| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 3, June 2023, pages 195-204
Impact of Aspirin Use on Outcomes in Patients With Hepatocellular Cancer: A Nationwide Analysis
Armaan Dhaliwala, g, h, Aalam Sohalb, g, Kanwal Bainsa, Hunza Chaudhryc, Ishandeep Singhd, Eva Kalrad, Kirti Arorad, Dino Dukovice, Alejandro Recio Boilesf
aDepartment of Internal Medicine, University of Arizona, South Campus, Tuscon, AZ, USA
bDepartment of Hepatology, Liver Institute Northwest, Seattle, WA, USA
cDepartment of Internal Medicine, University of California San Francisco, Fresno, CA, USA
dDepartment of Medicine, Dayanand Medical College and Hospital, Ludhiana, India
eDepartment of Internal Medicine, Ross University School of Medicine, Bridgetown, Barbados
fDepartment of Hematology & Medical Oncology, University of Arizona Cancer Center, Tuscon, AZ, USA
gThese authors contributed equally to this work.
hCorresponding Author: Armaan Dhaliwal, Department of Internal Medicine, University of Arizona, South Campus, PO Box 245005, Tucson, AZ, USA
Manuscript submitted April 21, 2023, accepted May 16, 2023, published online June 11, 2023
Short title: Aspirin Use on Outcomes in HCC Patients
doi: https://doi.org/10.14740/wjon1601
| Abstract | ▴Top |
Background: Despite the use of new immunotherapies, hepatocellular carcinoma (HCC) has a poor survival rate. Through multiple molecular mechanisms, aspirin (ASA) has demonstrated a reduced incidence of HCC, however, the impact of long-term ASA use on in-hospital outcomes has not been studied.
Methods: We queried the National Inpatient Sample (NIS) database from 2016 to 2020 to identify patients with HCC. Patients were stratified into two groups, based on long-term ASA use. Information was collected regarding patient demographics, Elixhauser comorbidities, interventions, etiology, and decompensations of liver disease. Outcomes studied included sepsis, shock, acute kidney injury (AKI), intensive care unit (ICU) admission, and in-hospital mortality. The association between long-term ASA use and outcomes was studied using multivariate analysis.
Results: A total of 224,735 patients were included in the study. Of them, 18,835 (8.4%) patients were on long-term ASA. The majority of the patients with ASA use were White (61.3%), men (78.2%), and aged > 65 years old (68.8%). Patients in the ASA group had a higher incidence of non-alcoholic steatohepatitis (NASH) and decreased rates of hepatic decompensation than those not on ASA. Patients with ASA use had lower incidence of sepsis (2.76% vs. 3.54%), shock (4.86% vs. 8.23%), AKI (30.9% vs. 33.4%), ICU admission (3.88% vs. 7.4%) and in-hospital mortality (5.18% vs. 9.87%). After adjusting for confounding factors, ASA use was associated with a 30% lower risk of in-hospital mortality (adjusted odds ratio (aOR): 0.70, 95% confidence interval (CI): 0.60 - 0.82, P < 0.001). ASA users also had 21% lower odds of developing shock (aOR: 0.79, 95% CI: 0.67 - 0.94, P = 0.007) and 31% lower odds of requiring ICU admission (aOR: 0.69, 95% CI: 0.54 - 0.78, P < 0.001).
Conclusions: Our study noted that patients on long-term ASA use had better in-hospital outcomes such as mortality, shock, and ICU admissions compared to non-ASA users. These findings are of interest, and further randomized clinical trials confirming the benefits of ASA in improving outcomes in HCC patients need to be conducted.
Keywords: HCC; Aspirin; NIS; Outcomes
| Introduction | ▴Top |
Aspirin’s usefulness in cancer started with experimental and clinical evidence demonstrating a reduced risk and mortality of colorectal cancer (CRC), corroborated by a 20-year follow-up of five randomized controlled trials (RCTs) [1]. In addition to CRC, aspirin use has been linked to reduced incidence of stomach, prostate, breast, endometrial cancers and basal cell carcinoma of skin [2-6]. Several studies have indicated overexpression of cyclooxygenase 2 (COX-2) in liver injury and well-differentiated types of hepatocellular carcinoma (HCC) [7, 8]. High COX-2 expression aided in invasion and metastasis in a study conducted in HCC cell lines [9] and a meta-analysis highlighting worse overall survival (OS) in HCC patients with COX-2 overexpression [10]. Therefore, aspirin, through its modulation of multiple molecular pathways, including inhibition of COX enzymes, has demonstrated anti-tumor activity with an insignificant increase in the incidence of gastrointestinal bleeding [11]. Additionally, its low cost and easy availability allow an opportunity to use aspirin in the management of HCC.
HCC represents about 80% of newly diagnosed liver cancers worldwide. It has a relative 5-year survival rate of less than 20% and is the third most common cause of cancer-related mortality in the world [12]. HCC has a dismal survival rate, with advanced HCC cases reported to have a median survival time of less than a year [13]. The poor prognosis of the disease can be explained by the lack of effective therapeutic options available. Despite the development of novel systemic therapies in the last 10 years, the survival rates in intermediate and advanced HCC cases are extremely low [14]. Therefore, research into newer interventions and modalities to improve the prognosis in this population of patients has received tremendous attention in recent years.
