P = 0.02). Increasing the specific activity indicates a way to enhance the therapeutic outcome. Further studies of the role of the specific activity are therefore important and justified.
Acknowledgments
This work was supported by grants from the Swedish Cancer Society, the King Gustaf V Jubilee Clinic Research Foundation, and the Assar Gabrielsson Foundation in Gothenburg, Sweden. Kanita Cukur at the electron microscopy unit (EMU), Sahlgrenska Academy, University of Gothenburg, is acknowledged for the preparation of the specimen intended for electron microscopy.
Conflict of Interest
The authors have no conflict of interest.
| References | ▴Top |
- Zalutsky MR, Reardon DA, Pozzi OR, Vaidyanathan G, Bigner DD. Targeted alpha-particle radiotherapy with 211At-labeled monoclonal antibodies. Nucl Med Biol. 2007;34(7):779-785.
pubmed doi - Elgqvist J, Bernhardt P, Hultborn R, Jensen H, Karlsson B, Lindegren S, Warnhammar E,
et al . Myelotoxicity and RBE of 211At-conjugated monoclonal antibodies compared with 99mTc-conjugated monoclonal antibodies and 6°Co irradiation in nude mice. J Nucl Med. 2005;46(3):464-471.
pubmed - McQuarrie S, Mercer J, Syme A, Suresh M, Miller G. Preliminary results of nanopharmaceuticals used in the radioimmunotherapy of ovarian cancer. J Pharm Pharm Sci. 2005;7(4):29-34.
pubmed - Janssen ML, Pels W, Massuger LF, Oyen WJ, Boonstra H, Corstens FH, Boerman OC. Intraperitoneal radioimmunotherapy in an ovarian carcinoma mouse model: Effect of the radionuclide. Int J Gynecol Cancer. 2003;13(5):607-613.
pubmed doi - Borchardt PE, Quadri SM, Freedman RS, Vriesendorp HM. Intraperitoneal radioimmunotherapy with human monoclonal IGM in nude mice with peritoneal carcinomatosis. Cancer Biother Radiopharm. 2000;15(1):53-64.
pubmed doi - Grana C, Bartolomei M, Handkiewicz D, Rocca P, Bodei L, Colombo N, Chinol M,
et al . Radioimmunotherapy in advanced ovarian cancer: is there a role for pre-targeting with (90)Y-biotin? Gynecol Oncol. 2004;93(3):691-698.
pubmed doi - Meredith RF, Alvarez RD, Partridge EE, Khazaeli MB, Lin CY, Macey DJ, Austin JM
Jr ,et al . Intraperitoneal radioimmunochemotherapy of ovarian cancer: a phase I study. Cancer Biother Radiopharm. 2001;16(4):305-315.
pubmed doi - Mahe MA, Fumoleau P, Fabbro M, Guastalla JP, Faurous P, Chauvot P, Chetanoud L,
et al . A phase II study of intraperitoneal radioimmunotherapy with iodine-131-labeled monoclonal antibody OC-125 in patients with residual ovarian carcinoma. Clin Cancer Res. 1999;5(10 Suppl):3249s-3253s.
pubmed - Buchsbaum DJ, Khazaeli MB, Axworthy DB, Schultz J, Chaudhuri TR, Zinn KR, Carpenter M,
et al . Intraperitoneal pretarget radioimmunotherapy with CC49 fusion protein. Clin Cancer Res. 2005;11(22):8180-8185.
pubmed doi - Persson M, Gedda L, Lundqvist H, Tolmachev V, Nordgren H, Malmstrom PU, Carlsson J. 177Lu-Pertuzumab: experimental therapy of HER-2-expressing xenografts. Cancer Res. 2007;67(1):326-331.
pubmed doi - Tolmachev V, Orlova A, Pehrson R, Galli J, Baastrup B, Andersson K, Sandstrom M,
et al . Radionuclide therapy of HER2-positive microxenografts using a 177Lu-labeled HER2-specific Affibody molecule. Cancer Res. 2007;67(6):2773-2782.
