| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 4, August 2023, pages 266-276
Anticancer and Antimutagenic Properties of Pogonatherum paniceum on Colorectal Cancer Cells
Ratsada Praphasawata , Sinittra Thakaewb, Anchalee Rawangkanb, Rungthip Thongboonthoc, Pornchai Sooksaena, Sarunya Laovittayangkoond, Warangkhana Klajinge, Pongnared Jaengprommae, Paween Kunsornf, Prasit Suwannalertf, Witchuda Payuhakritf, g
aDepartment of Pathology, School of Medicine, University of Phayao, Phayao, Thailand
bDivision of Microbiology, School of Medical Sciences, University of Phayao, Phayao, Thailand
cDivision of Biochemistry, School of Medical Science, University of Phayao, Phayao, Thailand
dExpert Centre of Innovative Herbal Products (InnoHerb), Thailand Institute of Scientific and Technological Research (TISTR), Techno Polis, Khlong Luang District, Pathum Thani, Thailand
eDepartment of Traditional Chinese Medicine, School of Public Health, University of Phayao, Phayao, Thailand
fDepartment of Pathobiology, Faculty of Science, Mahidol University, Bangkok, Thailand
gCorresponding Author: Witchuda Payuhakrit, Department of Pathobiology, Faculty of Science, Mahidol University, Bangkok 10400, Thailand
Manuscript submitted April 20, 2023, accepted June 27, 2023, published online August 4, 2023
Short title: Anticancer and Antimutagenic Properties of P. paniceum
doi: https://doi.org/10.14740/wjon1602
| Abstract | ▴Top |
Background: Pogonatherum paniceum (P. paniceum) (Lam.) Hack. plays an important role in detoxification. However, its anticancer activity has not yet been elucidated. The aim of our study was to examine the suppressive proliferation, anti-migration and mutagenic/antimutagenic properties of P. paniceum. Moreover, we set out to determine the cellular mechanism underlying its antiproliferation.
Methods: To investigate P. paniceum’s anticancer ability, HCT116 and HT29 cell lines were treated with a water extract containing P. paniceum, and then the cell viability was examined using the trypan blue exclusion method which were compared to HEK293 (non-cancerous cells). The anticancer effects were investigated by MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) and colony formation assay. Apoptosis induction, cell cycle distribution, and migration abilities were assessed by cell death detection enzyme-linked immunoassay (ELISA), flow cytometry, and wound healing assay. Finally, the mutagenicity and antimutagenicity were evaluated using the micronucleus assay.
Results: Treatment with P. paniceum caused a loss of cell viability in HCT116 and HT29 cells (not found in HEK293), which had an IC50 (half-maximal inhibitory concentration) of 1,156.2 and 1,207.0 µg/mL, respectively. We found that P. paniceum significantly inhibited the proliferative function of HCT116 and HT29 cells. To find the mechanism that exerts a suppressive proliferation effect on P. paniceum, we determined the DNA fragmentation and cell cycle distribution. We also found that P. paniceum treatment increased apoptosis and arrested of the cell cycle at G0/G1 remarkably when compared with the control group. Moreover, P. paniceum could decrease the migration of HCT116 and HT29 cancer cells. Finally, the treatment of P. paniceum did not induce micronucleus formation but did decrease the micronucleus frequency against mutagen-mitomycin C.
Conclusions: P. paniceum did not possess any toxicity (cytotoxic and mutagenic) but has the potential for anticancer activity against human colorectal cells by increasing apoptosis, which leads to the suppression of cell proliferation. P. paniceum also inhibits cell migration and exerts antimutagenicity, thereby suggesting that P. paniceum might be useful for colorectal cancer treatment.
Keywords: Colorectal cancer cells; Anticancer; Antimutagenicity; Pogonatherum paniceum
| Introduction | ▴Top |
Cancer continues to be a major cause of death worldwide [1]. The greatest number of cancer cases relate to the detection of cancer in the breast, lungs, colon, and rectum. Among incidences of cancer, colorectal cancer (CRC) is the most commonly occurring cancer, accounting for approximately one-third of all cancer cases worldwide and is the second leading cause of cancer-related mortality in the world [2-4]. A diverse amount of stimuli contributes to genomic alterations that accumulate over time, which then drive the transformation of normal colonic epithelium cells into a colorectal carcinoma. CRC cells have a frequency characterized by excessive cell proliferation, most of which are able to evade apoptosis. These are important hallmarks of cancer [5, 6]. Apoptosis is one mechanism leading to cell death and is characterized by specific morphological and biochemical changes to cells, including cell blebbing, cytoplasmic condensation, loss of cell contact and cleavage of chromosomal DNA. Therefore, the evasion of apoptosis is a major cause of cancer, but it is also providing opportunities for anticancer drug design [7-9].
Moreover, most cancer patients develop metastasis leading to poor prognosis due to limited therapeutic options [10, 11]. Tumor metastasis is a crucial mechanism consisting of a series of biological processes by which cancer cells move from the primary neoplasm to distant locations. Migration is a key cellular mechanism for cancer cells moving out, thereby disturbance in this process eliminates cancer progression [12, 13].
Various research studies have demonstrated that natural products, in particular plants, play a role as potential cancer agents. The World Health Organization (WHO) has estimated that approximately 75-80% of the world’s population depends mainly on traditional medicines for their health care [14, 15]. In addition, there are several natural products and their analogs that are currently in the preclinical and clinical stages of development. In cancer treatment, natural products can be more efficient with fewer side effects, which makes them a promising substitute for chemotherapy, which has deleterious side effects and does not meet clinical needs [16, 17].
Pogonatherum paniceum (P. paniceum) is a perennial grass that belongs to the family of Poaceae. This plant is widespread in tropical and subtropical regions of Asia, Africa, and Oceania. A previous study has shown that P. paniceum can differentially respond to a number of environmental stress factors by the activation of various abiotic stress-specific proteins through alternative transcript splicing [18, 19]. Another study has shown that expression of GDP-D-mannose pyrophosphorylase (GMPase) was upregulated in the leaves of P. paniceum under stressed conditions (salinity and drought), and this contributes to increasing the content of L-ascorbic, which plays a crucial role in the detoxification of reactive oxygen species (ROS) [20]. P. paniceum might be a good candidate for the treatment of cancer due to its exertion of biological functions (e.g., detoxification). However, it has not yet been studied in the field of medical research. Therefore, the aim of our study was to assess suppressive cell proliferation and any possible mechanism responsible for anticancer activity on CRC cells. We selected human colorectal HCT116 and HT29 cancer cells as a research object, and a preliminary cytotoxicity test revealed that water extract containing P. paniceum exerts selected cytotoxic activity on those CRC cell lines and significantly inhibits the proliferative function of those cells, suggesting that it might be of potential as an anticancer agent. Interestingly, to further highlight the mechanisms of its proliferative suppression, we first evaluated its effects on programmed cell death or apoptosis and on the cell cycle progression of both CRC cells. Finally, we also explored, for the first time, mutagenicity and antimutagenicity of P. paniceum.
| Materials and Methods | ▴Top |
Sample collection and polyphenolic analysis
P. paniceum plants were collected in May 2020 from a local medicinal herb store in Kamphaengphet Province, Thailand. Leaves from the P. paniceum plants were extracted using aqueous according to previously reported with some modification [21]. A stock solution for our experiments was prepared in deionized water. The solution was then filtered and stored in a sterilized container and stored in the refrigerator (2 - 4 °C). Due to its average polyphenolic concentration, the P. paniceum extract was further tested using high performance liquid chromatography (HPLC) from the Central Laboratory Co., Ltd. Service Center (Chiangmai, Thailand). The HPLC analysis used 25 g of P. paniceum extract. The final solvent was filtered through 0.22-µm sterile membrane filters, and 20 µL was injected for HPLC analysis. Analytical HPLC analyses were performed on a binary solvent system (absolute methanol + 1% acetic acid). The column used was a 3.5-mm Kromasil reversed-phase column (150 × 4 mm) protected by a Kromasil C 18 (10 mm pre-column) (Scantec Lab, Savedalen, Sweden). The ultraviolet (UV) detection was monitored at 280, 360 and 530 nm. The concentration of each component was quantified with reference to samples of the commercial standards.