Numerous retrospective cohort studies and meta-analyses have demonstrated the chemoprotective effect of aspirin in HCC [15-25]. However, these studies are limited by non-randomization, various causes of cirrhosis, hepatitis B virus (HBV)/hepatitis C Virus (HCV) status, confounders (age, chemopreventive medications), and alcohol use. Moreover, its utility as an adjuvant chemotherapeutic agent for managing HCC is also gaining traction. The use of aspirin in reducing the risk and mortality associated with HCC is dose and duration dependent, with benefits evident after 5 or more years of use [17]. Till now, no studies have assessed the impact of long-term aspirin use on inpatient outcomes in patients with HCC at a national level. The present study sought to measure this impact.
| Materials and Methods | ▴Top |
Data source
The National Inpatient Sample (NIS) is under the maintenance of the Healthcare Cost and Utilization Project (HCUP). It is the largest database of inpatient hospital stays in the United States [26]. The NIS data can be reliably used to estimate disease burden and outcomes. Each hospitalization is deidentified and maintained in the NIS as a unique entry.
Ethics approval statement
The data used in this study was obtained from NIS which includes deidentified patient information. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as the Helsinki Declaration. The Institutional Review Board (IRB) approval was not required for this study as it is publicly available deidentified data.
Study population
The International Classification of Diseases 10th Version, Clinical Modification (ICD-10 CM) diagnosis codes were used to identify patients (≥ 18 years) hospitalized with HCC between 2016 and 2020. Patients were stratified into two groups based on the presence or absence of long-term aspirin use (Z79.82). All patients with missing mortality data or demographics were excluded. In total, 224,735 cases met the inclusion criteria. This is shown in Figure 1.
 Click for large image | Figure 1. Inclusion flow diagram of patients with HCC. HCC: hepatocellular carcinoma. |
Study outcomes and variables
The primary outcome was comparing inpatient mortality between aspirin and non-aspirin-using HCC patients. We also compared other outcomes such as sepsis, shock, acute kidney injury (AKI), and intensive care unit (ICU) admissions.
Our primary exposure variable was aspirin usage by HCC patients. We also collected information on other variables such as age, gender, race, primary insurance, median income, hospital characteristics such as region, bed size, rural/urban location prespecified by HCUP, and transfer status. Data were also collected on the common causes of liver disease, such as alcoholic liver disease, hepatitis B, hepatitis C, and non-alcoholic fatty liver disease (NAFLD). The comorbidity burden was assessed using the Elixhauser comorbidity index [27]. This is a well-validated index based on ICD-10-CM codes meant to be used in large administrative data to predict mortality and hospital resource use. The index has 31 comorbid categories. We also studied the various decompensations of the disease. These included ascites, hepatic encephalopathy, spontaneous bacterial peritonitis (SBP), and hepatorenal syndrome (HRS).
Statistical analysis
Hospital-level discharge weights provided by NIS were used to generate national estimates. We used Chi-square and independent sample t-tests to compare categorical and continuous variables. Univariate regression was done to study the effect of variables on outcomes. A P value of 0.1 was considered a cutoff. All the variables that met the criteria were included for multivariate logistic regression for categorical outcomes. The adjusted odds ratio (aOR) was calculated with a 95% confidence interval (CI). A type I error of < 0.05 was considered statistically significant. Data analysis was done using STATA 17.0 (Texas).
| Results | ▴Top |
Patient characteristics
Out of 142,338,643 US hospitalizations between 2016 and 2019, 237,730 adult patients were diagnosed with HCC, and 12,995 patients had missing data. There were 224,735 patients in the HCC study population. Out of them, 18,835 (8.4%) were aspirin users, while 205,900 (91.6%) were non-aspirin users. The majority of the patients in the aspirin group were aged > 65 years (68.75%), males (78.2%), Whites (61.3%), and had Medicare insurance (67.5%). More than 90% of the patients in the aspirin group had three or more comorbidities. The results are described in Table 1.
 Click to view | Table 1. Patient Characteristics, Stratified by the Use of Aspirin |
Comorbidities
Patients who were aspirin users had a higher incidence of congestive heart failure, cardiac arrhythmias, valvular disease, peripheral vascular disorders, and chronic pulmonary disease. A lower incidence of metastatic cancer, alcohol abuse, and coagulopathy was noted in the aspirin group compared to non-aspirin users. The results are presented in Table 2.
 Click to view | Table 2. Comorbidities, Stratified by the Presence of Aspirin Use |
Etiology and decompensations of liver disease
A higher incidence of non-alcoholic steatohepatitis (NASH) was noted in the aspirin group compared to non-aspirin users. A lower incidence of hepatitis B, hepatitis C, and alcohol-related liver disease was noted in the aspirin group compared to non-aspirin users. Aspirin users had lower rates of decompensations, such as hepatic encephalopathy, ascites, variceal bleeding, SBP, and HRS. This information is presented in Table 3.
