| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 3, June 2022, pages 145-154
Abdominopelvic Lymphatic Drainage Area Irradiation for Consolidative Radiotherapy of Advanced Ovarian Carcinoma: Analysis of Clinical Application Efficacy and Dosimetric Verification
Jing Shena, Yin Jie Taoa, Hui Guana, Hong Nan Zhena, Ting Ting Donga, Zhi Kai Liua, b, Fu Quan Zhanga
aDepartment of Radiation Oncology, Peking Union Medical College Hospital, Beijing 100730, China
bCorresponding Author: Zhikai Liu, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
Manuscript submitted February 5, 2022, accepted March 25, 2022, published online June 22, 2022
Short title: Efficiency and Safe Alternative Method of WART
doi: https://doi.org/10.14740/wjon1454
| Abstract | ▴Top |
Background: The aim of the study was to evaluate the efficacy of abdominopelvic lymphatic drainage area irradiation (APLN), instead of whole abdominal radiotherapy (WART), in the consolidative radiotherapy of advanced ovarian carcinoma patients.
Methods: We conducted a retrospective analysis collecting 99 patients with locally advanced ovarian cancer treated by APLN with 45 - 50 Gy/25- 28 fractions/5-7#, instead of WART. We evaluated the clinical outcome of APLN. Five patients were selected for dosimetric verifications verses WART (30 Gy/20 fractions). The normal tissue complication probability (NTCP) was calculated for the two treatment methods.
Results: The mean follow-up time was 64.10 months (5.5 - 113.2 months), after APLN consolidative radiotherapy, 1-, 3-, and 5-year overall survival (OS) was 87.9%, 81.3%, and 61.5%, median disease-free survival (DFS) was 40.8 months, 5-year local recurrence free survival (LRFS) was 75.9%, and 5-year distant metastasis free survival (DMFS) was 49.2%. One patient died due to intestinal perforation. Local recurrence in the area between WART and APLN was rare (3/99 patients). The number of surgical procedures < 2 was an independent risk factor for LRFS (P = 0.023). Dosimetric comparison showed that comparing with WART, APLN significantly reduced the organ at risk (OAR) dose: 25.37 ± 3.63 Gy (25%) for liver, 8.77 ± 5.03 Gy (25%) for kidney, 8.14 ± 1.51 Gy (25%) for small intestine, etc. NTCP was reduced by 0.04-1.04% for liver, kidney, and small intestine.
Conclusion: For consolidative radiotherapy in locally advanced ovarian cancer, APLN (intensity-modulated radiotherapy 45 - 50 Gy/25 - 28 fractions) could be an alternative to WART, resulting in excellent LRFS and DFS, with acceptable toxicities, comparing with previous literature reports. Dosimetric analysis also showed the benefits of APLN in NTCP.
Keywords: Ovarian cancer; Intensity-modulated radiotherapy; Lymphatic drainage area; TCP; NTCP
| Introduction | ▴Top |
Ovarian cancer is the third most common malignancy of the female reproductive system [1], with a 5-year mortality rate of more than 50% according to the International Agency for Research on Cancer (IARC) GLOBOSCAN 2018 report [2]. It is estimated that the global burden of ovarian cancer will increase by 47% (295,414 to 434,184 women) from 2018 to 2040 [3]. Due to the insidious nature of ovarian cancer symptoms and lack of effective screening, approximately 75% of patients are locally advanced (stage III-IV) at initial diagnosis, and for patients with locally advanced primary ovarian cancer, more than 70-80% of patients will recur even after standard treatment such as surgery and adjuvant chemotherapy [4].
Whole abdominal radiotherapy (WART) is recommended for the consolidative radiotherapy of locally advanced ovarian cancer patients, aiming to reduce the chance of abdomen or pelvic recurrence, and kill subclinical lesions, but its wide application is limited by the fact that WART includes the whole abdominal cavity and the whole pelvic cavity, with correspondingly large toxic side effects [5, 6] and the limited irradiation dose that can be given. With the advancement of radiotherapy technology, it has crossed from the era of two-dimensional radiotherapy to our current widely used intensity-modulated radiation therapy technology (IMRT), which defines the volume of the target area with a three-dimensional imaging method and uniformly concentrates high doses to the target area, which can achieve higher irradiation doses while minimizing the amount of normal tissues and organs exposed. It makes the application of higher dose radiotherapy in ovarian cancer patients a reality [7-10]. With the advancement of the IMRT, IMRT has also been used in WART [11], and there are related studies that have performed simultaneous integrated boost-IMRT with increased irradiation dose, although relatively low toxic effects [12, 13].
