| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 6, December 2023, pages 457-463
The Role of Crohn Disease on Breast Cancer Incidence: A Clinical Analysis
Raina K. Patela , Lexi Frankela, Matthew Cardeiroa, Wade Hansena, Kazuaki Takabeb, c, Omar M. Rashida, d, e, f, g, h, i, j, k, l
aNova Southeastern University Dr. Kiran C. Patel College of Allopathic Medicine, Fort Lauderdale, FL, USA
bDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA
cDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, The State University of New York, Buffalo, NY, USA
dMichael and Dianne Bienes Comprehensive Cancer Center, Holy Cross Health, Fort Lauderdale, FL, USA
eUniversity of Miami Leonard M. School of Medicine, Miami, FL, USA
fMassachusetts General Hospital, Boston, MA, USA
gBroward Health, Fort Lauderdale, FL, USA
hTopline MD Alliance, FL, USA
iMemorial Health, Pembroke Pines, FL, USA
jDelray Medical Center, Delray, FL, USA
kComplex General Surgical Oncology, General and Robotic Surgery, TopLine MD Alliance, Fort Lauderdale, FL 33308, USA
lCorresponding Author: Omar M. Rashid, Complex General Surgical Oncology, General and Robotic Surgery, TopLine MD Alliance, Fort Lauderdale, FL 33308, USA
Manuscript submitted June 28, 2023, accepted August 26, 2023, published online October 21, 2023
Short title: The Role of CD on BC Incidence
doi: https://doi.org/10.14740/wjon1644
| Abstract | ▴Top |
Background: Crohn disease is a chronic inflammatory disease that can affect the entire gastrointestinal tract. The pathophysiology of this disease characteristically involves transmural inflammation, which predisposes patients to various gastrointestinal cancers such as colon cancer. Although the increased risk of gastrointestinal cancers in Crohn disease has been well established, the risk of extra-gastrointestinal cancers remains unknown. We sought to study the risk of breast cancer in patients with Crohn disease.
Methods: The data for this retrospective study were compiled using the International Classification of Disease Ninth Revision (ICD-9) and ICD 10th Revision (ICD-10) codes from the national Health Insurance Portability and Accountability Act (HIPAA)-compliant PearlDiver database from 2010 to 2019. Patients were matched for age, sex, and Charlson Comorbidity Index (CCI). Statistical analyses were implemented to assess Chi-squared, logistic regression, and odds ratio.
Results: The database query resulted in 70,027 patients in both the control and Crohn disease groups. The incidence of breast cancer was 4,087 in the control group compared to 654 in the Crohn disease group. The P value was < 2.2 × 10-16 and the odds ratio was 0.15 (95% confidence interval (CI)). Patients without Crohn disease had an increased prevalence of breast cancer throughout all age ranges compared to patients with Crohn disease. Additionally, patients without Crohn disease had higher rates of breast cancer throughout the four major regions of the United States. In terms of healthcare costs, patients with breast cancer and a history of Crohn disease paid $23.87 more per hospital visit compared to patients with breast cancer and no history of Crohn disease.
Conclusions: The results of this study indicate a statistically significant correlation between Crohn disease and a reduced incidence of breast cancer. This finding is true across all age groups and across the United States. Further study is required to investigate a possible mechanism between the pathophysiology of Crohn disease ultimately leading to reduced tumorigenesis in the breast.
Keywords: Crohn disease; Breast cancer; Inflammation; Oncogenesis; Immunology
| Introduction | ▴Top |
Crohn disease (CD) is a chronic inflammatory disease of the gastrointestinal tract that affects approximately half a million Americans [1]. There is no cure for this disease and the quality of life for patients with CD is exceedingly poor. CD exhibits a bimodal distribution of disease onset, peaking around age 20 and again around age 50 [2]. This may reflect different phenotypes of the disease and/or the diverse influence of environmental or genetic factors on disease presentation and progression. The established risk factors for this disease include smoking, positive family history, and genetic mutations such as in NOD2 [3, 4].
CD can involve any portion of the gastrointestinal tract and is characterized by skip lesions and transmural inflammation. The pathophysiology of CD remains poorly understood but likely involves a complex interplay between genetic makeup, environmental influences, and the gut microbiome [5]. Patients with CD commonly experience periods of remission interspersed between disease flares, which often occur at unpredictable times [6]. These flares are characterized by the release of proinflammatory cytokines which provoke a heightened immune response, ultimately producing the characteristic symptoms of the disease such as diarrhea, rectal bleeding, and abdominal pain [7]. This inflammatory response is the target of numerous therapies for CD, many of which have been successful at reducing symptoms and preventing remission.
The persistence of localized inflammation in CD is thought to increase the risk of various gastrointestinal cancers through alteration of the composition of the gut mucosa. For instance, diseased small bowel in CD has been shown to display various molecular abnormalities, even before evidence of dysplasia or neoplasia is present [8]. Colon cancer is the most commonly diagnosed cancer in these patients and is a leading cause of mortality. The risk of colon cancer is thought to increase with various factors, such as longer duration of disease, extent of bowel involvement, and concomitant inflammatory conditions [8-10]. The link between CD and gastrointestinal cancers has been readily established, but the same cannot be said for extra-gastrointestinal cancers.