 Click to view | Table 3. Etiology and Decompensations, Stratified by the Presence of Aspirin Use |
Interventions
Our study noted lower rates of blood transfusion (10.35% vs. 13.91%, P < 0.001) and endoscopy (10.17% vs. 12.18%) in the aspirin group, compared to the non-aspirin group. Lower rates of transjugular intrahepatic portosystemic shunt (TIPS) were also noted in the aspirin group compared to another group (0.05% vs. 0.28%, P = 0.009). No statistically significant difference was noted in the rates of liver transplantation between the two groups (P = 0.47).
Outcomes
In-hospital mortality
Total in-hospital mortality in the study population was 21,295 (9.48%). The mortality rate in patients with aspirin use was 5.18% compared to 9.87% in patients without aspirin. Outcomes stratified by aspirin use is presented in Figure 2. On multivariate analysis, patients with aspirin use had a statistically significant lower risk of mortality than patients without aspirin use (aOR = 0.70, P < 0.001). The results of multivariate analysis are presented in Table 4.
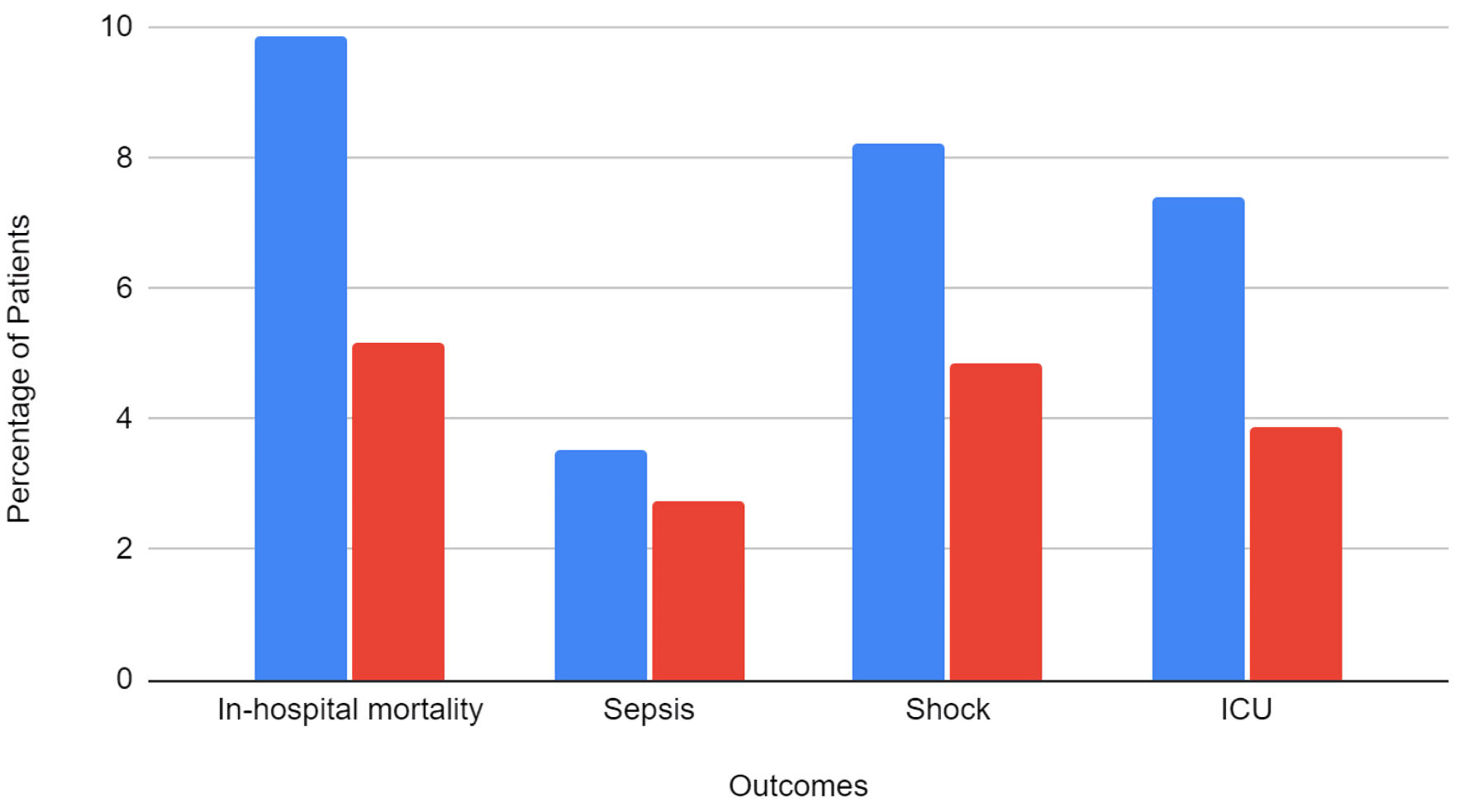 Click for large image | Figure 2. Categorical outcomes, stratified by the presence of aspirin use. ICU: intensive care unit. |
 Click to view | Table 4. Results of Multivariate Logistic Regression Identifying Association Between Aspirin and Categorical Outcomes |
Sepsis
The total incidence of sepsis in the study population was 3.48%. About 2.76% of patients with aspirin use and 3.54% of patients without aspirin use developed sepsis. On multivariate analysis, the results were not statistically significant between the groups.
Shock
A total of 17,865 (7.95%) patients developed shock. The incidence of shock was 4.86% in patients with aspirin use compared to 8.23% in patients without aspirin use. On multivariate analysis, patients with aspirin use had a statistically significant lower risk of shock (aOR = 0.79, P = 0.007).