Local failure patterns after surgery for locally advanced ovarian cancer are mostly within the region of large vessels [4, 14], so our center applied abdominopelvic lymphatic drainage area irradiation (APLN), instead of WART for consolidative radiotherapy of locally advanced ovarian cancer, using IMRT with an increased irradiation dose of 45 - 50 Gy to observe the clinical efficacy, toxic effects and failure mode. Dosimetric validation was also performed. To the best of our knowledge, this is the first reported application of APLN instead of WART for consolidative radiotherapy in locally advanced ovarian cancer, which has strong clinical guiding significance.
| Materials and Methods | ▴Top |
Collection of patient data
A total of 99 patients with locally advanced ovarian cancer treated at the Department of Radiotherapy, Peking Union Medical College Hospital from 2013 to 2020 were collected, all of whom were surgically pathologically confirmed ovarian cancer patients and underwent standard ovarian cancer staging surgery (70 cases with complete resection, 21 cases with basic resection, and eight cases with major resection) and completed four to nine cycles of standard first-line regimen chemotherapy after surgery (40 cases had four to six courses of chemotherapy, and 39 cases had more than six courses of chemotherapy). The median age was 50 years (16 - 75 years). Eighty-nine patients had stage III and 10 patients had stage IV. There were 92 cases of epithelial carcinoma (59 plasmacytosis, 11 endometrial carcinomas, 12 clear cell carcinomas, two mucinous carcinomas, and eight other epithelial carcinomas), five interstitial tumors of the sex cords, and two germ cell tumors. Among them, 56 patients had only one time standard surgery and 43 patients had recurrence after standard surgery (34 cases with first recurrence, two cases with second recurrence, and seven cases with more than two recurrences).
APLN radiotherapy methods
Ninety-nine patients with locally advanced ovarian cancer after postoperative chemotherapy were treated with APLN at our center using IMRT, irradiating the abdominopelvic lymphatic drainage area at a dose of 45 - 50 Gy/25 - 28 times. Cone-beam computed tomography (CBCT) image acquisition was performed once a week to ensure the accuracy of treatment.
Para-aortic lymphatic drainage area (PALN), vaginal stump and pelvic lymphatic drainage area (common iliac, external iliac, internal iliac, closed foramen region, and anterior sacral region), abdominal aorta PALN (upper border at the level of T12, lower border at the bifurcation of the abdominal aorta): 2 cm external dilatation of left-lateral para-aortic (PALN-LPA), 1 cm external dilatation of the right para-caval (PALN-RPC), and 5 mm external dilatation of aorto-caval (PALN-AC); common iliac lymphatic drainage area (upper border at the bifurcation of the abdominal aorta, lower border at the bifurcation of the common iliac artery): 7 mm external dilatation of the common iliac artery, including bilaterally to the inner edge of the psoas major muscle; internal and external iliac level: 7 mm external dilatation of the internal and external iliac artery, 17 mm anterolateral to the external iliac group. The anterior sacral region: 15 mm anterior to the vertebrae; the closed foramen region: 18 mm of the pelvic wall connecting the internal and external iliac parts. The specific illustration is shown in Figure 1.
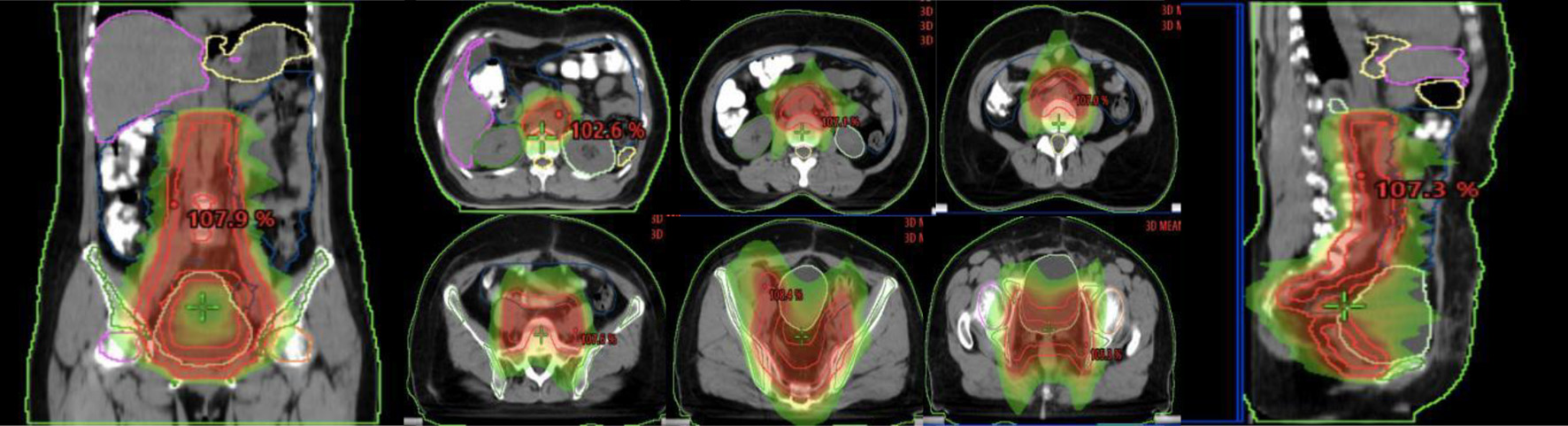 Click for large image | Figure 1. Illustration of the abdominopelvic lymphatic drainage area irradiation. |
Follow-up and efficacy assessment methods
The clinical data of all 99 patients were retrospectively studied by reviewing medical records and telephone follow-up, and follow-up was conducted every 3 months for 2 years after treatment, and every 6 months for 2 - 5 years. The follow-up included: gender, age, blood tumor markers, surgical stage, whether it was the first treatment, number of recurrence, blood tumor markers at the time of recurrence, and treatment mode after recurrence. Toxic side effects were evaluated by Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0.