Breast cancer (BC) is the most commonly diagnosed type of cancer worldwide [11]. It is estimated that 297,790 women will be newly diagnosed with BC in the United States in 2023. Moreover, the rate of incidence of BC has been steadily increasing in females since the mid-2000s [12]. Numerous risk factors have been established for the development of BC, including smoking, obesity, mammographic density, positive family history, and prolonged exposure to estrogen [13-15]. It has been proposed that chronic inflammation, which is an important factor in the development of many cancers, may be an additional risk factor for breast carcinogenesis [16]. As CD is a disease that manifests with local in addition to systemic chronic inflammation, we hypothesized that patients with CD may have a different risk of developing BC compared to the general population [17].
Despite the predominance of both CD and BC, current literature on this topic fails to establish a strong association between CD and the development of BC. While some studies find a positive association between CD and BC development [18, 19], others find a negative association [20] or even no association [21-23] between the two, making this relationship all the more unclear. Furthermore, the majority of studies researching this association do not take place in the United States and thus may not be truly representative of the desired population. Finally, many studies look at both CD and its sister disease, ulcerative colitis (UC), together through the lens of irritable bowel disease (IBD), and therefore do not stratify BC risk based solely on the disease of interest. For these reasons, we chose to study the large-scale association of CD and BC in the United States. The overall purpose of this study was to assess the correlation between prior history of CD and risk of BC development.
| Materials and Methods | ▴Top |
Patient cohort
Access to a Humana Health Insurance Portability and Accountability Act (HIPAA) compliant national database was provided by Holy Cross Health, Fort Lauderdale, Florida, for the sole purpose of academic research. The data were analyzed retrospectively from January 2010 to December 2019 using the International Classification of Disease Ninth Revision (ICD-9) and ICD 10th Revision (ICD-10) codes for CD and BC. The control group consisted of patients without a history of CD while the experimental group consisted of patients with a history of CD. Both groups were then matched by age range, sex, and Charlson Comorbidity Index (CCI). The incidence of BC among the control and experimental groups was then assessed. Statistical analysis was performed using the PearlDiver Mariner database. PearlDiver contains over 41 billion HIPPA-compliant patient records compiled from various insurance organizations such as Humana, United Healthcare, and Medicare claims. To meet the inclusion criteria, patients required active status in the database for at least 8 years. BC incidence was the primary outcome measure of this study. Additionally, the demographics of the patient population were assessed and analyzed. Females and males were grouped together for analysis due to the small sample size of males preventing individual analysis by gender. Chi-square analyses, relative risk, and odds ratios (ORs) were utilized to analyze the database results and assess the statistical significance.
Literature review
A literature review was conducted to establish previous research on CD, BC, and their possible association. The review was conducted using Google Scholar and PubMed, and included papers published from 2000 to present. The review was guided using the following search terms: “Crohn disease”, “cancer”, “breast”, “inflammation”, and “carcinogenesis”. The literature review resulted in a diverse array of findings. A small majority of papers found no association between CD and BC. The remainder of the papers found either a positive or negative association between CD and BC. Ultimately, the review revealed a lack of consensus on this topic.
Institutional Review Board (IRB) approval
This study is exempt from IRB approval due to the use of a deidentified patient database. Ethical compliance with human study was not applicable.
| Results | ▴Top |
Patients with a history of CD had a significantly lower incidence of BC
Initial matching resulted in 70,027 patients without a history of CD and 70,027 patients with a history of CD. The total number of 70,027 matched patients was the largest sample of CD matched with control that was computed by the PearlDiver database. In the control group (light blue flowchart on left in Fig. 1), 4,087 out of 70,027 patients (5.84%) subsequently developed BC. Of these 4,087 patients, 3,993 were female and 94 were male. In the experimental group (dark blue flowchart on right in Fig. 1), 654 out of 70,027 (0.93%) patients subsequently developed BC. Of these 654 patients, 638 were female and 16 were male (Fig. 1). The difference in BC incidence between the control (light blue column on left in Fig. 2) and experimental groups (dark blue column on right in Fig. 2) was statistically significant (P < 2.2 × 10-16) (Fig. 2). Logistic regression also indicated that CD was associated with a decreased incidence of BC (OR = 0.15, 95% confidence interval (CI): (0.14, 0.17)).