AKI
A total of 74,655 (33.22%) patients developed AKI. There were 5,810 (30.9%) patients with aspirin use who developed AKI compared to 68,845 (33.44%) patients without aspirin use. On multivariate analysis, the results were not statistically significant between the groups (P = 0.73).
ICU admission
A total of 15,975 (7.11%) patients required ICU admission. There were 730 (3.88%) patients with aspirin use who required ICU admission compared to 15,245 (7.4%) patients without aspirin use. On multivariate analysis, patients with aspirin use had a statistically significant lower risk of ICU admission (aOR = 0.65, P < 0.001).
| Discussion | ▴Top |
In our national analysis of 224,735 patients in the United States, we noted that long-term aspirin users had lower odds of mortality than the controls. Our study is the first retrospective study evaluating inpatient clinical outcomes observed in HCC patients in aspirin users vs. patients not on aspirin. A point of interest highlighting the significance of the difference in mortality rates pertains to the age demographic of the population studied. The aspirin group was significantly older, with 68.75% of the users greater than 65 years of age compared to only 50.57% of the non-aspirin group. Our results align with the findings of a meta-analysis consisting of six studies in HCC patients treated with liver resection, or trans-arterial chemoembolization (TACE) that reported a decrease in all-cause mortality in aspirin users by 41% compared to non-aspirin users [23]. Another meta-analysis of two studies showed a statistically significant reduction in 2-year and 4-year mortality in HCC patients using aspirin [24]. A Japanese study of HCC patients by Hayashi et al corroborated the improved OS and reduced liver-associated mortality without any difference in gastrointestinal bleeding risk [28]. Conversely, researchers using the National Cohort Study of Korean Adults demonstrated a reduction in HCC incidence risk with aspirin use, but no changes in the HCC-related mortality in HBV patients [18].
The mechanism by which aspirin affects the incidence and prognosis of HCC is unclear. However, several hypotheses have been suggested. HCC is inflammation-driven cancer with cycles of hepatocyte damage and regeneration, resulting in chronic inflammation progressing to fibrosis and cirrhosis. Platelets can promote inflammation and immune-related hepatocyte injury through the proliferation of inflammatory and immune cells [29]. A meta-analysis demonstrated a poor prognosis in advanced HCC patients with higher platelet-to-lymphocyte ratio, reinforcing the key role played by platelets in determining the outcomes in HCC patients [30]. Reports of aspirin in preventing fibrosis and progression to HCC through its inhibition of intrahepatic platelet, immune cell activation, suppression of platelet-derived growth factor-β and consequently, suppressing hepatic stellate cell activity, are present in the literature [31, 32]. Furthermore, a study conducted in a nude mouse xenograft model demonstrated that increased interferon (INF) α-mediated apoptosis and anti-proliferation of HCC cells through signal transducer and activator of transcription could lead to increased deposition of collagen and tumor growth, and aspirin has shown to reduce the expression of P4HA2, exhibiting another mechanism of action responsible for reducing fibrosis and HCC growth [33].
Our study also showed that aspirin users had lower incidence in rate of hepatic decompensation, such as hepatic encephalopathy, ascites, varices, SBP, and HRS. This finding is interesting as the association between aspirin use and rates of decompensation has not been studied previously. Imbalance in arachidonic acid metabolite levels like thromboxane and prostaglandins has been implicated in the pathogenesis of HRS, which can explain the beneficial effects of aspirin in HRS [34]. A lower severity of the disease in aspirin users, as evidenced by rates of shock and ICU admissions in the aspirin group, was also noted. Our study suggests that the use of aspirin not only reduces in-hospital mortality but might also play a role in decreasing the severity of liver disease and admissions.
Our study showed lower incidence of blood transfusion as well as endoscopy in aspirin users. There are currently mixed data on this finding, with the study by Hayashi et al of HCC patients reporting no increase in the gastrointestinal bleeding risk [28]. On the contrary, Ma et al in their meta-analysis, reported an increased bleeding risk in the aspirin group [35]. The increased risk of bleeding with a low dose of aspirin was suggested by another meta-analysis consisting of 31 RCTs [36]. A study on rats with portal hypertension demonstrated reduced hemorrhage rates in rats injected with ultra-low doses of aspirin [37]. The study was conducted after ultra-low doses of aspirin showed improved platelet aggregation [38]. Moreover, a 26-patient case study concluded a combination of low-dose aspirin with thrombopoietin (TPO) receptor agonists showed improved platelet recovery in cirrhosis-associated thrombocytopenia without any hemorrhagic complications [39].
A higher incidence of NASH was noted in the aspirin group. This is likely because patients with NASH have a higher incidence of cardiovascular comorbidities. Furthermore, the aspirin group had a lower proportion of patients with alcohol-related liver disease, hepatitis B and C. The data regarding the mortality benefit of aspirin use in patients with hepatitis B are mixed. A Taiwanese study analyzed data related to HBV-associated HCC undergoing liver resection from the National Health Insurance Research Database and found a 23% decrease in recurrence and a 43% drop in mortality in patients on aspirin and/or clopidogrel compared to non-users [40, 41]. Another retrospective cohort study conducted employing the nationwide Swedish patient registries showed a 31% drop in HCC risk and a 27% lower risk of mortality in HBV and HCV patients [42]. Conversely, another Taiwanese veterans study did not show any improvement in OS in aspirin users in HCC patients secondary to HBV infection, treated with tumor resection, despite an 82% decrease in HCC recurrence [22].