The follow-up period was defined as from the date of surgery to the last follow-up date or the time of death, and the deadline for follow-up was September 30, 2021. Overall survival (OS) was defined as the time between the completion of radiotherapy and the appearance of patients’ death, and disease-free survival (DFS) was defined as the time between the completion of radiotherapy and the appearance of disease progression. Local recurrence free survival (LRFS) was defined as the time between the completion of radiotherapy and the appearance of local recurrence in the irradiated field of radiotherapy. Distant metastasis free survival (DMFS) was defined as the time from completion of radiotherapy to the appearance of disease progression in the non-irradiated field or area.
Dosimetric evaluation method
Five patients with ovarian cancer irradiated in the APLN were selected and designed a WART plan for further comparison (30 Gy/20 fractions, irradiation area including the whole abdominopelvic cavity from the top of the diaphragm to the pelvic floor), normal tissue, and target area dose volume histograms (DVHs) based on those two treatment plans were collected, computer code was written and saved as a Matlab executable program, parameters (a, m, D50 (Gy), γ50) of the target area, organ at risk (OAR) (small intestine, liver, kidney, etc.) were obtained, equivalent uniform dose (EUD) of PTV and OAR were calculated, and then normal tissue complication probability (NTCP) was calculated.
Statistical methods
Statistical analysis was performed using SPSS version 25.0. The Chi-square test was used for categorical variables. The Kolmogorov-Smirnov method was used to test for normality for continuous variables. Student’s t-test was used to assess normally distributed variables and the Mann-Whitney U test was used for non-normally distributed variables. The Kaplan-Meier method was used to estimate the incidence of OS, DFS, and local control (LC), and the significance of prognostic factors on survival was evaluated using the univariate log-rank test. Cox proportional regression was used for multivariate analysis of covariates selected in univariate analysis. P value < 0.05 was considered statistically significant.
| Results | ▴Top |
General patient profile
All 99 patients underwent standard ovarian cancer staging surgery, including 56 patients after initial standard surgery and 43 patients after standard surgery of recurrence (34 cases for the first recurrence, two cases for the second recurrence, and seven cases for more than two recurrences). The surgical situation was complete resection in 70 cases, basic resection in 21 cases, and major resection in eight cases, and the standard first-line regimen of postoperative chemotherapy was completed in four to nine cycles (four to six courses of chemotherapy in 40 cases and more than six courses of chemotherapy in 39 cases). The patients’ age, pathological type, preoperative carbohydrate antigen 125 (CA125) elevation, postoperative chemotherapy courses, and the number of chemotherapy courses after which CA125 decreased to normal are detailed in Table 1.
 Click to view | Table 1. Baseline and Clinical Characteristics of the Patients (n = 99) |
Clinical outcomes
Efficacy analysis showed 99 patients with a mean follow-up of 64.10 months (5.5 - 113.2 months). The 5-year OS was 61.5%, 5-year DFS was 40.9%, median time to DFS was 40.8 months, 5-year LRFS was 75.9%, and 5-year DMFS was 49.2%. Details are shown in Figure 2.