pubmed doi - Turner JH, Rose AH, Glancy RJ, Penhale WJ. Orthotopic xenografts of human melanoma and colonic and ovarian carcinoma in sheep to evaluate radioimmunotherapy. Br J Cancer. 1998;78(4):486-494.
pubmed - Kievit E, Pinedo HM, Schluper HM, Haisma HJ, Boven E. Comparison of monoclonal antibodies 17-1A and 323/A3: the influence of the affinity on tumour uptake and efficacy of radioimmunotherapy in human ovarian cancer xenografts. Br J Cancer. 1996;73(4):457-464.
pubmed - Molthoff CF, Pinedo HM, Schluper HM, Boven E. Influence of dose and schedule on the therapeutic efficacy of 131I-labelled monoclonal antibody 139H2 in a human ovarian cancer xenograft model. Int J Cancer. 1992;50(3):474-480.
pubmed - Epenetos AA, Hird V, Lambert H, Mason P, Coulter C. Long term survival of patients with advanced ovarian cancer treated with intraperitoneal radioimmunotherapy. Int J Gynecol Cancer. 2000;10(S1):44-46.
pubmed doi - Alvarez RD, Partridge EE, Khazaeli MB, Plott G, Austin M, Kilgore L, Russell CD,
et al . Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: a phase I/II study. Gynecol Oncol. 1997;65(1):94-101.
pubmed doi - Alvarez RD, Huh WK, Khazaeli MB, Meredith RF, Partridge EE, Kilgore LC, Grizzle WE,
et al . A Phase I study of combined modality 90Yttrium-CC49 intraperitoneal radioimmunotherapy for ovarian cancer. Clin Cancer Res. 2002;8(9):2806-2811.
pubmed - Stewart JS, Hird V, Snook D, Dhokia B, Sivolapenko G, Hooker G, Papadimitriou JT,
et al . Intraperitoneal yttrium-90-labeled monoclonal antibody in ovarian cancer. J Clin Oncol. 1990;8(12):1941-1950.
pubmed - Verheijen RH, Massuger LF, Benigno BB, Epenetos AA, Lopes A, Soper JT, Markowska J,
et al . Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J Clin Oncol. 2006;24(4):571-578.
pubmed doi - Oei AL, Verheijen RH, Seiden MV, Benigno BB, Lopes A, Soper JT, Epenetos AA,
et al . Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int J Cancer. 2007;120(12):2710-2714.
pubmed doi - Andersson H, Elgqvist J, Horvath G, Hultborn R, Jacobsson L, Jensen H, Karlsson B,
et al . Astatine-211-labeled antibodies for treatment of disseminated ovarian cancer: an overview of results in an ovarian tumor model. Clin Cancer Res 2003;9(10 Pt. 2):3914S-3921S.
pubmed - Andersson H, Lindegren S, Back T, Jacobsson L, Leser G, Horvath G. Radioimmunotherapy of nude mice with intraperitoneally growing ovarian cancer xenograft utilizing 211At-labelled monoclonal antibody MOv18. Anticancer Res. 2000;20(1A):459-462.
pubmed - Andersson H, Lindegren S, Back T, Jacobsson L, Leser G, Horvath G. The curative and palliative potential of the monoclonal antibody MOv18 labelled with 211At in nude mice with intraperitoneally growing ovarian cancer xenografts—a long-term study. Acta Oncol. 2000;39(6):741-745.
pubmed doi - Andersson H, Palm S, Lindegren S, Back T, Jacobsson L, Leser G, Horvath G. Comparison of the therapeutic efficacy of 211At- and 131I-labelled monoclonal antibody MOv18 in nude mice with intraperitoneal growth of human ovarian cancer. Anticancer Res. 2001;21(1A):409-412.
pubmed - Elgqvist J, Andersson H, Back T, Hultborn R, Jensen H, Karlsson B, Lindegren S,
et al . Therapeutic efficacy and tumor dose estimations in radioimmunotherapy of intraperitoneally growing OVCAR-3 cells in nude mice with 211At-labeled monoclonal antibody MX35. J Nucl Med. 2005;46(11):1907-1915.