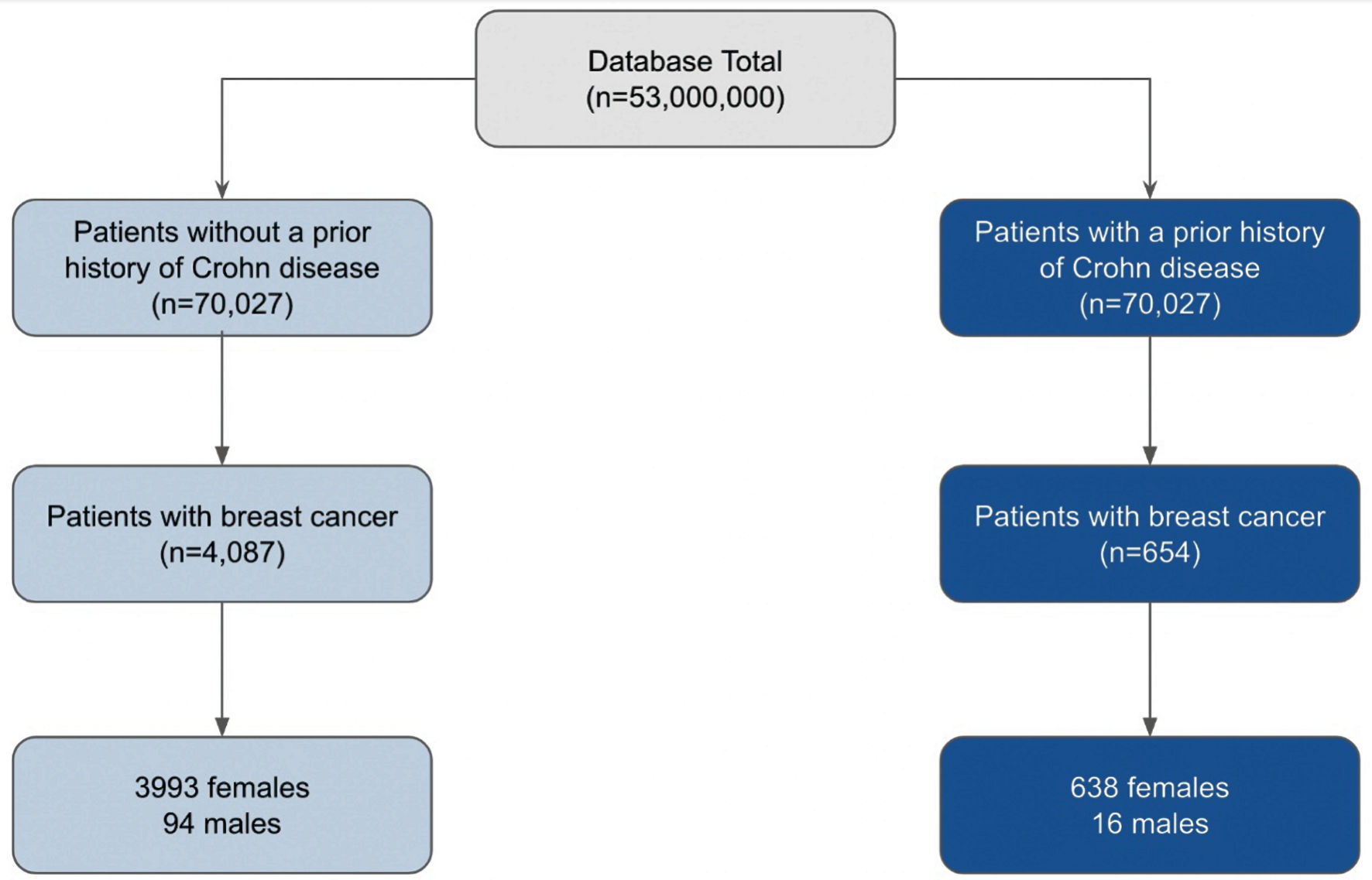 Click for large image | Figure 1. Analysis of the incidence of breast cancer in patients with (dark blue) and without (light blue) a history of CD, matched by age range and CCI score. From the total of 53,000,000 patients in the database, 70,027 patients were matched into either the group of patients with (dark blue) and without (light blue) CD. The incidence of breast cancer was then subsequently measured in each group. The gender breakdown of these cases was then evaluated. CD: Crohn disease; CCI: Charlson Comorbidity Index. |
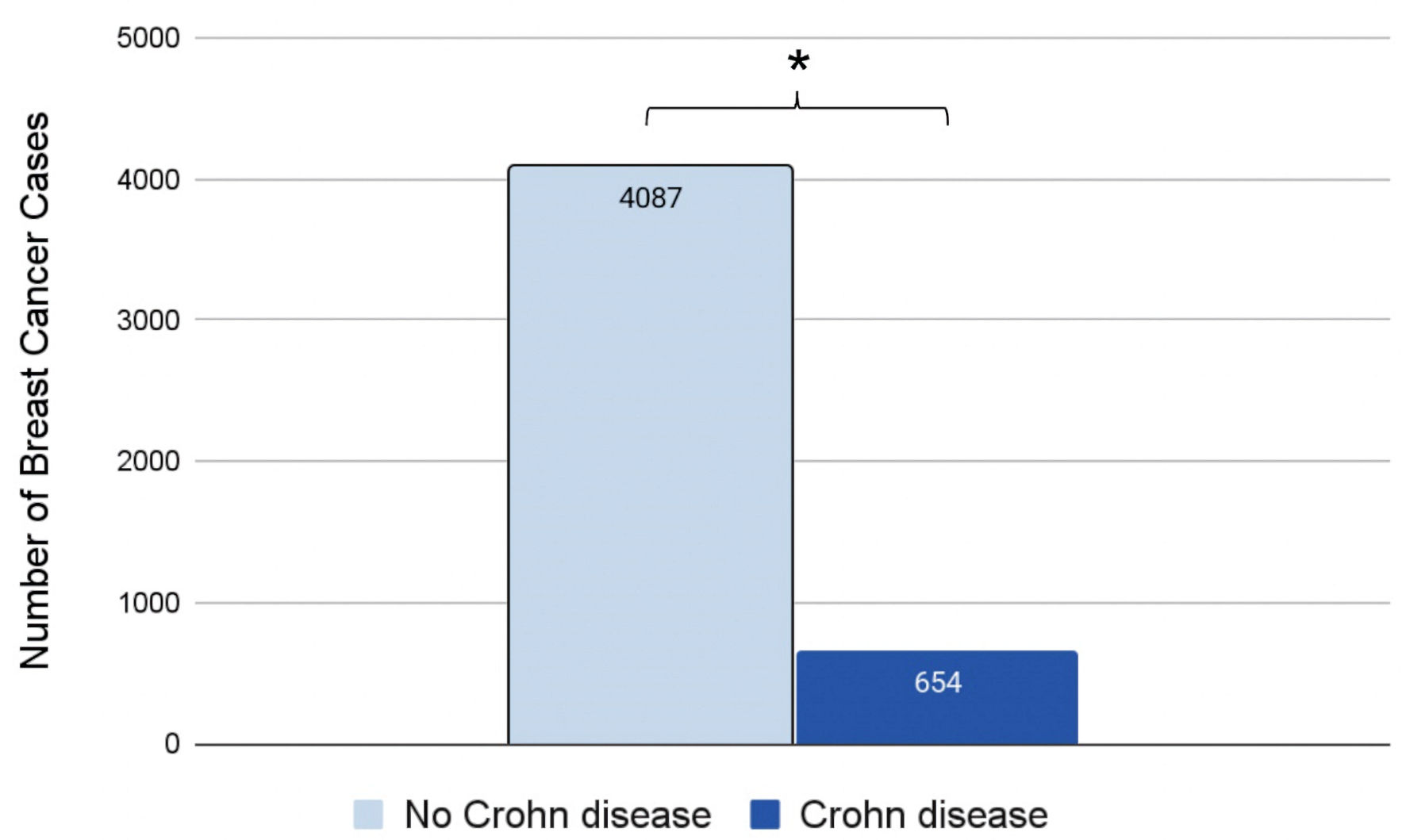 Click for large image | Figure 2. Incidence of breast cancer in patients with (dark blue) or without (light blue) a prior history of CD. Significance was measured using the Student’s t-test. *P < 0.05. CD: Crohn disease. |
Patients with CD of all ages had a consistently decreased prevalence of BC
Age stratification of the data revealed a difference in the prevalence of BC between patients with and without a history of CD. In both the control and experimental groups, the number of BC cases increased throughout adulthood, peaked at ages 60 - 64, and then declined. Our data indicate that patients with CD (dark blue columns in Fig. 3) display a markedly decreased prevalence of BC throughout all age groups compared to patients without CD (light blue columns in Fig. 3) (Fig. 3). Additionally, the number of BC cases in patients with CD remained more stable over various age groups, as opposed to the sharp increase and decrease in the number of BC cases in patients’ lifetimes in those without CD.