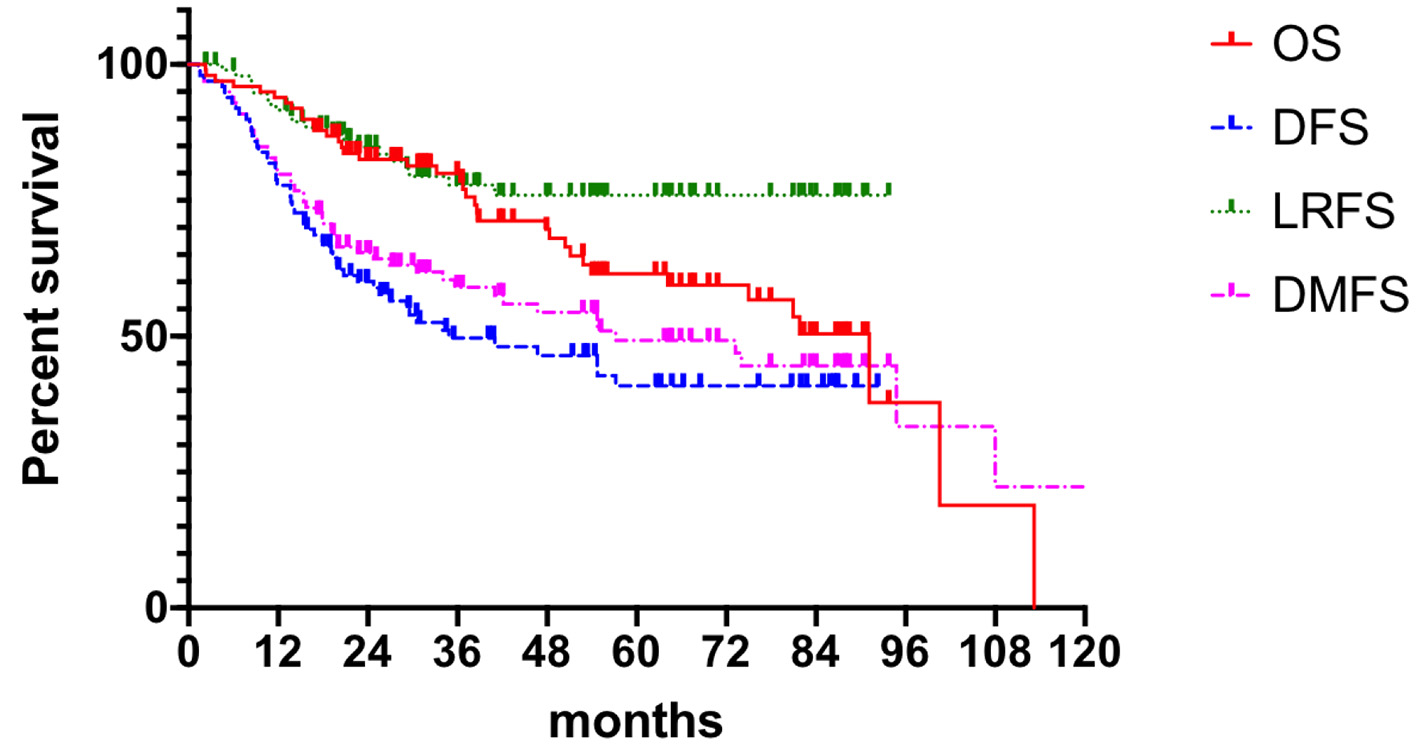 Click for large image | Figure 2. Clinical outcomes. OS: overall survival; DFS: disease-free survival; LRFS: local recurrence free survival; DMFS: distant metastasis free survival. |
Failure of patterns
A total of 52 patients presented with disease failure, including 20 cases of local recurrence, 50 cases of distant metastasis, and 18 cases of combined local recurrence and distant metastasis at the same time. Among the 20 patients with local recurrence: seven patients with recurrence in the para-aortic lymph nodes, six patients with recurrence in the pelvic lymph node area, two patients with recurrence in the vaginal stump, three patients with recurrence in the mesenteric area, and one patient each with recurrence in the inguinal area and abdominal wall. Among the 50 patients with distant metastases: nine cases of lymph node metastases in the clavicular region, eight cases of liver metastases, five cases of lung metastases, four cases of mediastinal metastases, three cases of brain metastases, two cases of bone metastases, and 19 cases of other sites (breast, axilla, etc.), as detailed in Table 2 and Figure 3.