Cell culture and in vitro experiments
Human colorectal HCT116 (p53wt tumor suppressor gene) (CCL-247™) and HT29 (p53R273H tumor suppressor gene) (HTB-38™) cancer cells were supplied from American Type Culture Collection (ATCC). The cells were maintained in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (p/s) in a CO2 incubator at 37 °C. A non-cancer HEK293 (human kidney epithelium) cell line was purchased from ATCC (CRL-1573™) and grown in completed medium consisting of Eagle’s minimum essential medium (EMEM), 10% FBS, 1% p/s (all from Gibco). For subculturing, the media were discarded, and the cells were washed twice with phosphate buffered saline (PBS). The cells were treated with 0.25% trypsin-ethylenediaminetetraacetic acid solution (0.25% trypsin/EDTA) and centrifuged at 1,500 rpm for 5 min. The cells were collected and transferred to a new dish. The cells were sub-cultured every 2 to 3 days or at 80-90% confluency.
To assess the anticancer effect of P. paniceum, the HCT116 and HT29 cells were exposed to serial concentrations of P. paniceum for 24 - 48 h. Cell viability was evaluated using the trypan blue exclusion method. Then, a 50% reduction in cell survival (half-maximal inhibitory concentration (IC50) value) was indicated. To analyze the effect of P. paniceum on antiproliferative ability, the HCT116 and HT29 cells were exposed to noncytotoxic doses of P. paniceum in DMEM containing 10% FBS and then subsequently incubated for 48 h. Furthermore, a study of the mechanism responsible for proliferative suppression was conducted. In this study, we investigated the effect of P. paniceum on apoptosis induction and cell cycle distribution through the evaluation of DNA fragmentation and cell cycle analysis, respectively. Inhibition of the migration of cancer cells was also evaluated using the scratch wound healing method. Finally, we performed mutagenicity and antimutagenicity using the in vitro cytokinesis-block micronucleus (CBMN) assay.
Cell viability assessment
The cytotoxicity of P. paniceum was assessed using the trypan blue exclusion method. The HCT116 and HT29 cell suspensions were seeded at a density of 8 × 104 cells/mL in a 12-well plate and allowed to attach for 24 - 48 h. The cells were exposed to P. paniceum at concentrations of 0, 500, 1,000, 1,250, and 1,500 µg/mL for 24 and 48 h in the condition mentioned above. After the treatment period, the chemical-containing medium was removed and washed several times with PBS. Then, the treated cells were collected using trypsinization and centrifugation at 1,500 rpm, for 5 min. The cell suspensions were analyzed after being mixed with 0.4% trypan blue dye, and the cells were counted using a hemocytometer under an inverted microscope. The percentage of viable cells was calculated and the cytotoxicity of the P. paniceum on those cell lines was indicated as an IC50 value, which was calculated by a linear equation of the relative viability rate versus the concentrations of P. paniceum.
The cytotoxicity in non-cancerous cells
The selective cytotoxicity of P. paniceum for CRC cells was evaluated by screening the IC50 value of P. paniceum against the non-cancerous HEK293 cell line. After 24 - 48 h of treatment periods, cell viability was assessed by cell counting using trypan blue dye. Then, the IC50 value was determined as described above.
Antiproliferative ability evaluation
Cell viability was investigated using the colorimetric MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) reduction assay (Promega, Madison, WI). Briefly, 2,000 cells of HCT116 and HT29 cell suspensions were seeded into a 96-well plate with 100 µL seeded into each well. Subsequently, P. paniceum was added to the culture at final concentrations of 0, 600, 800, and 1,000 µg/mL, and it was then further incubated for 48 h. At the end of the incubation period, the medium containing P. paniceum was replaced with a new medium, and 20 µL of MTS solution was added to each well. The solution was incubated further for 1 h at 37 °C, after which the plate was shaken, and the absorbance at a wavelength of 492 nm was detected using a microplate reader (Synergy H1, Biotek, USA).
Colony formation assay
Cell lines at a density of 6 × 102 cells/well were plated in a six-well plate, and then treated with varying concentrations of P. paniceum extract (600, 800, and 1,000 µg/mL). The control cells were incubated without a test sample. After the cells were incubated for 48 h at 37 °C, the treated cells were replaced with fresh medium and incubated for another 7 - 9 days. Next, the medium was discarded, and the treated cells were washed with cold PBS before being fixed with ice-cold methanol. Then, the treated cells were stained with crystal violet (1%) and the cellular colonies attained in each well plate were photographed with a phase-contrast microscope. The number of colonies was counted.
Cell cycle analysis
HCT116 and HT29 cells were grown in six-well plates (2 × 105 cells/well) and then incubated with or without various concentrations of P. paniceum extract (0 - 800 µg/mL) for 48 h. The cell suspension was then pelleted, centrifuged, washed twice with cold PBS, and fixed with 70% ethanol at -20 °C for 24 h. Following this, the cells were stained with 50 µg/mL of propidium iodide (Biolegend, California) containing RNaseA (Geneaid, Taiwan) solution (20 µg/mL) for 30 min in the dark. The DNA contents at different phases of the cell cycle were measured by flow cytometry using a FACScan apparatus (Becton Dickinson, USA).
Apoptosis induction determination by cell death detection enzyme-linked immunoassay (ELISA)
Apoptosis was quantified using a cell death detection ELISA PLUS kit (Sigma-Aldrich, Mannheim, Germany) in accordance with previous study [22]. Briefly, the cells were seeded at a density of 6 × 104 cells/well in a 12-well plate and led to attach overnight. The cells were treated with P. paniceum at concentrations of 600 and 800 µg/mL and incubated for 72 h. Next, the cells were lysed with lysis solution and incubated at room temperature for 30 min. The lysate cells were transferred to the ELISA plate and incubated with anti-histone-biotin and anti-DNA-peroxidase before analysis, according to the manufacturer’s protocol. The absorbance level was measured at 405 nm using the microplate reader (Synergy H1, Biotek, USA).
Wound-healing anti-migratory assay
The in vitro cell migration ability was determined by the wound healing assay. HCT116 and HT29 cells (1 × 106 cells/mL) were seeded in each 12-well plate. After incubation in a 5% CO2 incubator at 37 °C to complete confluency, the old medium was replaced by a serum-free culture medium, which was then further incubated overnight. Then, the cultures were scratched to produce a wound using sterile 200 µL pipette tips. After removing the cell debris, the cells were exposed to P. paniceum (600, 800, and 1,000 µg/mL) and the untreated control was included for 24 - 48 h. The gap width was measured at 0, 24, or 48 h under an inverted microscope at magnification × 10. The percentage of wound closure was measured by using the following formula:
Mutagenicity and antimutagenicity using in vitro CBMN test
The CBMN test was carried out to evaluate the mutagenicity and anti-mutagenicity of the P. paniceum extract using the protocol described by Fenech [23]. Chinese hamster lung fibroblast cells (V79 cells) were cultured at a density of 1 × 105 cells/mL. Following overnight incubation, the cells were treated with P. paniceum extract at various concentrations (600 and 1,000 µg/mL) for 24 h. A negative (untreated) and positive (mitomycin C (MMC): 1.25 µg/mL) controls were also evaluated. Eighteen hours before harvesting, cytochalasin B at a final concentration of 6 µg/mL was added to the cells to block the cytokinesis, which led to the cells being produced at the binucleated stage. After being washed by centrifugation at 800 rpm for 10 min at 4 °C, the cell pellets were resuspended in hypotonic solution (0.56% KCl) for 10 min at room temperature. Then, the pellets were incubated with fixing solution 1 (methanol: acetic acid: 0.9% NaCl) and fixing solution 2 (methanol: acetic acid) for 10 min at 4 °C, followed by centrifugation. The cell pellets were washed twice with the fixative, and then the cell suspension was dropped onto a clean microscope slide. After drying, the slides were stained with 4’,6-diamidino-2-phenylindole (DAPI).