Due to the increasing literature on this topic, aspirin use in HCC management has been gaining traction. Adjuvant aspirin use has been shown to be associated with improved OS in patients receiving sorafenib, the first-line protein kinase inhibitor approved for HCC [43]. An Italian study demonstrated improvement in OS and progression-free survival (PFS) in HCC patients on sorafenib concomitantly using aspirin, but the positive results did come at the cost of increased bleeding risk [44]. Pre-clinical studies evaluating the effects of adjuvant aspirin demonstrated increased sensitivity of HCC to sorafenib mediated by upregulation of mammalian target of rapamycin complex 2 (mTORC2) and silencing of long-chain fatty acid -CoA ligase 4 (ACSL4) targets [45, 46]. Sorafenib has been shown to have pro-metastatic effects, and aspirin can minimize this adverse effect through upregulation of human immunodeficiency virus (HIV)-1 Tat interactive protein 2 (HTATIP2) and downregulation of stromal cell-derived factor 1 SDF1-α [47, 48]. Through its effect on the inhibition of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), Li et al were able to show aspirin’s ability to overcome HCC resistance to sorafenib [49]. A trial is currently underway in China and is actively recruiting surgically resected liver cancer patients to compare the effectiveness of antiviral and aspirin treatment (NCT01936233). COX-2 overexpression in HCC has prompted researchers to evaluate the role of celecoxib, a COX-2 inhibitor in HCC, with studies performed in human HCC cell lines and mice demonstrating the benefits of celecoxib in enhancing the antitumor activity when combined with other HCC drugs [50, 51]. Further studies evaluating the role of aspirin and selective COX-2 inhibitors in HCC will be beneficial in understanding its long-term effects.
Numerous studies have highlighted the reduced incidence and recurrence of HCC development in cirrhotic patients with aspirin use, with ongoing discussions about its use as prophylaxis after the diagnosis of cirrhosis currently underway. A clinical trial (NCT02748304) evaluating tumor recurrence in high-risk HCC patients receiving a combination of sorafenib and aspirin was terminated in 2019 due to low recruitment rates after lenvatinib was approved for HCC, with most of the patients choosing to receive the new tyrosine kinase inhibitor. There is ample evidence in the literature to propel the use of aspirin in HCC management in combination with immunotherapies, provided there is more attention and expansion to clinical trials to reap the benefits of aspirin’s therapeutic potential in cancer, particularly HCC treatment. Our retrospective study is one of the few studies done on a North American patient population.
The systematic review and meta-analysis conducted by Ma et al involving six countries, mostly from the Asian continent, and including 3.2 million participants demonstrated a 30% reduction in HCC-related mortality in aspirin users compared to HCC patients not using aspirin [35]. Moreover, the review showed reduced HCC incidence and recurrence of 25% and 21%, respectively, in aspirin users. Taking a step further into the beneficial effects of aspirin, the study exhibited a dose and duration-dependent improvement in the outcomes of HCC patients. However, the bleeding risk was observed to be increased in aspirin users. A second meta-analysis by Zhou et al found HCC mortality rates to be lower by 29% in aspirin users compared to the non-aspirin group [52]. In contrast, a meta-analysis of 12 studies demonstrated no change in HCC-related mortality with non-aspirin NSAIDs [53].
Despite the presence of several studies conducted to evaluate the benefits of aspirin in HCC, no strict guidelines for using aspirin in HCC management are in place. Little support can be expected from the pharmaceutical industry to promote trials on potentially using an out-of-patent medication for HCC treatment which might present as an obstacle.
Despite the large sample size of our study, it has several limitations. Our study is retrospective in nature and, therefore, prone to bias. We used ICD-10 codes to identify aspirin users. Due to the nature of the database, it is difficult to ascertain the dose, duration and indication of aspirin use. The observational nature of the NIS study involved selecting aspirin users and therefore, carries selection bias as a fundamental limitation. The NIS database, albeit large, cannot provide information about individual patients. Moreover, there is no method to differentiate between initial and recurrent cases of HCC using the database. The findings of the study depend on accurate coding and appropriate documentation, which can be inconsistent depending on institutional and individual practices. Coding for HCC, aspirin use, and concomitant morbidities was conducted through the employment of distinct ICD-10 codes to minimize the errors involved with coding and to maintain the reproducibility of the study. Concomitant use of metformin or statins was not considered in our study, which requires a deeper understanding of their effects as these medications have shown to be independent predictors of improving outcomes in HCC patients [54, 55]. Additionally, our study was unable to differentiate the patients based on the HCC staging and the associated treatments. However, our study was successful in stratifying patients with HCC secondary to multiple etiologies, including alcoholic liver disease, NASH, and hepatitis B or C infections. Also, data on over-the-counter aspirin use was not available. Since aspirin use is associated with bleeding and patients with liver disorders have the predisposition to develop bleeding complications, further studies using the database can be conducted to study the bleeding outcomes observed in the HCC population using aspirin. Longitudinal follow-up of patients cannot be conducted using the information provided by the HCUP. Nevertheless, our study includes data over a period of 4 years and hence provides a large sample size to offset some of the limitations of the study. A large database allows our study to have a greater impact in comparison to the single-center studies, including a small population of patients.