 Click to view | Table 2. Failure Pattern for Patients Treated With APLN |
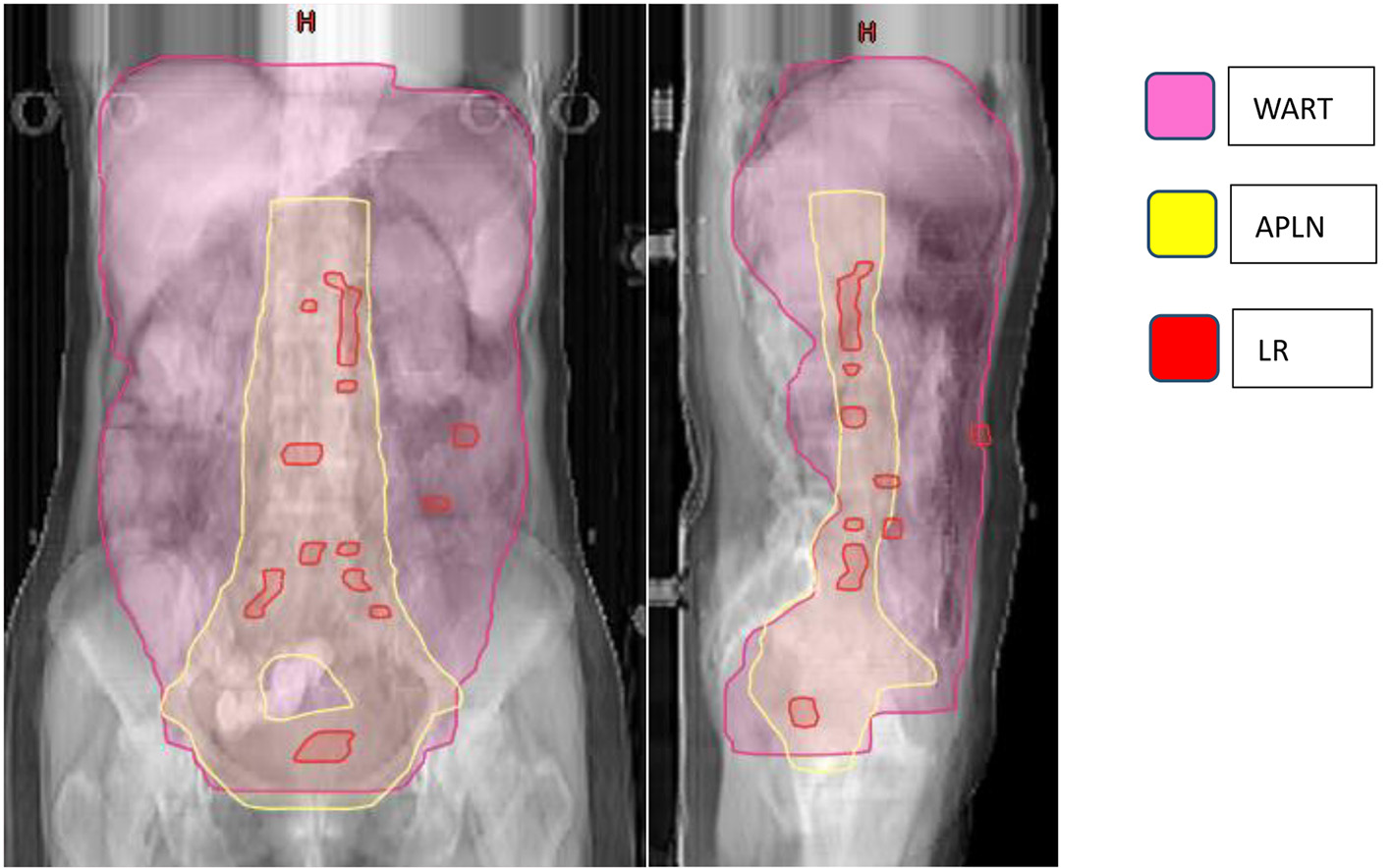 Click for large image | Figure 3. Failure map for patients treated with APLN. *The yellow line represents the area of APLN (vaginal stump and pelvic lymphatic drainage area (common iliac, external iliac, internal iliac, closed foramen region, and anterior sacral region), abdominal aorta PALN (upper border at the level of T12). The pink line represents the radiation area of WART. The red rods represent LR. The failure map shows 20 patients with LR: 13 para-aortic or pelvic lymph nodes, two vaginal stump, three mesenteric area, and one inguinal and one abdominal wall, and three with recurrence between the area of WART and APLN. APLN: abdominopelvic lymphatic drainage area irradiation; PLAN: para-aortic lymphatic drainage area; WART: whole abdominal radiotherapy; LR: local recurrence. |
Toxic side effects
Acute side effects included: grade 3 hematological toxicity in 67/99 cases and grade 2 gastrointestinal disorders in 30/99 cases. As for late toxic side effects, a total of 10 patients showed grade 3 gastrointestinal disorders, including one case of gastrointestinal fistula and nine cases of gastrointestinal obstruction, of which one patient died due to severe toxic side effects (who had already received three operations).
Univariate and multifactorial analyses
In univariate analysis, the number of surgeries was an independent influencing factor for patients’ 5-year LRFS. The age, the number of surgeries, standard surgery resection status, histology, Federation of Gynecology and Obstetrics (FIGO) stage, preoperative CA125 level, postoperative chemotherapy courses, and CA125 decreased to normal chemotherapy courses were analyzed in OS, DFS, LRFS and DMFS, showing no significant influence (Table 3).
 Click to view | Table 3. Univariate Analysis of Factors Influencing 5-year OS, DFS, LRFS and DMFS |
In multifactorial analysis, the number of surgeries was an independent influencing factor for patients’ 5-year LRFS (P = 0.048, hazard ratio (HR) 2.708 (95% confidence interval (CI): 1.009 - 7.266)) (Table 4).