For micronucleus (MN) scoring, all slides were coded and analyzed blindly using a fluorescence microscope (Metafer, Germany) following the criteria for MN scoring in binucleated cells (BNC) as described by Fenech [23]. MN frequency was expressed as the number of micronucleated cells in 2,000 BNC scored. The cell cycle alterations, including cytotoxic and cytostatic effects, were concurrently expressed as a cytokinesis-blocked proliferation index (CBPI). Five hundred cells per concentrations were examined to determine the CBPI value, which was calculated from the following formula:
M1 - M4 represent the number of cells with one to four nuclei and N as a total number of cells count.
Statistical analysis
Data are presented as the mean ± standard deviation. Differences between the mean values for individual groups were assessed using a one-way analysis of variance (ANOVA) with Tukey’s test applied to compare the mean frequencies of treatment groups with the control. Statistical analysis was conducted using SPSS version 25 (IBM). P < 0.05 was identified as indicating a statistically significant difference.
| Results | ▴Top |
P. paniceum possess cytotoxic effect on CRC cells
In order to study the effect of P. paniceum on proliferative inhibition activity in CRC cells, we first evaluated cell cytotoxicity. This allowed us to establish the IC50 value for P. paniceum on HCT116 and HT29 human CRC cell lines. When those cells were exposed to P. paniceum extract for 24 h, it had no effect on reducing the cell viability of the cell lines tested (Fig. 1a), as evidenced by there being no difference in cell morphology and cell population between the treatment groups and the control. After 48 h of P. paniceum treatment, we observed that the cells became damaged, starting from 500 to 1,500 µg/mL, and that the cell cultures exhibited a decrease in cell population after P. paniceum treatment compared to the control (Fig. 1a). Moreover, the treated cells became round in shape and detached from the surface leading to loss of cell viability. We further evaluated the cell viability using the trypan blue exclusion method. The results showed that P. paniceum significantly decreased the viability of both cells in a dose-dependent manner (P < 0.05, P < 0.01) (500 - 1,500 µg/mL), as shown in Figure 1b, demonstrating that P. paniceum treatment induces a cytotoxic effect on the cell line tested. Then, the IC50 value of each cell was determined to be 1,156.2 µg/mL for the HCT116 and 1,207.0 µg/mL for the HT29 cells after 48 h of treatment. According to these IC50 values, non-cytotoxic doses were used for subsequent experiments. Moreover, the viability of normal human epithelium (HEK293) with P. paniceum was similar to the control group at all selected concentrations for 24 and 48 h (excepted for the highest doses at 48 h, IC50 = 2,691.589 µg/mL), as evidenced by the non-neoplastic cell lines remaining above 70% even with the extract dose of 1,250 µg/mL at 48 h (Fig. 1b).
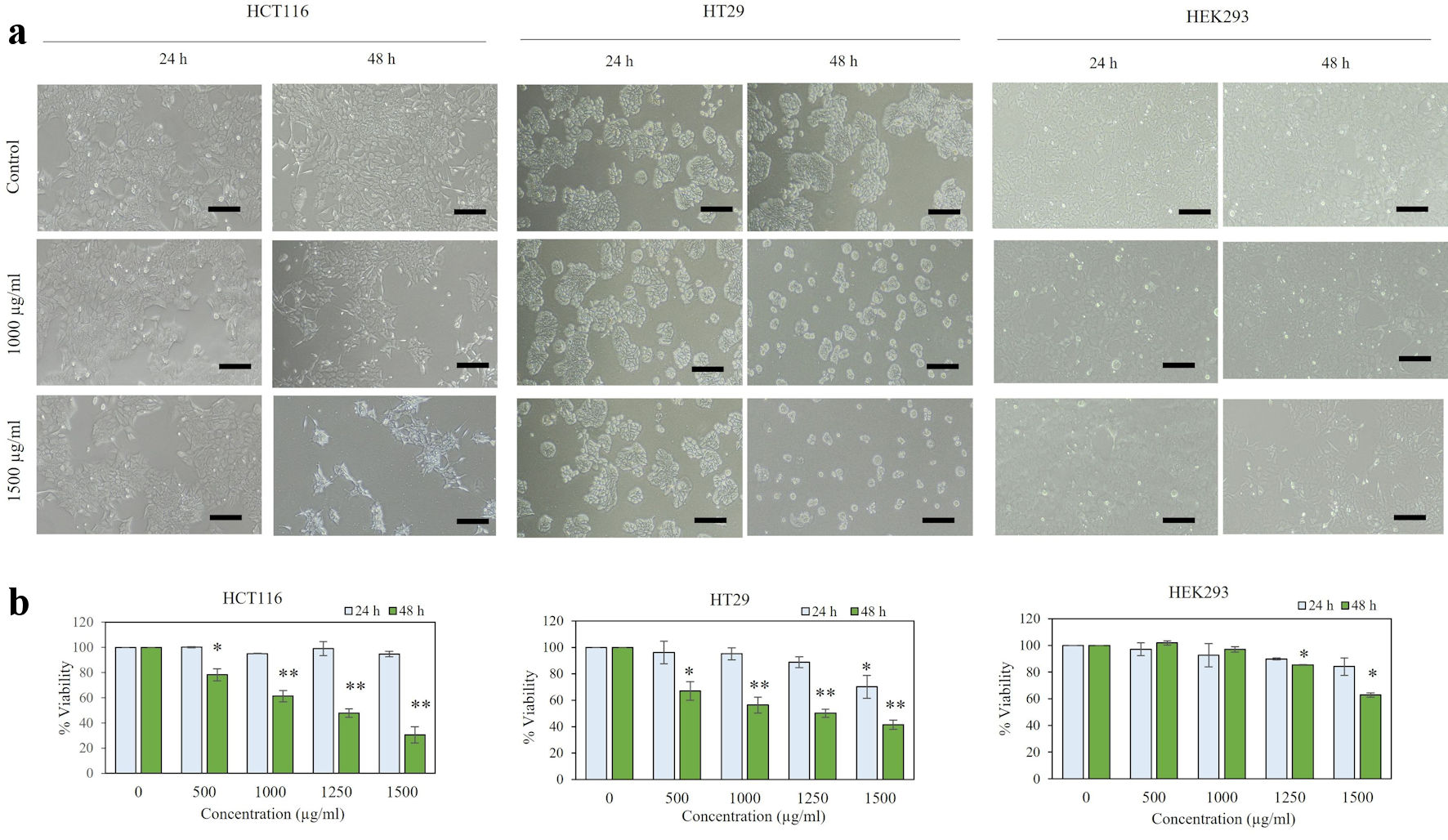 Click for large image | Figure 1. Viability of P. paniceum in HCT116, HT29, and HEK293 cells. (a) Representative images (scale bar = 50 µm) are shown. (b) Cells were treated for 24 and 48 h with P. paniceum, and cell viability was measured using trypan blue exclusion method. Data shown are mean ± SD. *P < 0.05, **P < 0.01, significantly different from control (untreated group). P. paniceum: Pogonatherum paniceum; SD: standard deviation. |
Polyphenolic content from the leaves of P. paniceum
The HPLC analysis of P. paniceum revealed the presence of several polyphenolic constituents and data are presented in Table 1.