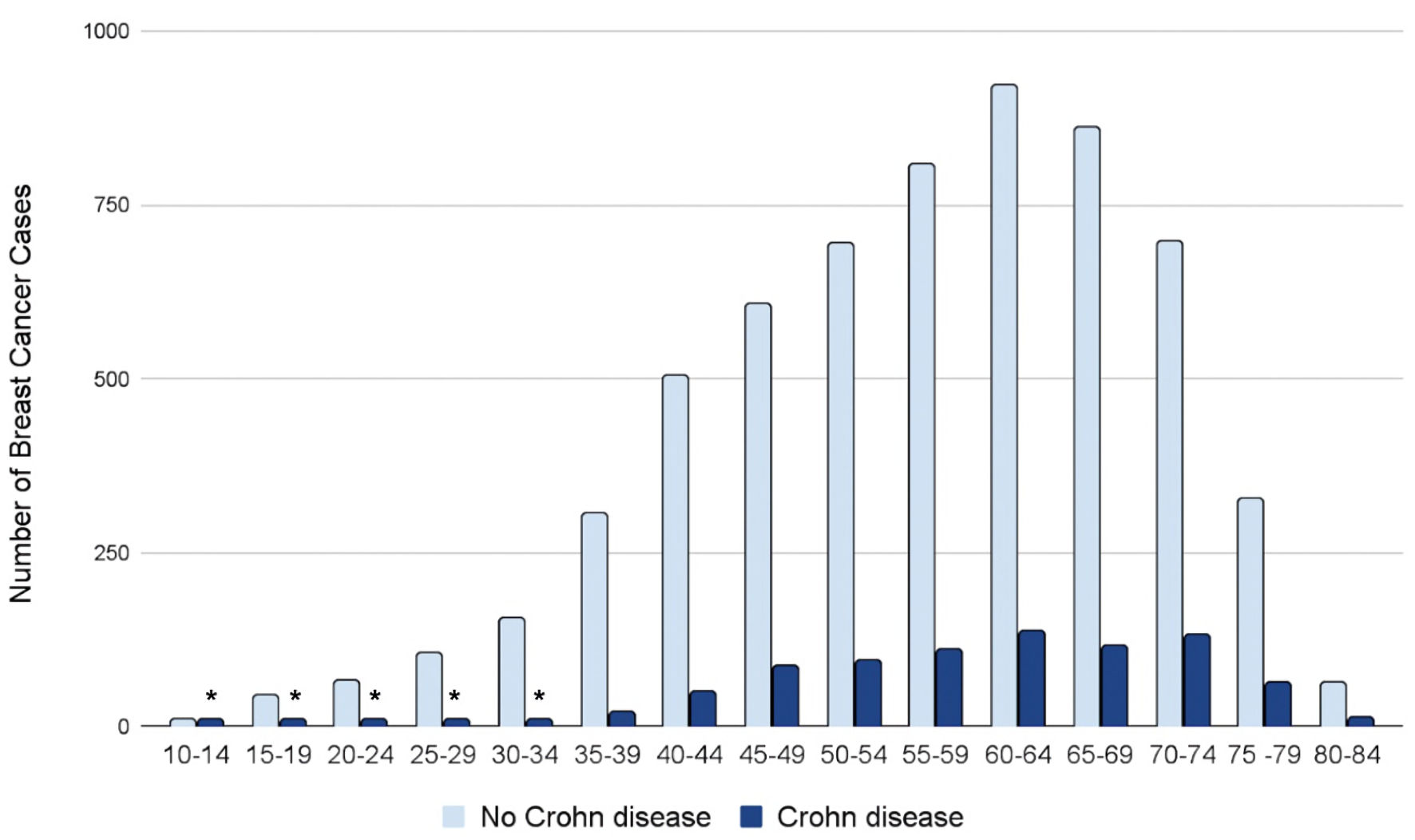 Click for large image | Figure 3. Prevalence of breast cancer by age group and presence (dark blue) or absence (light blue) of CD. *Number of patients ≤ 11. The exact number of patients could not be provided due to HIPAA constraints. CD: Crohn disease; HIPAA: Health Insurance Portability and Accountability Act (HIPAA). |
The incidence of BC in patients with CD displays demographic disparities
Further analysis of the data revealed various similarities and differences in demographic data among the control and experimental groups. Across all four regions of the United States, patients without a history of CD (light blue columns in Fig. 4) had higher rates of BC compared to patients with a history of CD (dark blue columns in Fig. 4) (Fig. 4). Interestingly, this difference was most apparent in the Southern United States compared to the Midwest, Northeast, and Western regions.
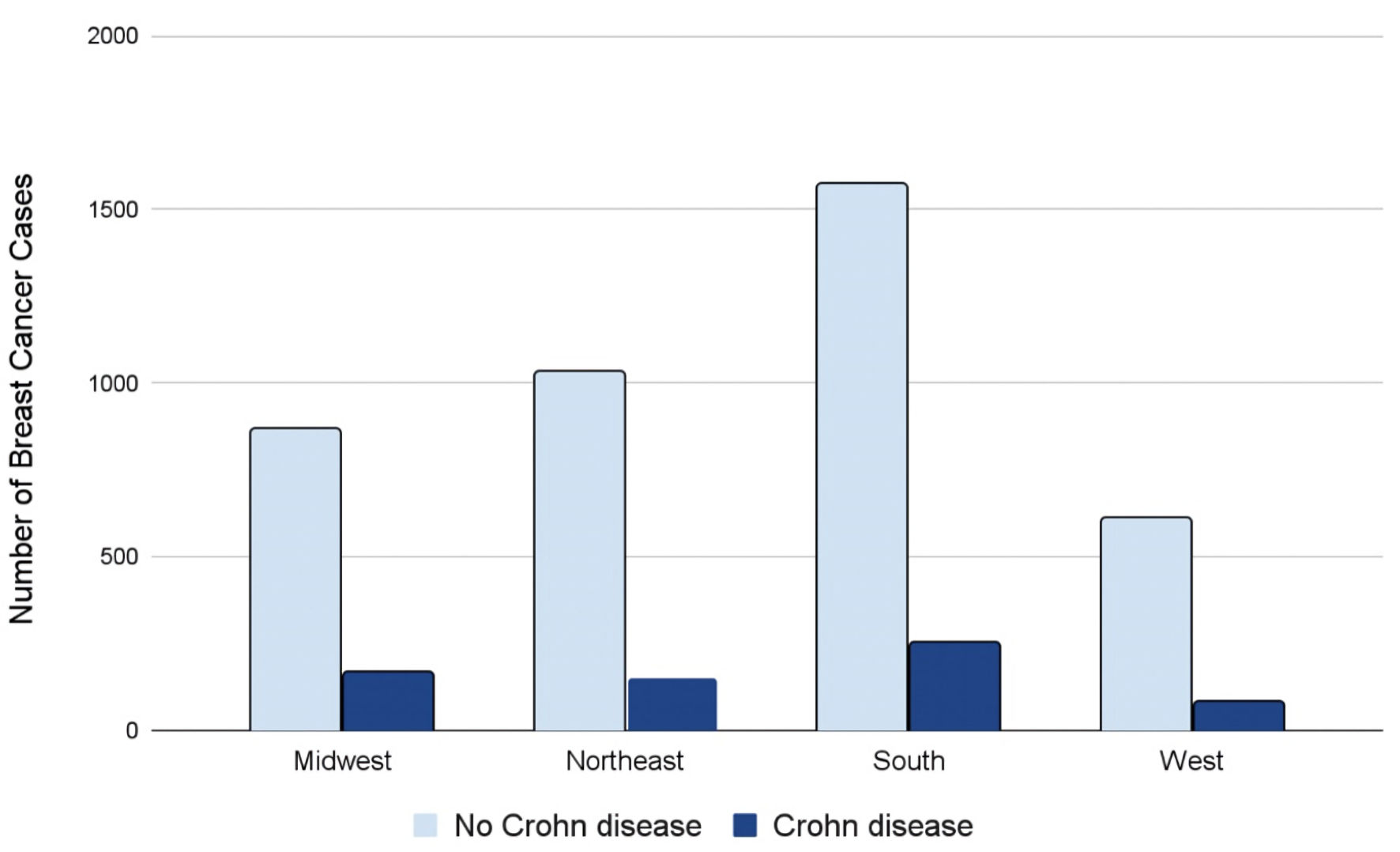 Click for large image | Figure 4. Regions of breast cancer development in the USA amongst individuals with (dark blue) and without (light blue) CD. CD: Crohn disease. |
Patients with CD and BC face a higher healthcare cost burden
Finally, a cost analysis revealed a disparity in healthcare costs between patients with a history of CD who later developed BC compared to patients without a history of CD who later developed BC. As expected, patients without a history of CD who later developed BC (light blue column on left in Fig. 5) paid less in healthcare costs on average compared to patients with a history of CD who also later developed BC (dark blue column on right in Fig. 5). This difference was quantified as $23.87 per hospital visit (Fig. 5).