 Click to view | Table 4. Multivariate Analysis of Factors Influencing 5-Year LRFS |
Dosimetric differences
The dosimetric comparison showed that APLN significantly increased the mean planning target volume (PTV) dose by 15.08 ± 0.43 Gy (50.3%) compared to WART. It significant decrease the OAR dose: 25.37 ± 3.63 Gy for liver, 24.71 ± 3.91 Gy for spleen, 8.77 ± 5.03 Gy for the right kidney, 8.68 ± 5.89 Gy for the left kidney, 8.14 ± 1.51 Gy for the small intestine, 24.42 ± 5.72 Gy for the stomach, and 3.05 ± 2.00 Gy for the spine cord, meanwhile it increased the OAR dose comparing with WART: 6.32 ± 2.61 Gy for the right femoral, 7.26 ± 1.44 Gy for the left femoral, 5.70 ± 4.24 Gy for bladder, 14.04 ± 4.01 Gy for rectum, and 4.93 ± 2.90 Gy for bone marrow (Tables 5 and 6).
 Click to view | Table 5. Dosimetric Differences Between Two Groups |
 Click to view | Table 6. TCP and NTCP Comparison of APLN and WART |
NTCP in the liver, kidney, and small intestine were decreased by 0.04-1.04%.
| Discussion | ▴Top |
The recommended treatment protocol for locally advanced ovarian cancer is first open surgery to remove as much of the lesion as possible [15], followed by first-line adjuvant chemotherapy [4]. WART has been suggested as a postoperative adjuvant treatment modality for patients with intermediate- to high-risk ovaries, but there has been controversy as to whether consolidation radiotherapy is recommended due to the toxic side effects caused by WART in the previous era of two-dimensional radiotherapy, as well as uncertain efficacy [8, 16, 17]. In earlier years, Bruzzone et al [18] and Lambert et al [19] randomized ovarian cancer patients to compare clinical outcomes between postoperative consolidation WART or continuation of the same chemotherapy regimen and showed no statistical difference in DFS and OS between the two groups. In 1993, a review by Thomas et al included 28 studies (713 patients) and this review showed no clinical benefit of WAI in the consolidation of patients with advanced ovarian cancer [19]. However, encouraging results were shown in two subsequent randomized trials [20, 21]. A randomized study by Pickel et al in 1999 evaluated WAI consolidation therapy after surgery for stage IC-IV ovarian cancer, with significantly higher 5-year DFS and OS compared to chemotherapy alone group [20]. A prospective study by Sorbe et al in 2003, for stage III ovarian cancer patients, observed a progression-free survival benefit in the group undergoing consolidation radiotherapy [21], and with the advancement of the IMRT, IMRT has also been used in WART [11], and there are related studies that have performed simultaneous integrated boost-IMRT with increased irradiation dose, although there are only small dosimetric [12, 13] and phase I clinical studies, but also achieved good clinical efficacy with a DFS of 77 months and relatively low toxic effects [22]. Table 7 lists the relevant literature and reported results on WART for consolidative radiotherapy in recent years.
 Click to view | Table 7. Literature and Reported Results on WART for Consolidative Radiotherapy |
The local failure mode in patients with locally advanced ovarian cancer is mostly the lymphatic drainage area near blood vessels [23, 24], and our center innovatively applied preventive irradiation of the abdominopelvic lymphatic drainage area instead of total abdominopelvic irradiation, which greatly reduced the normal tissue exposure and increased the target area irradiation dose to 45 - 50 Gy. The mean follow-up time was 64.10 months (5.5 - 113.2 months), 5-year OS was 61.5%, 5-year DFS was 40.9%, and median DFS was 40.8 months after a course of adjuvant chemotherapy with the application of IMRT technique for radiotherapy to the abdominopelvic lymphatic drainage area, with improved median survival and OS in patients with advanced ovarian cancer compared to the SEER database (40,692 patients in the 1995 - 2007 database), and Baldwin et al reported relative 5-year survival rates of 36% and 17% for patients with FIGO stage III and IV epithelial ovarian cancer, respectively [14], compared with a median DFS of 27.6 months and 5-year OS of 33.0% in the OVAR-IMRT-01 study, both of which were greatly improved. Patients who underwent APLN were followed up, with distant metastasis as the main failure mode and local recurrence in the irradiated field in fewer patients, accounting for only 2/99 cases, confirming the relevance of performing abdominopelvic lymphatic drainage area prophylactic radiotherapy in patients with locally advanced ovarian cancer. Moreover, the associated toxic side effects were small due to the reduction of irradiation field, with acute side effects: grade 3 hematological toxicity in 67/99 cases, and for late toxic side effects, a total of 10 patients showed grade 3 gastrointestinal disorders, one patient died due to the repeated three times operations. Comparing with the reported literature above, the severe advanced toxic side effects were small.
In subgroup analysis, the number of surgeries was an independent risk factor for LRFS (P = 0.023). It is suggested that postoperative prophylactic radiotherapy should be administered as early as possible, as patients who received radiotherapy immediately after initial surgery had a longer duration of local progression-free survival and a better effect compared to patients with a history of two or more surgeries. It is suggested that for patients with locally advanced ovarian cancer, it may be more clinically relevant to receive postoperative radiotherapy as early as possible after standard surgery and chemotherapy.