 Click to view | Table 1. HPLC Analysis Revealed the Presence of Phenolic Components in Aqueous Extract of P. paniceum |
P. paniceum exerts proliferative suppression on CRC cells
To explore the anticancer potential of P. paniceum in CRC cells, the effect of P. paniceum, at concentrations that do not cause massive cell death, was evaluated for cell proliferation in HCT116 and HT29 cells using MTS assay. We treated those cells with different concentrations of P. paniceum (600, 800, and 1,000 µg/mL) for 48 h. The results revealed that the proliferative abilities were different when comparing the control and treatment groups (Fig. 2a). Treatment with P. paniceum led to a very evident decrease in cell proliferation on both cells in a dose-dependent manner. To confirm the proliferative suppression ability, a colony-forming assay was conducted in both cancer cells. The results revealed that the number of colonies of HCT116 and HT29 cells in the P. paniceum treatment groups was significantly less than in the control group (P < 0.05) (Fig. 2b). Collectively, P. paniceum exerts the antiproliferation of HCT116 and HT29 cells. However, the mechanism responsible for proliferative suppression remains unclear.
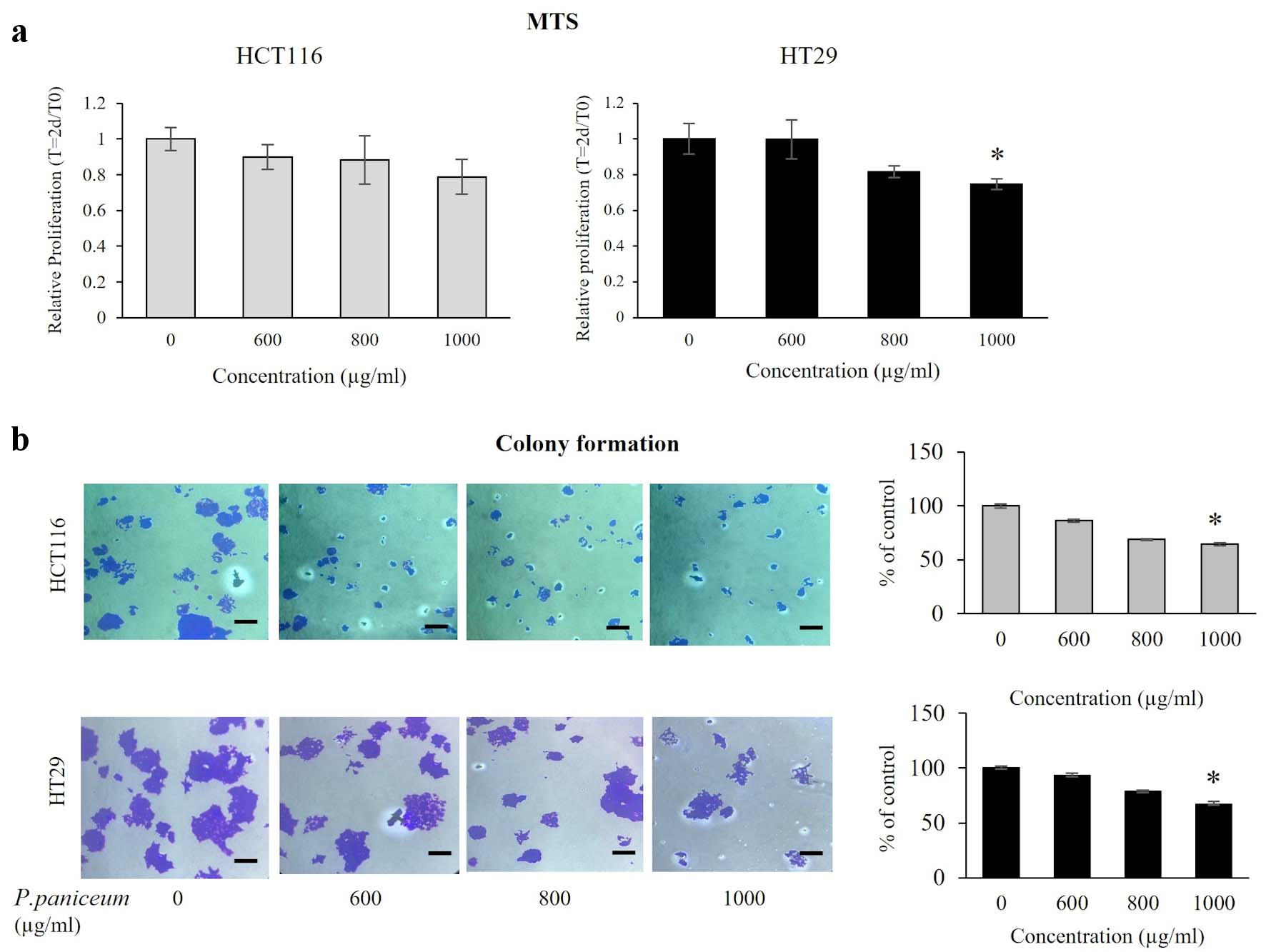 Click for large image | Figure 2. Proliferation and clonogenic property of HCT116 and HT29 cancer cells. (a) Cells were treated with varying doses of P. paniceum for 48 h, and proliferation was measured using MTS assay. (b) The anticolony formation activity of P. paniceum (48 h). The scale bar is 50 µm with magnification (× 20). The data shown are mean value ± SD. *P < 0.05, significantly different from control (untreated group). MTS: 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; P. paniceum: Pogonatherum paniceum; SD: standard deviation. |
P. paniceum induced cell cycle arrest on CRC cells
To elucidate the mechanism underlying cellular suppression, we carried out a cell cycle phase analysis of HCT116 and HT29 cells following treatment with P. paniceum (600 and 800 µg/mL). The flow cytometry results showed that P. paniceum caused a G0/G1 phase arrest in both HCT116 and HT29 cells compared to the control group (P < 0.05) (Fig. 3). In the G0/G1 phase after treatment with P. paniceum, it was found that the highest accumulations of HCT116 cells and HT29 cells was 75.35% for 800 µg/mL and 82.40% for 600 µg/mL, respectively. Concomitantly, a significant decrease in the number of cells in the S phase was also observed (P < 0.05).