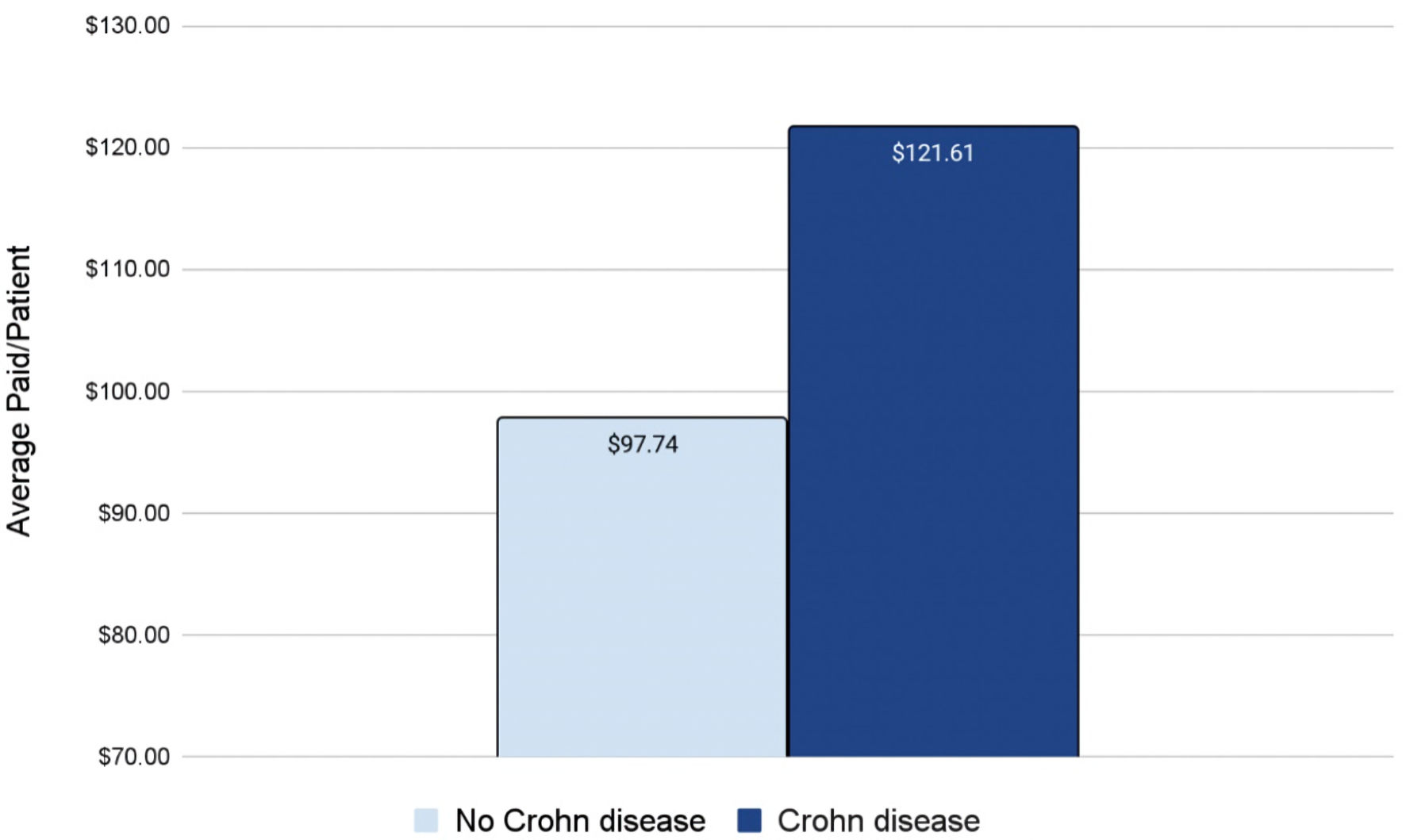 Click for large image | Figure 5. Average paid per visit for a breast cancer patient with (dark blue) or without (light blue) a prior history of CD. CD: Crohn disease. |
| Discussion | ▴Top |
CD is a chronic inflammatory disease with multifactorial etiology. The inflammation in CD characteristically affects localized areas of the gastrointestinal tract. Additionally, CD also results in a systemic inflammatory response characterized by extraintestinal symptoms such as enteropathic arthritis, uveitis, and erythema nodosum [17]. This chronic widespread inflammation may alter the risk of various extragastrointestinal cancers in CD patients, namely, BC. The data gathered in this study ultimately demonstrates an increased need to investigate the association between CD and BC.
Our data indicate a statistically significant correlation between CD and a reduced incidence of BC. Further data are needed to understand the pathophysiology underlying this finding. A proposed mechanism that may explain this may involve the presence of a subpopulation of CD4+ T-helper cells called T-helper 17 (Th17) cells. These cells are thought to play a role in numerous autoimmune and inflammatory conditions such as rheumatoid arthritis, multiple sclerosis, psoriasis, and inflammatory bowel disease [24]. The dysregulation of Th17 cells in CD is believed to promote the localized proinflammatory response characteristic of the disease [6].
The role of Th17 cells in cancer is poorly understood. Literature reveals that these cells may have a protumor or antitumor effect depending on the specific cancer phenotype. For instance, a study of patients with gastric cancer found a significantly increased expression of interleukin (IL)-17 