Conclusions
Our study noted that patients on long-term aspirin use had better in-hospital outcomes such as mortality, shock, and ICU admissions compared to non-aspirin users. More research needs to be conducted in the form of clinical trials to provide reasonable evidence for the timing of initiation, duration of aspirin use, and response when combined with the current treatments in HCC management.
Acknowledgments
None to declare.
Financial Disclosure
The work of the authors is funded by 2P30 CA023074 supplement of the Cancer Center Support Grant from the NCI/NIH to the University of Arizona Cancer Center.
Conflict of Interest
All authors have no conflict of interest to declare.
Informed Consent
Not applicable.
Author Contributions
Armaan Dhaliwal, Aalam Sohal, Kanwal Bains, Hunza Chaudhry, Ishandeep Singh, Eva Kalra, Kirti Arora, Dino Dukovic and Alejandro Recio Boiles reviewed the literature, interpreted the data, drafted the manuscript, revised it for important intellectual content and were involved in the final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Data Availability
The data used in this study were obtained from NIS which includes deidentified patient information.
| References | ▴Top |
- Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741-1750.
doi pubmed - Niikura R, Hirata Y, Hayakawa Y, Kawahara T, Yamada A, Koike K. Effect of aspirin use on gastric cancer incidence and survival: A systematic review and meta-analysis. JGH Open. 2020;4(2):117-125.
doi pubmed pmc - Fan LL, Xie CP, Wu YM, Gu XJ, Chen YH, Wang YJ. Aspirin exposure and mortality risk among prostate cancer patients: a systematic review and meta-analysis. Biomed Res Int. 2019;2019:9379602.
doi pubmed pmc - Ma S, Guo C, Sun C, Han T, Zhang H, Qu G, Jiang Y, et al. Aspirin use and risk of breast cancer: a meta-analysis of observational studies from 1989 to 2019. Clin Breast Cancer. 2021;21(6):552-565.
doi pubmed - Wang Y, Zhao J, Chen X, Zhang F, Li X. Aspirin use and endometrial cancer risk: a meta-analysis and systematic review. Ann Transl Med. 2020;8(7):461.
doi pubmed pmc - Frankel L, Ardeljan AD, Takabe K, Rashid OM. The association between aspirin and basal cell carcinoma: a clinical and financial analysis. World J Oncol. 2022;13(6):343-349.
doi pubmed pmc - Cheng AS, Chan HL, Leung WK, To KF, Go MY, Chan JY, Liew CT, et al. Expression of HBx and COX-2 in chronic hepatitis B, cirrhosis and hepatocellular carcinoma: implication of HBx in upregulation of COX-2. Mod Pathol. 2004;17(10):1169-1179.
doi pubmed - Shiota G, Okubo M, Noumi T, Noguchi N, Oyama K, Takano Y, Yashima K, et al. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999;46(25):407-412.
pubmed - Guo Z, Jiang JH, Zhang J, Yang HJ, Yang FQ, Qi YP, Zhong YP, et al. COX-2 promotes migration and invasion by the side population of cancer stem cell-like hepatocellular carcinoma cells. Medicine (Baltimore). 2015;94(44):e1806.
doi pubmed pmc - Chen G, Li X, Yang J, Li J, Wang X, He J, Huang Z. Prognostic significance of cyclooxygenase-2 expression in patients with hepatocellular carcinoma: a meta-analysis. Arch Med Sci. 2016;12(5):1110-1117.
doi pubmed pmc - Ricciotti E, Wangensteen KJ, FitzGerald GA. Aspirin in hepatocellular carcinoma. Cancer Res. 2021;81(14):3751-3761.
doi pubmed pmc - Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33.
doi pubmed - Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A. Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2020;17(3):139-152.
doi pubmed - Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018.
doi pubmed - Sahasrabuddhe VV, Gunja MZ, Graubard BI, Trabert B, Schwartz LM, Park Y, Hollenbeck AR, et al. Nonsteroidal anti-inflammatory drug use, chronic liver disease, and hepatocellular carcinoma. J Natl Cancer Inst. 2012;104(23):1808-1814.
doi pubmed pmc - Petrick JL, Sahasrabuddhe VV, Chan AT, Alavanja MC, Beane-Freeman LE, Buring JE, Chen J, et al. NSAID Use and Risk of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: The Liver Cancer Pooling Project. Cancer Prev Res (Phila). 2015;8(12):1156-1162.
doi pubmed pmc - Simon TG, Ma Y, Ludvigsson JF, Chong DQ, Giovannucci EL, Fuchs CS, Meyerhardt JA, et al. Association Between Aspirin Use and Risk of Hepatocellular Carcinoma. JAMA Oncol. 2018;4(12):1683-1690.
doi pubmed pmc - Lee M, Chung GE, Lee JH, Oh S, Nam JY, Chang Y, Cho H, et al. Antiplatelet therapy and the risk of hepatocellular carcinoma in chronic hepatitis B patients on antiviral treatment. Hepatology. 2017;66(5):1556-1569.