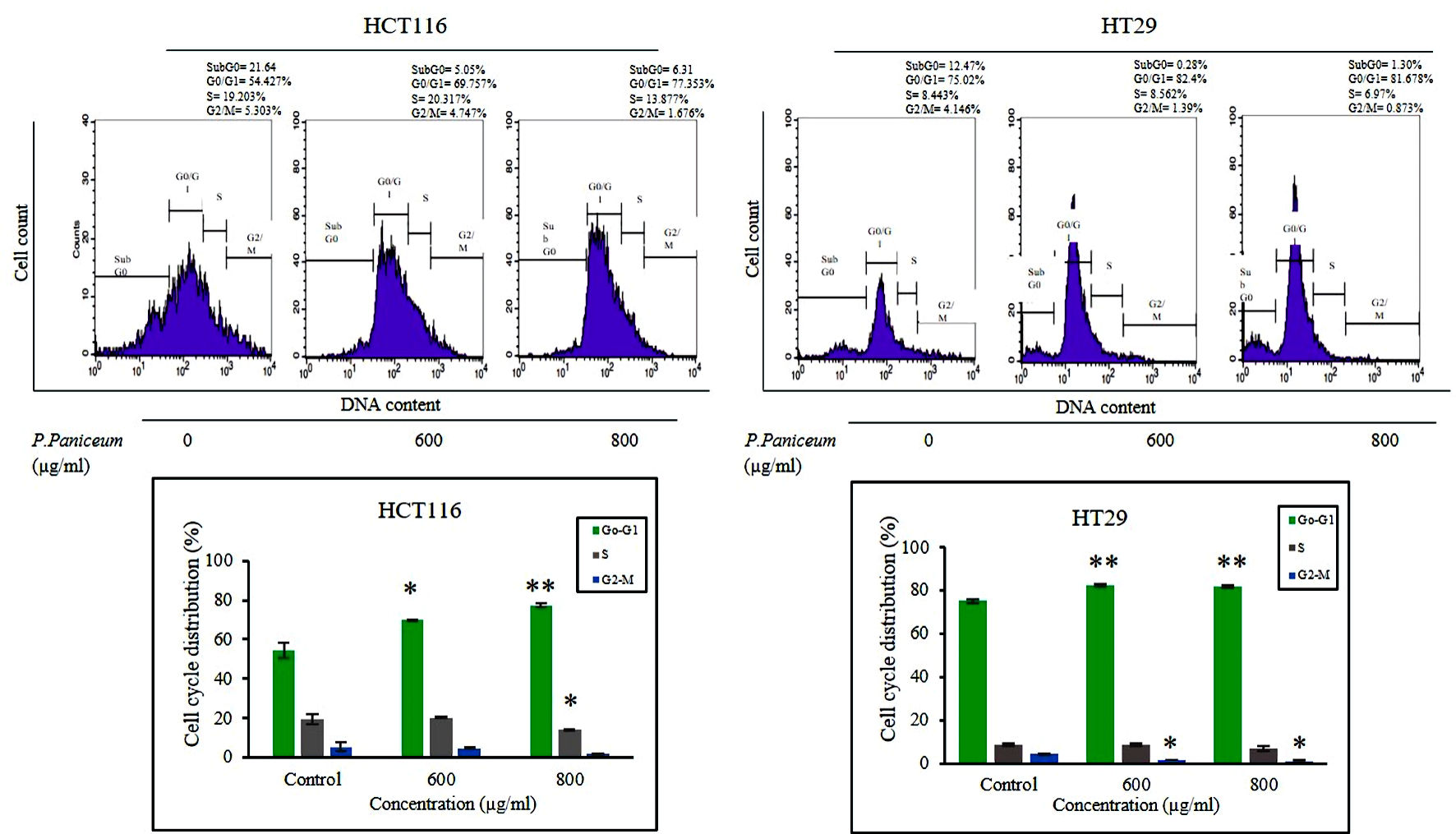 Click for large image | Figure 3. P. paniceum induced G0 - G1 cell cycle arrest of HCT116 and HT29 cancer cells. Cells were treated for 48 h with P. paniceum, and flow cytometry was used to determine the cell cycle distribution. All data are expressed as the mean ± SD. *P < 0.05, **P < 0.01 compared to the control. P. paniceum: Pogonatherum paniceum; SD: standard deviation. |
P. paniceum induces apoptosis on CRC cells
We conducted a further experiment to investigate the effects on apoptosis induction. This experiment was carried out to clarify whether apoptosis induction upon proliferative suppression on CRC cells is caused by P. paniceum. We determined this on the basis of DNA fragmentation, which is a biochemical hallmark of apoptosis, using a cell death detection ELISA assay. This assay is used for the quantitative in vitro determination of cytoplasmic histone-associated DNA fragments after induced cell death. The results revealed that the treatment of non-cytotoxic concentrations of P. paniceum on HCT116 and HT29 cells led to morphological changes, which were observed using inverted microscopy. Representative results are shown in Figure 4a. We found that there were more cell shrinkages and a tendency for them to float in the medium, which was seen more in the P. paniceum treatment groups when compared to the control. Interestingly, the apoptosis analysis results show that as the concentrations of P. paniceum increased (600 and 800 µg/mL), the apoptotic induction in both cells increased compared to the untreated control (P < 0.05) (Fig. 4b).
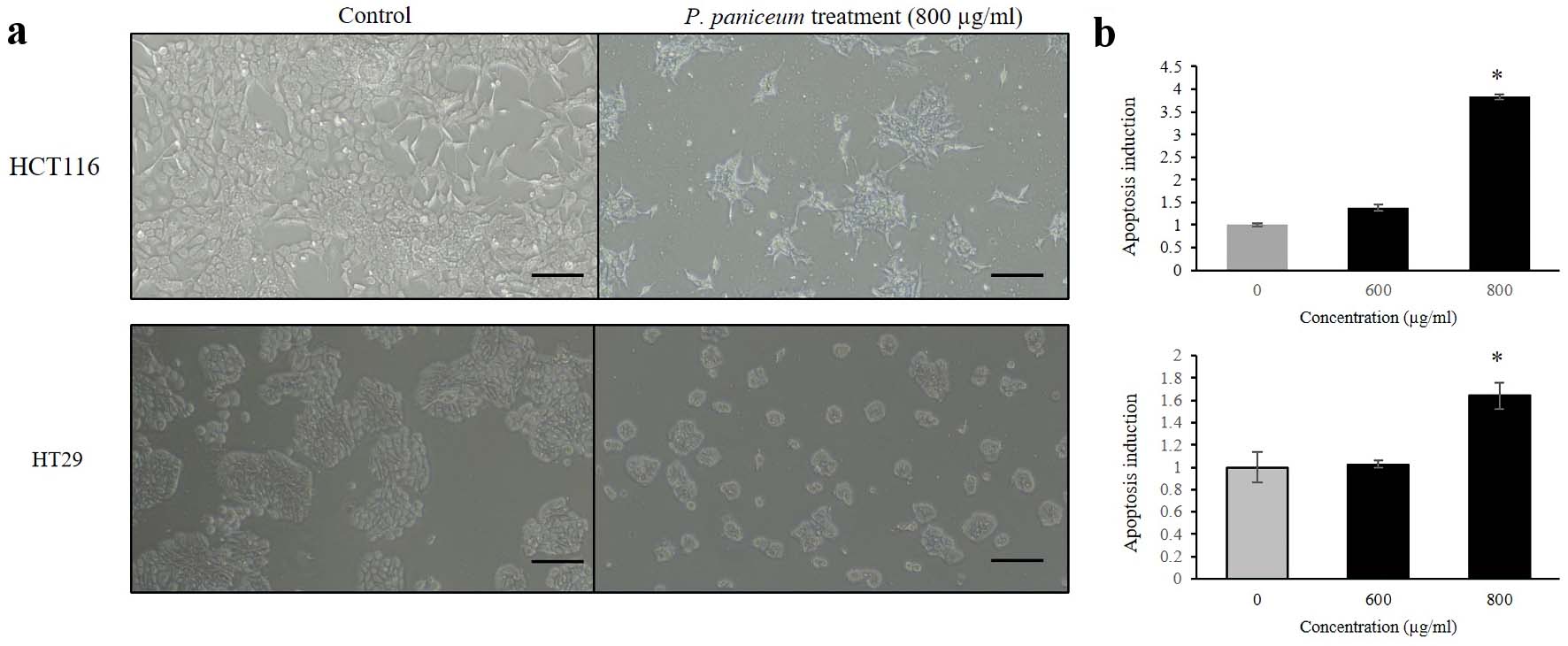 Click for large image | Figure 4. Apoptosis induction of HCT116 and HT29 cancer cells. (a) Cells were treated for 48 h with P. paniceum, and (b) apoptosis induction was measured using cell death detection ELISA (scale bar = 50 µm). The data shown are the mean value ± SD. *P < 0.05, significantly different from control (untreated group). P. paniceum: Pogonatherum paniceum; SD: standard deviation; ELISA: enzyme-linked immunoassay. |
P. paniceum abolishes migration activity of CRC cells
Next, we evaluated the effect of P. paniceum on the migration of CRC cells, which was investigated by scratch assays. The migration ability of HCT116 and HT29 cells was observed following treatment with P. paniceum at 600, 800, and 1,000 µg/mL for 24 and 44 h, respectively. The differences in the wideness of the scratch area were measured under an inverted microscope. While the untreated control increased the migration, treatment with P. paniceum resulted in a remarkable reduction in the migration of both HCT116 and HT29 cells (Fig. 5a). The treatment of HCT116 with 600, 800 and 1,000 µg/mL of P. paniceum for 24 h suppressed wound healing by 41.98%, 29.66%, and 29.17%, respectively, whereas in HT29 after 44 h, the width was 44.14%, 34.45%, and 25.32%, respectively. This result suggests that P. paniceum inhibits the in vitro migration of HCT116 and HT29 cells (Fig. 5b).