in patients with highly advanced gastric cancers, with the level of expression correlating with disease severity [25]. Contrastingly, another study found that Th17 cells were instrumental in the eradication of lung metastatic tumors in a murine model [26]. The plasticity of these cells is largely dependent on the stimuli they face and contributes to their divergent role in tumor immunity [27, 28].
In CD, these cells have been shown to be specifically recruited to breast tissue by cytokines secreted from the intestinal tract through the IL-17 and nuclear factor kappa B (NF-κB) signaling pathways, which are common pathways shared by both CD and BC [29]. We hypothesize that the Th17 cells recruited to the site of the breast may be of an antitumor phenotype, ultimately decreasing the risk of BC in these patients. Ultimately, the immunomodulatory role of Th17 cells is likely to be dependent on the specific cancer phenotype. Given these findings, future studies are recommended to investigate the role of the immune system and specifically Th17 cells in the prevention and treatment of BC.
Inherent limitations were sustained due to this study being of retrospective observational design. Potential limitations faced in our study include selection bias and data stratification limitations; for instance, the inability to determine the severity of CD, differentiate the specific subtype of BC diagnosis, and quantify the duration of CD before BC diagnosis. Having this information may allow us to better understand the nuances of the association between these two diseases and establish a timeframe between diagnoses. Patients with CD were also unable to be matched by the duration or type of