doi pubmed - Shin S, Lee SH, Lee M, Kim JH, Lee W, Lee HW, Park MS, et al. Aspirin and the risk of hepatocellular carcinoma development in patients with alcoholic cirrhosis. Medicine (Baltimore). 2020;99(9):e19008.
doi pubmed pmc - Lee TY, Hsu YC, Tseng HC, Lin JT, Wu MS, Wu CY. Association of daily aspirin therapy with hepatocellular carcinoma risk in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2020;18(12):2784-2792.e2787.
doi pubmed - Liao YH, Hsu RJ, Wang TH, Wu CT, Huang SY, Hsu CY, Su YC, et al. Aspirin decreases hepatocellular carcinoma risk in hepatitis C virus carriers: a nationwide cohort study. BMC Gastroenterol. 2020;20(1):6.
doi pubmed pmc - Lee TY, Hsu YC, Tseng HC, Yu SH, Lin JT, Wu MS, Wu CY. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern Med. 2019;179(5):633-640.
doi pubmed pmc - Li X, Yu Y, Liu L. Influence of aspirin use on clinical outcomes of patients with hepatocellular carcinoma: a meta-analysis. Clin Res Hepatol Gastroenterol. 2021;45(6):101545.
doi pubmed - Tao Y, Li Y, Liu X, Deng Q, Yu Y, Yang Z. Nonsteroidal anti-inflammatory drugs, especially aspirin, are linked to lower risk and better survival of hepatocellular carcinoma: a meta-analysis. Cancer Manag Res. 2018;10:2695-2709.
doi pubmed pmc - Li X, Wu S, Yu Y. Aspirin use and the incidence of hepatocellular carcinoma in patients with hepatitis B virus or hepatitis C virus infection: a meta-analysis of cohort studies. Front Med (Lausanne). 2020;7:569759.
doi pubmed pmc - HCUP National Inpatient Sample (NIS). Healthcare cost and utilization project (HCUP). Agency for Healthcare Research and Quality, Rockville, MD. 2012.
- Agency for healthcare research and quality. Elixhauser Comorbidity Software Refined for ICD-10-CM.
- Hayashi T, Shibata M, Oe S, Miyagawa K, Honma Y, Harada M. Antiplatelet therapy improves the prognosis of patients with hepatocellular carcinoma. Cancers (Basel). 2020;12(11):3215.
doi pubmed pmc - Iannacone M, Sitia G, Isogawa M, Marchese P, Castro MG, Lowenstein PR, Chisari FV, et al. Platelets mediate cytotoxic T lymphocyte-induced liver damage. Nat Med. 2005;11(11):1167-1169.
doi pubmed pmc - Lin WF, Zhong MF, Zhang YR, Wang H, Zhao HT, Cheng BB, Ling CQ. Prognostic role of platelet-to-lymphocyte ratio in hepatocellular carcinoma with different BCLC stages: a systematic review and meta-analysis. Gastroenterol Res Pract. 2018;2018:5670949.
doi pubmed pmc - Malehmir M, Pfister D, Gallage S, Szydlowska M, Inverso D, Kotsiliti E, Leone V, et al. Platelet GPIbalpha is a mediator and potential interventional target for NASH and subsequent liver cancer. Nat Med. 2019;25(4):641-655.
doi pubmed - Assy N, Hussein O, Khalil A, Luder A, Szvalb S, Paizi M, Spira G. The beneficial effect of aspirin and enoxaparin on fibrosis progression and regenerative activity in a rat model of cirrhosis. Dig Dis Sci. 2007;52(5):1187-1193.
doi pubmed - Wang T, Fu X, Jin T, Zhang L, Liu B, Wu Y, Xu F, et al. Aspirin targets P4HA2 through inhibiting NF-kappaB and LMCD1-AS1/let-7g to inhibit tumour growth and collagen deposition in hepatocellular carcinoma. EBioMedicine. 2019;45:168-180.
doi pubmed pmc - Zipser RD, Radvan GH, Kronborg IJ, Duke R, Little TE. Urinary thromboxane B2 and prostaglandin E2 in the hepatorenal syndrome: evidence for increased vasoconstrictor and decreased vasodilator factors. Gastroenterology. 1983;84(4):697-703.
pubmed - Ma S, Qu G, Sun C, Liu H, Jiang Y, Li N, Wu B, et al. Does aspirin reduce the incidence, recurrence, and mortality of hepatocellular carcinoma? A GRADE-assessed systematic review and dose-response meta-analysis. Eur J Clin Pharmacol. 2023;79(1):39-61.
doi pubmed - Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Baggish JS, Bhatt DL, Topol EJ. Analysis of risk of bleeding complications after different doses of aspirin in 192,036 patients enrolled in 31 randomized controlled trials. Am J Cardiol. 2005;95(10):1218-1222.
doi pubmed - Eizayaga FX, Aguejouf O, Belon P, Doutremepuich C. Platelet aggregation in portal hypertension and its modification by ultra-low doses of aspirin. Pathophysiol Haemost Thromb. 2005;34(1):29-34.
doi pubmed - Doutremepuich C, de Seze O, Le Roy D, Lalanne MC, Anne MC. Aspirin at very ultra low dosage in healthy volunteers: effects on bleeding time, platelet aggregation and coagulation. Haemostasis. 1990;20(2):99-105.
doi pubmed - Osuga T, Yoshiyasu KY, Yoshida S, Kim SK, Ikura Y, Kim SR, Funatsu E, et al. Effects of low-dose aspirin administration on cirrhosis-related thrombocytopenia: report of 26 cases. Int J Clin Exp Med. 2017;10(11):15376-15383.