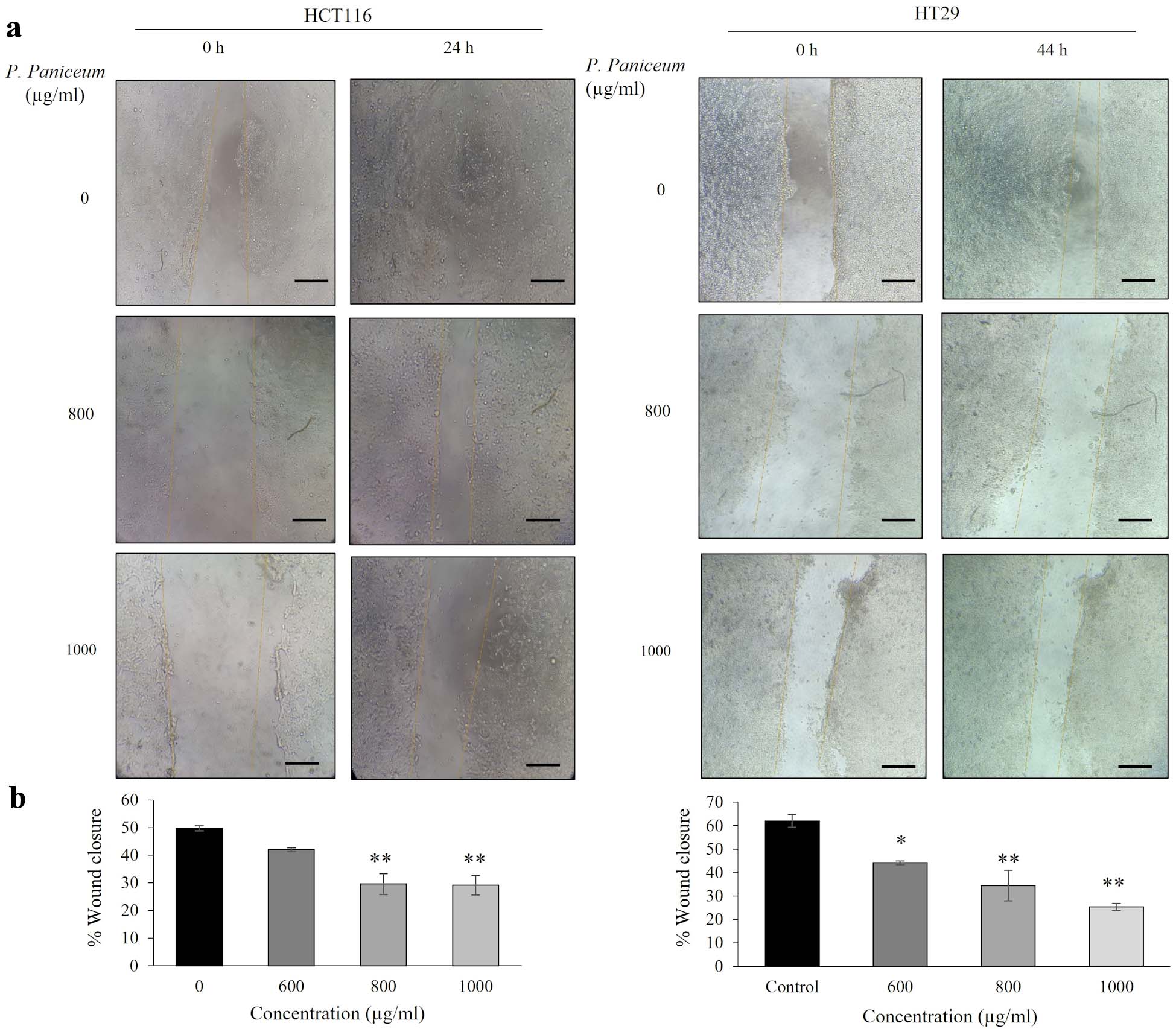 Click for large image | Figure 5. Migration of HCT116 and HT29 cancer cells following P. paniceum incubation for 24 and 44 h. (a) The photograph of migrating cells shown at magnification × 20 (scale bar = 50 µm). (b) The migration ability indicated as the percentage of wound healing. *P < 0.05, **P < 0.01 compared to the control. P. paniceum: Pogonatherum paniceum. |
Mutagenicity and antimutagenic properties of P. paniceum
The mutagenicity and antimutagenicity of P. paniceum on V79 cells were evaluated using an in vitro CBMN assay. Before the antimutagenicity evaluation, we performed the mutagenicity test of P. paniceum. The analysis of MN frequency revealed that the extract concentrations at 600 and 800 µg/mL did not induce a dramatic increase in the MN formation compared to the negative and positive control groups (Fig. 6a, b). In the untreated negative control, the baseline of MN frequency was 30.00 ± 1.78 MN/2,000 BNC. Similar to the negative control, the MN formation was found at 26.00 ± 1.08 and 32.50 ± 1.04 for P. paniceum doses of 600 and 800 µg/mL, whereas the MMC-treated positive control showed a significant increase of MN of up to 144.50 ± 6.76 compared to the negative control (P < 0.05). These results indicate that P. paniceum did not induce mutagenicity in the in vitro MN assay.
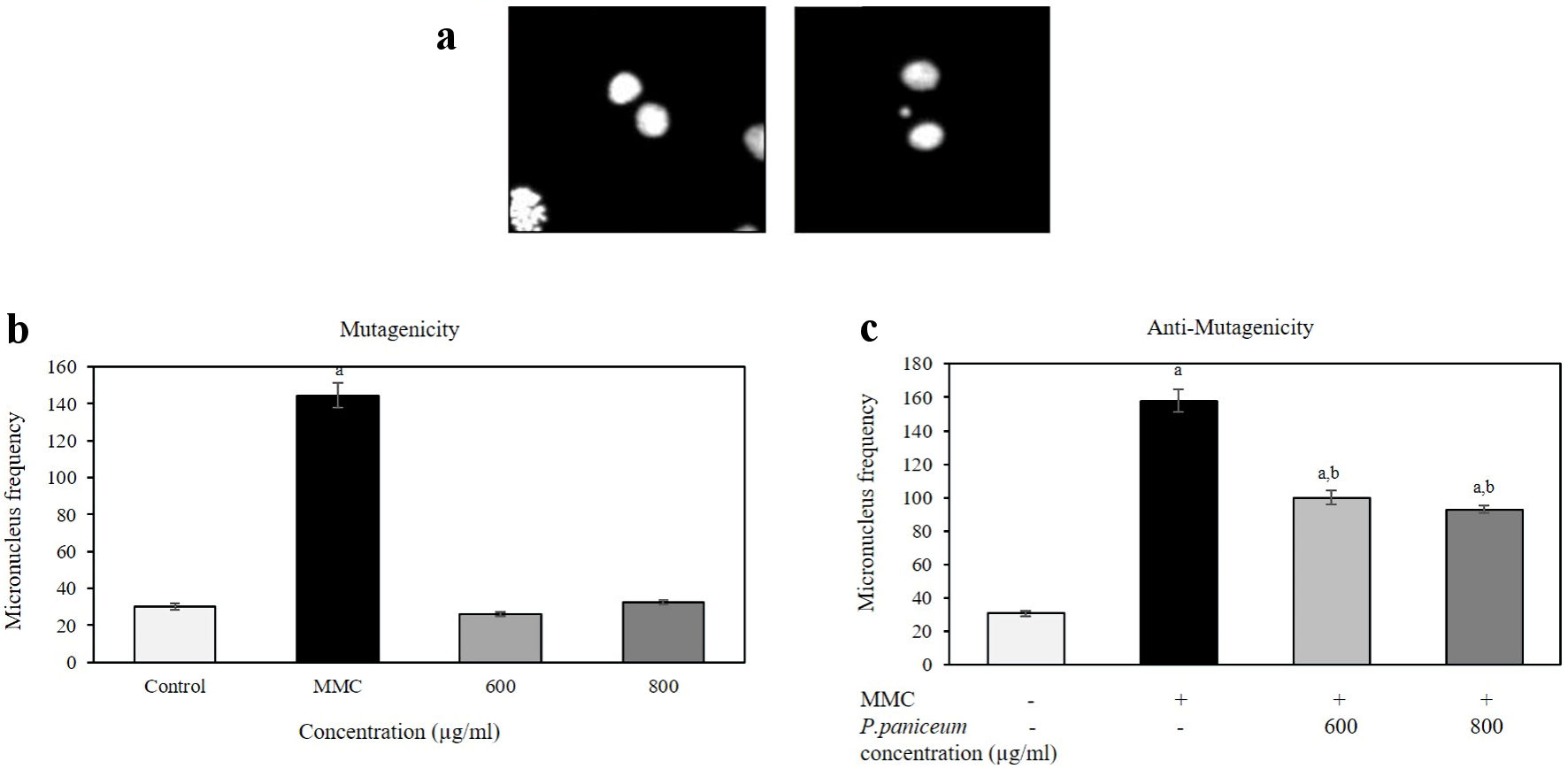 Click for large image | Figure 6. The frequency of micronuclei (MN) in V79 cells after the treatment of P. paniceum for 24 h. (a) Representative images shown binucleated cell (left) and MN in binucleated cell (right). (b) Cells were treated for 24 h with P. paniceum, and MN frequency was scored in 2,000 binucleated cells. (c) Cells were treated for 24 h with P. paniceum combined with mitomycin C ((MMC), 1.25 µg/mL). (a) and (b) significantly different from control (untreated group) and MMC, respectively. P. paniceum: Pogonatherum paniceum. |
The same concentrations used for mutagenicity testing were also used to evaluate the antimutagenic potential of the combination of P. paniceum and MMC (Fig. 6c). The frequency of MN in the negative control group was 30.75 ± 1.55 MN/2,000 BNC. In contrast, the MMC-treated group induced significant increase in MN frequency by 158.0 ± 6.76 (P < 0.05). For the combination of the extract with MMC, the data demonstrated that the combination treatment elicited a significant reduce in the MN frequency (P < 0.05). The MN frequencies were 100.0 ± 4.04 and 92.5 ± 2.31 MN/2,000 BNC when the cells were treated with doses of 600 and 800 µg/mL of P. paniceum.