treatment they received. This may introduce confounding variables, such as anti-inflammatory therapy, which may impact the incidence of BC but were unable to be controlled for in this study. Furthermore, although large, the PearlDiver database is limited by specific insurance types and may not accurately represent the entire population of interest. The database is also heavily dependent on meticulous record-keeping by healthcare professionals and insurance companies to gather accurate data for this study. Future studies should focus on obtaining large-scale yet detailed patient data in order to capture the fullest picture of CD and how it may influence the risk of subsequent BC diagnosis.
Acknowledgments
The authors acknowledge the support of Holy Cross Health and Nova Southeastern University Dr. Kiran C. Patel College of Allopathic Medicine.
Financial Disclosure
Grant support by Broward Community Foundation.
Conflict of Interest
K. Takabe was supported by US National Institutes of Health; R37CA248018, R01CA250412, R01CA251545, R01EB029596, as well as US Department of Defense BCRP grants; W81XWH-19-1-0674 and W81XWH-19-1-0111. Other authors have no conflict of interest to disclose.
Informed Consent
IRB approval: exempt (database study).
Author Contributions
Raina K. Patel, as the first author, is responsible for data analysis, figures, literature review, and manuscript writing. Matthew Cardeiro is responsible for database extraction and analysis, and manuscript editing. Lexi Frankel and Wade Hansen are responsible for supplemental editing. Authors Omar M. Rashid, as the corresponding author, and Kazuaki Takabe are responsible for manuscript writing, editing, and data analysis.