pubmed - Elgqvist J, Andersson H, Bernhardt P, Back T, Claesson I, Hultborn R, Jensen H,
et al . Administered activity and metastatic cure probability during radioimmunotherapy of ovarian cancer in nude mice with 211At-MX35 F(ab')2. Int J Radiat Oncol Biol Phys. 2006;66(4):1228-1237.
pubmed doi - Elgqvist J, Andersson H, Back T, Claesson I, Hultborn R, Jensen H, Johansson BR,
et al . Alpha-radioimmunotherapy of intraperitoneally growing OVCAR-3 tumors of variable dimensions: Outcome related to measured tumor size and mean absorbed dose. J Nucl Med. 2006;47(8):1342-1350.
pubmed - Elgqvist J, Andersson H, Haglund E, Jensen H, Kahu H, Lindegren S, Warnhammar E,
et al . Intraperitoneal alpha-radioimmunotherapy in mice using different specific activities. Cancer Biother Radiopharm. 2009;24(4):509-513.
pubmed doi - Elgqvist J, Andersson H, Jensen H, Kahu H, Lindegren S, Warnhammar E, Hultborn R. Repeated Intraperitoneal alpha-Radioimmunotherapy of Ovarian Cancer in Mice. J Oncol. 2010:394913.
pubmed - Back T, Andersson H, Divgi CR, Hultborn R, Jensen H, Lindegren S, Palm S,
et al . 211At radioimmunotherapy of subcutaneous human ovarian cancer xenografts: evaluation of relative biologic effectiveness of an alpha-emitter in vivo. J Nucl Med. 2005;46(12):2061-2067.
pubmed - Palm S, Andersson H, Back T, Claesson I, Delle U, Hultborn R, Jacobsson L,
et al . In vitro effects of free 211At, 211At-albumin and 211At-monoclonal antibody compared to external photon irradiation on two human cancer cell lines. Anticancer Res. 2000;20(2A):1005-1012.
pubmed - Lindegren S, Frost S, Back T, Haglund E, Elgqvist J, Jensen H. Direct procedure for the production of 211At-labeled antibodies with an epsilon-lysyl-3-(trimethylstannyl)benzamide immunoconjugate. J Nucl Med. 2008;49(9):1537-1545.
pubmed doi - Andersson H, Cederkrantz E, Back T, Divgi C, Elgqvist J, Himmelman J, Horvath G,
et al . Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of 211At-MX35 F(ab')2—a phase I study. J Nucl Med. 2009;50(7):1153-1160.
pubmed doi - Lindegren S, Back T, Jensen HJ. Dry-distillation of astatine-211 from irradiated bismuth targets: a time-saving procedure with high recovery yields. Appl Radiat Isot. 2001;55(2):157-160.
pubmed doi - Yin BW, Kiyamova R, Chua R, Caballero OL, Gout I, Gryshkova V, Bhaskaran N,
et al . Monoclonal antibody MX35 detects the membrane transporter NaPi2b (SLC34A2) in human carcinomas. Cancer Immun. 2008;8:3-11.
pubmed - Rubin SC, Kostakoglu L, Divgi C, Federici MG, Finstad CL, Lloyd KO, Larson SM,
et al . Biodistribution and intraoperative evaluation of radiolabeled monoclonal antibody MX35 in patients with epithelial ovarian cancer. Gynecol Oncol. 1993;51(1):61-66.
pubmed doi - Hamilton TC, Young RC, McKoy WM, Grotzinger KR, Green JA, Chu EW, Whang-Peng J,
et al . Characterization of a human ovarian carcinoma cell line (NIH:OVCAR-3) with androgen and estrogen receptors. Cancer Res. 1983;43(11):5379-5389.
pubmed - Lindmo T, Boven E, Cuttitta F, Fedorko J, Bunn PA
Jr . Determination of the immunoreactive fraction of radiolabeled monoclonal antibodies by linear extrapolation to binding at infinite antigen excess. J Immunol Methods. 1984;72(1):77-89.
pubmed doi - Friedman PL, Ellisman MH. Enhanced visualization of peripheral nerve and sensory receptors in the scanning electron microscope using cryofracture and osmium-thiocarbohydrazide-osmium impregnation. J Neurocytol. 1981;10(1):111-131.
pubmed doi
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