Dosimetric validation was collected for normal tissue and target area DVHs, and computer code was written and saved as a Matlab executable program to calculate the EUD for PTV and OAR, which in turn calculated NTCP and TCP. Comparison of planned designs for irradiation of the APLN and WART in five patients showed a significant increase in mean planning target compared to total abdominal irradiation radiotherapy volume (PTV) dose of 10.8 ± 4.4 Gy (25%). Reduced OAR dose was 10.8 ± 4.4 Gy (25%) for liver, 10.8 ± 4.4 Gy (25%) for kidney, 10.8 ± 4.4 Gy (25%) for the small intestine, etc. TCP was increased by 23% (± 21%) and NTCP was reduced by 23% (± 21%) for the liver, kidney, and small intestine. Dosimetric analysis showed that prophylactic irradiation of the abdominopelvic lymphatic drainage area increased the tumor control rate compared with total abdominal irradiation, while ensuring a low dose of abdominopelvic OAR, which validated the clinical efficacy of prophylactic irradiation of the abdominopelvic lymphatic drainage area.
Dosimetric comparison showed that APLN significantly increased the mean PTV dose by 15.08 ± 0.43Gy (50.3%) compared to WART. It significant decreased the OAR dose: 25.37 ± 3.63 Gy for liver, 24.71 ± 3.91 Gy for spleen, 8.77 ± 5.03 Gy for the right kidney, 8.68 ± 5.89 Gy for the left kidney, 8.14 ± 1.51 Gy for small intestine, 24.42 ± 5.72 Gy for stomach, and 3.05 ± 2.00 Gy for spine cord, meanwhile it increased the OAR dose comparing with WART: 6.32 ± 2.61 Gy for the right femoral, 7.26 ± 1.44 Gy for the left femoral, 5.70 ± 4.24 Gy for bladder, 14.04 ± 4.01 Gy for rectum, and 4.93 ± 2.90 Gy for bone marrow. NTCP in the liver, kidney, and small intestine were decreased by 0.04-1.04%.
This study was done as our center applied preventive radiotherapy to the abdominopelvic lymphatic drainage area in patients with locally advanced ovarian cancer with better clinical results, prolonged DFS and acceptable toxicities compared to previous literature. The dosimetric validation was also performed. However, being a retrospective single-center study, there may be selective bias. Secondly, there are many confounding factors related to the pathological type of patients, and there may be confounding bias, which is suitable for further clarification in a prospective, multicenter study.
Conclusion
Consolidative radiotherapy to the abdominopelvic lymphatic drainage area (IMRT at a dose of 45 - 50 Gy/25 - 28 fractions), as an alternative to WART, provides excellent local control rates for the consolidation of locally advanced ovarian cancer, and prolongs the disease-free progression time, improves survival, and has acceptable toxic effects compared to previous literature reports of WART. Subgroup analysis showed a longer time to local recurrence-free progression and a better effect in patients who received radiotherapy immediately after initial surgery, suggesting that APLN consolidative radiotherapy should be administered as early as possible. Dosimetric analysis based on the EUD calculation procedure showed that PALN increased TCP while maintaining a low dose of abdominopelvic NTCP compared to WART, which also validated the clinical efficacy of APLN to the WART.
Acknowledgments
None to declare.
Financial Disclosure
This study was funded by the National Key Research and Development Plan, the Ministry of Science and Technology of the People’s Republic of China [grant number 2016YFC0105207].
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
SJ was responsible for data collection, drafted the manuscript, and performed statistic analysis and data interpretation; TYJ was responsible for data collection and data interpretation; GH participated in the design of the study and data interpretation; ZHN participated in the design of the study; DTT performed data interpretation; HL was responsible for data collection; LZK designed the study and revised the manuscript. All authors read and approved the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Yossi S, Nguyen D, Khodri M, Reure J, Cervellera M, Lamberth F, Marchand V, et al. [Radiotherapy for ovarian carcinoma management: Literature review]. Cancer Radiother. 2020;24(2):159-165.
doi pubmed - Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Pineros M, Znaor A, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941-1953.
doi pubmed - Torre LA, Trabert B, DeSantis CE, Miller KD, Samimi G, Runowicz CD, Gaudet MM, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284-296.
doi pubmed - Armstrong DK, Alvarez RD, Bakkum-Gamez JN, Barroilhet L, Behbakht K, Berchuck A, Berek JS, et al. NCCN guidelines insights: ovarian cancer, Version 1.2019. J Natl Compr Canc Netw. 2019;17(8):896-909.