The effect of cytostatic for all treated groups, including negative and positive controls and sample cultures, were measured and determined as CBPI values in accordance with OECD 487 guidelines. The results show that there were no significant differences between the CBPI values of all treatment and untreated control groups (P > 0.05) (Table 2).
 Click to view | Table 2. The Cytokinesis-Block Proliferation Index (CBPI) in V79 Cells After the Treatments With P. paniceum and P. paniceum Combination With MMC |
| Discussion | ▴Top |
Colon cancer is a common form of cancer that occurs in the digestive system and is a leading cause of cancer-related death. Moreover, the incidence rate of colon cancer is still increasing year by year. The courses used for therapy generally include surgical intervention and chemotherapy. However, these therapies can cause unexpected outcomes due to serious toxic side effects occurring, such as leading to damage of normal cells/tissue, and most patients with CRC die as a result of this disease. As a consequence, the screening of new anticancer drugs has become an increasing challenge.
In this study, we present the effects of P. paniceum on CRC cells. We have shown that P. paniceum extract, at non-cytotoxic concentrations, exerts a sign of damage on cancer cells and contributes to proliferative suppression while also not being toxic to non-cancerous cells. To elucidate the compound association of the biological effect, we first explored the biochemical compound in the extract sample. Interestingly, we found that a variety of polyphenolic compounds are most abundant in P. paniceum. These include tannic acid, gallic acid, catechin, and rutin. In cancer research, it has been suggested that the bioactive component may also have anticancer properties. This is in agreement with previous studies which reported that phytochemicals in plants can induce cytotoxic activity in various types of cancer cells, when compared with known chemotherapy (e.g., doxorubicin), which is reliant on a rich source of active ingredients such as flavonoids, carotenoids, and phenolics [24, 25]. In addition, related research has indicated that active compounds and their antioxidants might have potential anticancer activities due to their inhibitory effect on free radicals [26]. Tannic acid is also known for its inhibitory action against breast cancer stem cells [27]. Another study demonstrated that tannic acid could attenuate the formation of cancer stem cells by inhibiting NF-κB activation and thereby preventing the phenotype transition of breast cancer cells [28]. Similarly, catechin has been reported to inhibit oxidative stress and to possess an anticancer effect on a variety of cancer cells, including breast and colon cancer cells [29, 30].
We first explored the relevance of P. paniceum for its anticancer effects by looking at its ability to help with proliferative suppression and its possibility for use in the induction of apoptosis in CRC cells. Excessive cell proliferation and evasion of apoptosis are characteristic hallmarks of cancer [31]. Morphological changes in apoptosis begin primarily with decreased cell volume and the condensation and subsequent fragmentation of its nucleus [32, 33]. Several studies have reported that targeting apoptosis can be used as a criterion for developing anticancer agents [32, 34]. Therefore, therapeutic approaches to the prevention or suppression of the proliferation and targeting of apoptosis can possibly be used as anticancer agents, as well as enhancing patient survival rates. The mechanism of apoptosis occurs due to several factors involving intrinsic and extrinsic pathways. These would lead to the conveyance of signals to initiate the execution process, resulting in the degradation of cytoskeletal and nuclear proteins and the gradual loss of cell size, leading to the formation of apoptotic bodies [7, 35, 36]. Consistent with previous research, the findings of our study revealed a marked increase in the morphological hallmarks of apoptosis with regard to cell size loss. Some previous reports have shown an inducement of apoptosis in several types of cancer cells. In those studies, it was found that at high concentrations (2 mg/mL), water extract from Momordica cochinchinensis causes apoptosis of breast and melanoma cancer cells in a dose- and time-dependent manner [37].
Additionally, the cell cycle arrest of CRC cells in P. paniceum may also indicate its potential anticancer activity. However, there is currently no data on the cell cycle distribution of P. paniceum. In this study, the extract of P. paniceum at the concentration used showed a slight inhibition in the G0/G1 phase of the cell cycle. In this regard, previous studies have demonstrated that inducing the cell cycle arrest of cancer cells reflects an accumulation of responses to DNA damage that sequentially affect cell growth and could contribute to inhibiting cancer cell proliferation [38]. However, there may also be some activation of unique processes following the treatment of P. paniceum.
Cell migration is a crucial process of spreading cancer into the surrounding area. It is involved in many processes, such as metastasis, which is the main cause of mortality. Therefore, disturbance of the metastasis pathway is clinically promising, and inhibition in this process eliminates cancer progression [12, 39]. However, this important property of P. paniceum has not been extensively investigated. We demonstrated the capability of P. paniceum to inhibit the migration of HCT116 and HT29 cells in a dose-dependent manner. Our findings reveal that P. paniceum has an antimigration effect against those human CRC cells.
It is important to knowledge the properties of P. paniceum regarding its effect on human genetic material. In our current study, we used the in vitro CBMN which is the widely used method to evaluate genetic damage. In this work, we followed the regulatory guidelines to evaluate the genetic potential according to OECD guidelines [40], and some modifications of Fenech [23]. The MN are small chromatin bodies surrounded by a nuclear envelope, which are not incorporated into the nucleus of the daughter cell after nuclear division, thereby indicating DNA damage as clastogenic or aneugenic activities [41-43]. In the present study, the extract of P. paniceum did not possess significant mutagenicity up to a dose level of 800 µg/mL in the MN test. Interestingly, P. paniceum exhibited antimutagenic activity against mitomycin C. However, there was no previous evidence of the mutagenic/antimutagenic potentials of P. paniceum detected in any in vitro and in vivo studies, and the MN test alone does not provide direct information on the mutagenic and antimutagenic potency.
Moreover, there are some limitations in this study that should be noted. The in vivo studies were not completely understood. Future study studies may be conducted to find out the antiproliferative and its toxicity effects in vivo of P. paniceum. The information from these future tests will be beneficial for the usage of P. paniceum as an anticancer agent.