- Lee PC, Yeh CM, Hu YW, Chen CC, Liu CJ, Su CW, Huo TI, et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann Surg Oncol. 2016;23(Suppl 5):874-883.
doi pubmed - Jang H, Lee YB, Moon H, Chung JW, Nam JY, Cho EJ, Lee JH, et al. Aspirin use and risk of hepatocellular carcinoma in patients with chronic hepatitis B with or without cirrhosis. Hepatology. 2022;76(2):492-501.
doi pubmed - Simon TG, Duberg AS, Aleman S, Chung RT, Chan AT, Ludvigsson JF. Association of aspirin with hepatocellular carcinoma and liver-related mortality. N Engl J Med. 2020;382(11):1018-1028.
doi pubmed pmc - Casadei-Gardini A, Rovesti G, Dadduzio V, Vivaldi C, Lai E, Lonardi S, Fornaro L, et al. Impact of Aspirin on clinical outcome in advanced HCC patients receiving sorafenib and regorafenib. HPB (Oxford). 2021;23(6):915-920.
doi pubmed - Ielasi L, Tovoli F, Tonnini M, Tortora R, Magini G, Sacco R, Pressiani T, et al. Beneficial prognostic effects of aspirin in patients receiving sorafenib for hepatocellular carcinoma: a tale of multiple confounders. Cancers (Basel). 2021;13(24):6376.
doi pubmed pmc - Gao M, Kong Q, Hua H, Yin Y, Wang J, Luo T, Jiang Y. AMPK-mediated up-regulation of mTORC2 and MCL-1 compromises the anti-cancer effects of aspirin. Oncotarget. 2016;7(13):16349-16361.
doi pubmed pmc - Xia H, Lee KW, Chen J, Kong SN, Sekar K, Deivasigamani A, Seshachalam VP, et al. Simultaneous silencing of ACSL4 and induction of GADD45B in hepatocellular carcinoma cells amplifies the synergistic therapeutic effect of aspirin and sorafenib. Cell Death Discov. 2017;3:17058.
doi pubmed pmc - Lu L, Sun HC, Zhang W, Chai ZT, Zhu XD, Kong LQ, Wang WQ, et al. Aspirin minimized the pro-metastasis effect of sorafenib and improved survival by up-regulating HTATIP2 in hepatocellular carcinoma. PLoS One. 2013;8(5):e65023.
doi pubmed pmc - Lu L, Lu M, Pei Y, Chen J, Qin L, Zhu W, Jia H. Down-regulation of SDF1-alpha expression in tumor microenvironment is associated with aspirin-mediated suppression of the pro-metastasis effect of sorafenib in hepatocellular carcinoma. Acta Biochim Biophys Sin (Shanghai). 2015;47(12):988-996.
doi pubmed - Li S, Dai W, Mo W, Li J, Feng J, Wu L, Liu T, et al. By inhibiting PFKFB3, aspirin overcomes sorafenib resistance in hepatocellular carcinoma. Int J Cancer. 2017;141(12):2571-2584.
doi pubmed - Chu TH, Chan HH, Hu TH, Wang EM, Ma YL, Huang SC, Wu JC, et al. Celecoxib enhances the therapeutic efficacy of epirubicin for Novikoff hepatoma in rats. Cancer Med. 2018;7(6):2567-2580.
doi pubmed pmc - Hu JW, Chen B, Zhang J, Qi YP, Liang JH, Zhong JH, Xiang BD. Novel combination of celecoxib and metformin improves the antitumor effect by inhibiting the growth of Hepatocellular Carcinoma. J Cancer. 2020;11(21):6437-6444.
doi pubmed pmc - Zhou X, Zhang T, Sun Y, Li C, Ding X, Zhu Y, Li L, et al. Systematic review and meta-analysis: association of aspirin with incidence of hepatocellular carcinoma. Front Pharmacol. 2022;13:764854.
doi pubmed pmc - Pang Q, Jin H, Qu K, Man Z, Wang Y, Yang S, Zhou L, et al. The effects of nonsteroidal anti-inflammatory drugs in the incident and recurrent risk of hepatocellular carcinoma: a meta-analysis. Onco Targets Ther. 2017;10:4645-4656.
doi pubmed pmc - Chiu HF, Ho SC, Chen CC, Yang CY. Statin use and the risk of liver cancer: a population-based case-control study. Am J Gastroenterol. 2011;106(5):894-898.
doi pubmed - Ford RJ, Fullerton MD, Pinkosky SL, Day EA, Scott JW, Oakhill JS, Bujak AL, et al. Metformin and salicylate synergistically activate liver AMPK, inhibit lipogenesis and improve insulin sensitivity. Biochem J. 2015;468(1):125-132.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