doi pubmed - Schray MF, Martinez A, Howes AE, Ballon SC, Podratz KC, Sikic BI, Malkasian GD. Advanced epithelial ovarian cancer: toxicity of whole abdominal irradiation after operation, combination chemotherapy, and reoperation. Gynecol Oncol. 1986;24(1):68-80.
doi - Shetty MR. Toxicity of whole abdominal irradiation after operation, combination chemotherapy, and reoperation in advanced epithelial ovarian cancer. Gynecol Oncol. 1987;26(3):403-404.
doi - Rochet N, Jensen AD, Sterzing F, Munter MW, Eichbaum MH, Schneeweiss A, Sohn C, et al. Adjuvant whole abdominal intensity modulated radiotherapy (IMRT) for high risk stage FIGO III patients with ovarian cancer (OVAR-IMRT-01) - Pilot trial of a phase I/II study: study protocol. BMC Cancer. 2007;7:227.
doi pubmed - Iorio GC, Martini S, Arcadipane F, Ricardi U, Franco P. The role of radiotherapy in epithelial ovarian cancer: a literature overview. Med Oncol. 2019;36(7):64.
doi pubmed - Arians N, Kieser M, Benner L, Rochet N, Schroder L, Katayama S, Herfarth K, et al. Adjuvant intensity modulated whole-abdominal radiation therapy for high-risk patients with ovarian cancer FIGO stage III: final results of a prospective phase 2 study. Radiat Oncol. 2019;14(1):179.
doi pubmed - Kumar R, De Jesus O. Radiation effects on the fetus. In: StatPearls. Treasure Island (FL), 2022.
- Rai B, Bansal A, Patel FD, Sharma SC. Radiotherapy for ovarian cancers - redefining the role. Asian Pac J Cancer Prev. 2014;15(12):4759-4763.
doi pubmed - Mahantshetty U, Jamema S, Engineer R, Deshpande D, Sarin R, Fogliata A, Nicolini G, et al. Whole abdomen radiation therapy in ovarian cancers: a comparison between fixed beam and volumetric arc based intensity modulation. Radiat Oncol. 2010;5:106.
doi pubmed - Shetty UM, Shankar S, Engineer R, Chopra S, Gupta S, Maheshwari A, Kerkar R, et al. Image-guided intensity-modulated whole abdominal radiation therapy in relapsed epithelial ovarian cancers: a feasibility study. J Cancer Res Ther. 2013;9(1):17-21.
doi pubmed - Baldwin LA, Huang B, Miller RW, Tucker T, Goodrich ST, Podzielinski I, DeSimone CP, et al. Ten-year relative survival for epithelial ovarian cancer. Obstet Gynecol. 2012;120(3):612-618.
doi pubmed - Hacker NF, Rao A. Surgery for advanced epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:71-87.
doi pubmed - McCormack M. Radiation therapy in ovarian cancer: an overview and future directions. Clin Oncol (R Coll Radiol). 2018;30(8):504-506.
doi pubmed - Fields EC, McGuire WP, Lin L, Temkin SM. Radiation treatment in women with ovarian cancer: past, present, and future. Front Oncol. 2017;7:177.
doi pubmed - Bruzzone M, Repetto L, Chiara S, Campora E, Conte PF, Orsatti M, Vitale V, et al. Chemotherapy versus radiotherapy in the management of ovarian cancer patients with pathological complete response or minimal residual disease at second look. Gynecol Oncol. 1990;38(3):392-395.
doi - Lambert HE, Rustin GJ, Gregory WM, Nelstrop AE. A randomized trial comparing single-agent carboplatin with carboplatin followed by radiotherapy for advanced ovarian cancer: a North Thames Ovary Group study. J Clin Oncol. 1993;11(3):440-448.
doi pubmed - Pickel H, Lahousen M, Petru E, Stettner H, Hackl A, Kapp K, Winter R. Consolidation radiotherapy after carboplatin-based chemotherapy in radically operated advanced ovarian cancer. Gynecol Oncol. 1999;72(2):215-219.
doi pubmed - Sorbe B. Consolidation treatment of advanced ovarian carcinoma with radiotherapy after induction chemotherapy. Int J Gynecol Cancer. 2003;13(Suppl 2):192-195.
doi pubmed - Stevens MJ, West S, Gard G, Renaud C, Nevell D, Roderick S, Le A. Utility of adjuvant whole abdominal radiation therapy in ovarian clear cell cancer (OCCC): a pragmatic cohort study of women with classic immuno-phenotypic signature. Radiat Oncol. 2021;16(1):29.
doi pubmed - Gupta S, Nag S, Aggarwal S, Rauthan A, Warrier N. Maintenance therapy for recurrent epithelial ovarian cancer: current therapies and future perspectives - a review. J Ovarian Res. 2019;12(1):103.
doi pubmed - Stewart C, Ralyea C, Lockwood S. Ovarian cancer: an integrated review. Semin Oncol Nurs. 2019;35(2):151-156.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