Conclusions
In conclusion, we reported for the first time that P. paniceum extract exhibits antiproliferative and antimigration activities against CRC cells. The antiproliferative effect was shown to be mediated through the induction of apoptosis and cell cycle arrest. Our results also reveal that P. paniceum caused an absence of cytotoxic or mutagenic potential at all concentrations tested but exerts antimutagenicity effects under the condition of the micronucleus assay. These results provide a scientific basis for the traditional use of P. paniceum as a potential anticancer agent.
Acknowledgments
The authors would like to thank the Deanship of School of Medicine, University of Phayao, for supporting the facilities of this project.
Financial Disclosure
The research was funded by the Fundamental Fund of University of Phayao (grant no. FF66-RIM039, FF66-RIM093, FF66-RIM098).
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
RP and WP designed and performed the study. RP drafted the manuscript and did critical editing. ST, WK, PJ, PK, and SL did the experiments. RT supported sample collection and extraction. PS provided cell lines. AR, PS, WP, and PCS have carefully supervised this manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available with the article.
| References | ▴Top |
- Cancer (IARC) TIA for R on. Global Cancer Observatory n.d. https://gco.iarc.fr/. Accessed February 26, 2022.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115-132.
doi pubmed - Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
doi pubmed - Thanikachalam K, Khan G. Colorectal cancer and nutrition. Nutrients. 2019;11(1):164.
doi pubmed pmc - Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646-674.
doi pubmed - Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495-516.
doi pubmed pmc - Kuno T, Tsukamoto T, Hara A, Tanaka T. Cancer chemoprevention through the induction of apoptosis by natural compounds. J Biophys Chem. 2012;03:156-173.
doi - Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012;45(6):487-498.
doi pubmed pmc - Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5(1):28.
doi pubmed pmc - Wan L, Pantel K, Kang Y. Tumor metastasis: moving new biological insights into the clinic. Nat Med. 2013;19(11):1450-1464.
doi pubmed - Steeg PS. Targeting metastasis. Nat Rev Cancer. 2016;16(4):201-218.
doi pubmed pmc - Steeg PS. Invasion and metastasis. Curr Opin Oncol. 1992;4(1):134-141.
doi pubmed - Khalifa SAM, Elias N, Farag MA, Chen L, Saeed A, Hegazy MF, Moustafa MS, et al. Marine natural products: a source of novel anticancer drugs. Mar Drugs. 2019;17(9):491.
doi pubmed pmc - Bhanot A, Sharma R, Noolvi MN. Natural sources as potential anti-cancer agents: A review. Int J Phytomedicine. 2011;3:09-26.
- Kiyokawa T, Fukagawa T. Recent trends from the results of clinical trials on gastric cancer surgery. Cancer Commun (Lond). 2019;39(1):11.
doi pubmed pmc - Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, Kong AN. Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12(10):1281-1305.
doi pubmed pmc - Zhuang G, Ma D, Wang W, Wang S, Chen F. Genetic diversity of Pogonatherum paniceum (Lam.) Hack. in Southwest China revealed by AFLP markers. Afr J Biotechnol. 2009;8.
doi - Hong C, Haiyang W. Differentiation of population structure of Pogonatherum paniceum on different substrates. Xinan Nongye Daxue Xuebao China. 2004.
- Ai T, Liao X, Li R, Fan L, Luo F, Xu Y, Wang S. GDP-D-mannose pyrophosphorylase from Pogonatherum paniceum enhances salinity and drought tolerance of transgenic tobacco. Z Naturforsch C J Biosci. 2016;71(7-8):243-252.
doi pubmed - Thongboontho R, Petcharat K, Munkong N, Khonthun C, Boondech A, Phromnoi K, et al. Effects of Pogonatherum paniceum (Lamk) Hack extract on anti- mitochondrial DNA mediated inflammation by attenuating Tlr9 expression in LPS-induced macrophages. Nutr Res Pract. 2022;17.
- Ratsada P, Hijiya N, Hidano S, Tsukamoto Y, Nakada C, Uchida T, Kobayashi T, et al. DUSP4 is involved in the enhanced proliferation and survival of DUSP4-overexpressing cancer cells. Biochem Biophys Res Commun. 2020;528(3):586-593.
doi pubmed - Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2(5):1084-1104.
doi pubmed - Ghasemzadeh A, Karbalaii MT, Jaafar HZE, Rahmat A. Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem Cent J. 2018;12(1):17.
doi pubmed pmc - Kim C, Kim B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: a review. Nutrients. 2018;10(8):1021.
doi pubmed pmc - Beliveau R, Gingras D. Role of nutrition in preventing cancer. Can Fam Physician. 2007;53(11):1905-1911.
pubmed pmc - Choi HS, Kim DA, Chung H, Park IH, Kim BH, Oh ES, Kang DH. Screening of breast cancer stem cell inhibitors using a protein kinase inhibitor library. Cancer Cell Int. 2017;17:25.
doi pubmed pmc - Kim DA, Choi HS, Ryu ES, Ko J, Shin HS, Lee JM, Chung H, et al. Tannic acid attenuates the formation of cancer stem cells by inhibiting NF-kappaB-mediated phenotype transition of breast cancer cells. Am J Cancer Res. 2019;9(8):1664-1681.
pubmed pmc - Gan RY, Li HB, Sui ZQ, Corke H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit Rev Food Sci Nutr. 2018;58(6):924-941.
doi pubmed - Kumazaki M, Noguchi S, Yasui Y, Iwasaki J, Shinohara H, Yamada N, Akao Y. Anti-cancer effects of naturally occurring compounds through modulation of signal transduction and miRNA expression in human colon cancer cells. J Nutr Biochem. 2013;24(11):1849-1858.
doi pubmed - Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol. 2020;17(7):395-417.
doi pubmed pmc - Shangguan F, Zhou H, Ma N, Wu S, Huang H, Jin G, Wu S, et al. A novel mechanism of cannabidiol in suppressing hepatocellular carcinoma by inducing GSDME dependent pyroptosis. Front Cell Dev Biol. 2021;9:697832.
doi pubmed pmc - Letai A. Apoptosis and Cancer. Annu Rev Cancer Biol. 2017;1:275-294.
doi - Todorova J, Lazarov LI, Petrova M, Tzintzarov A, Ugrinova I. The antitumor activity of cannabidiol on lung cancer cell lines A549 and H1299: the role of apoptosis. Biotechnol Biotechnol Equip. 2021;35:873-879.
doi - Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845.
doi pubmed pmc - D'Arcy MS. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int. 2019;43(6):582-592.
doi pubmed - Wimalasiri D, Dekiwadia C, Fong SY, Piva TJ, Huynh T. Anticancer activity of Momordica cochinchinensis (red gac) aril and the impact of varietal diversity. BMC Complement Med Ther. 2020;20(1):365.
doi pubmed pmc - Dey DK, Kang SC. CopA3 peptide induces permanent cell-cycle arrest in colorectal cancer cells. Mech Ageing Dev. 2021;196:111497.
doi pubmed - Bergers G, Fendt SM. The metabolism of cancer cells during metastasis. Nat Rev Cancer. 2021;21(3):162-180.
doi pubmed pmc - OECD. Test No. 487: In vitro mammalian cell micronucleus test. Paris: Organisation for economic co-operation and development. 2016.
- Araldi RP, de Melo TC, Mendes TB, de Sa Junior PL, Nozima BH, Ito ET, de Carvalho RF, et al. Using the comet and micronucleus assays for genotoxicity studies: A review. Biomed Pharmacother. 2015;72:74-82.
doi pubmed - Decordier I, Kirsch-Volders M. The in vitro micronucleus test: from past to future. Mutat Res. 2006;607(1):2-4.
doi pubmed - Kirsch-Volders M, Elhajouji A, Cundari E, Van Hummelen P. The in vitro micronucleus test: a multi-endpoint assay to detect simultaneously mitotic delay, apoptosis, chromosome breakage, chromosome loss and non-disjunction. Mutat Res. 1997;392(1-2):19-30.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