Data Availability
Data were extracted from PearlDiver national database. Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Kappelman MD, Moore KR, Allen JK, Cook SF. Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured US population. Dig Dis Sci. 2013;58(2):519-525.
doi pubmed pmc - Polito JM, 2nd, Childs B, Mellits ED, Tokayer AZ, Harris ML, Bayless TM. Crohn's disease: influence of age at diagnosis on site and clinical type of disease. Gastroenterology. 1996;111(3):580-586.
doi pubmed - Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380(9853):1590-1605.
doi pubmed - Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV, Jr. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010;139(4):1147-1155.
doi pubmed pmc - Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389(10080):1741-1755.
doi pubmed - Petagna L, Antonelli A, Ganini C, Bellato V, Campanelli M, Divizia A, Efrati C, et al. Pathophysiology of Crohn's disease inflammation and recurrence. Biol Direct. 2020;15(1):23.
doi pubmed pmc - Veauthier B, Hornecker JR. Crohn's disease: diagnosis and management. Am Fam Physician. 2018;98(11):661-669.
pubmed - Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287(1):G7-17.
doi pubmed - Demb Joshua, Ashley Earles, Maria Elena Martinez, Ranier Bustamante, Bryant AK, Murphy JD, Lin Liu, et al. Risk factors for colorectal cancer significantly vary by anatomic site. BMJ Open Gastroenterology. 2019;6(1):e000313.
doi - von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The risk of cancer in patients with Crohn's disease. Dis Colon Rectum. 2007;50(6):839-855.
doi pubmed - Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, Vignat J, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15-23.
doi pubmed pmc - Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48.
doi pubmed - Checka CM, Chun JE, Schnabel FR, Lee J, Toth H. The relationship of mammographic density and age: implications for breast cancer screening. AJR Am J Roentgenol. 2012;198(3):W292-295.
doi pubmed - Escala-Garcia M, Morra A, Canisius S, Chang-Claude J, Kar S, Zheng W, Bojesen SE, et al. Breast cancer risk factors and their effects on survival: a Mendelian randomisation study. BMC Med. 2020;18(1):327.
doi pubmed pmc - Yue W, Wang JP, Li Y, Fan P, Liu G, Zhang N, Conaway M, et al. Effects of estrogen on breast cancer development: Role of estrogen receptor independent mechanisms. Int J Cancer. 2010;127(8):1748-1757.
doi pubmed pmc - Danforth DN. The role of chronic inflammation in the development of breast cancer. Cancers (Basel). 2021;13(15):3918.
doi pubmed pmc - Rivera ED, Coffey JC, Walsh D, Ehrenpreis ED. The Mesentery, Systemic Inflammation, and Crohn's Disease. Inflamm Bowel Dis. 2019;25(2):226-234.
doi pubmed - Hemminki K, Li X, Sundquist J, Sundquist K. Cancer risks in Crohn disease patients. Ann Oncol. 2009;20(3):574-580.
doi pubmed - Hovde O, Hoivik ML, Henriksen M, Solberg IC, Smastuen MC, Moum BA. Malignancies in patients with inflammatory bowel disease: results from 20 years of follow-up in the IBSEN study. J Crohns Colitis. 2017;11(5):571-577.
doi pubmed - van den Heuvel TR, Wintjens DS, Jeuring SF, Wassink MH, Romberg-Camps MJ, Oostenbrug LE, Sanduleanu S, et al. Inflammatory bowel disease, cancer and medication: Cancer risk in the Dutch population-based IBDSL cohort. Int J Cancer. 2016;139(6):1270-1280.
doi pubmed - Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91(4):854-862.
doi pubmed - Kappelman MD, Farkas DK, Long MD, Erichsen R, Sandler RS, Sorensen HT, Baron JA. Risk of cancer in patients with inflammatory bowel diseases: a nationwide population-based cohort study with 30 years of follow-up evaluation. Clin Gastroenterol Hepatol. 2014;12(2):265-273.e261.
doi pubmed pmc - Mark-Christensen A, Erichsen R, Veres K, Laurberg S, Sorensen HT. Extracolonic Cancer Risk After Total Colectomy for Inflammatory Bowel Disease: A Population-based Cohort Study. J Crohns Colitis. 2020;14(5):630-635.
doi pubmed - Yang J, Sundrud MS, Skepner J, Yamagata T. Targeting Th17 cells in autoimmune diseases. Trends Pharmacol Sci. 2014;35(10):493-500.
doi pubmed - Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374(3):533-537.
doi pubmed - Ankathatti Munegowda M, Deng Y, Mulligan SJ, Xiang J. Th17 and Th17-stimulated CD8(+) T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol Immunother. 2011;60(10):1473-1484.
doi pubmed - Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276.
doi pubmed pmc - Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184(3):1630-1641.
doi pubmed - Zhou S, Yu J. Crohn's disease and breast cancer: a literature review of the mechanisms and treatment. Intern Emerg Med. 2023;18(5):1303-1316.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


