| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 6, December 2023, pages 518-528
Addition of Olaparib to the New Hormonal Agent Regimen for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis
Syah Mirsya Warlia, b, e , Adrian Joshua Velaroc
, Naufal Nandita Firstyc
, Zaimah Zulkarnaini Talad
aDepartment of Urology, Universitas Sumatera Utara Hospital, Universitas Sumatera Utara, Medan, Indonesia
bDivision of Urology, Department of Surgery, Faculty of Medicine, Universitas Sumatera Utara-Haji Adam Malik General Hospital, Medan, Indonesia
cDepartment of Surgery, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
dDepartment of Nutrition, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
eCorresponding Author: Syah Mirsya Warli, Department of Urology, Universitas Sumatera Utara Hospital, Universitas Sumatera Utara, Medan, Indonesia
Manuscript submitted August 1, 2023, accepted September 25, 2023, published online October 21, 2023
Short title: Olaparib for mCRPC
doi: https://doi.org/10.14740/wjon1685
| Abstract | ▴Top |
Background: The emergence of olaparib, a poly (adenosine diphosphate (ADP)-ribose) polymerase (PARP) inhibitor to treat metastatic castration-resistant prostate cancer (mCRPC), created a measurable clinical question on whether the agent positively influences the treatment outcomes and acceptable safety factors. The objective was to elaborate on the efficacy and safety of olaparib-added regimens in treating mCRPC patients as compared to the established guideline.
Methods: The literature search was performed on several scientific databases, e.g., PubMed, Cochrane, and ScienceDirect, by applying the Boolean Term method. Statistical and risk of bias (RoB) analyses were calculated through RevMan 5.4.1. to investigate our outcomes, i.e., progression-free survival (PFS) and overall survival (OS) with the reported adverse effects (AEs). These outcomes were presented in hazard ratio (HR) and risk ratio (RR).
Results: Three trials consisting of 1,325 individuals with comparable baseline characteristics were investigated. The meta-analysis showed that introducing olaparib into the regimens significantly improved the PFS (HR 0.59 (0.48 - 0.73); P < 0.05), which disclosed even better outcomes among mutated homologous recombinant repair (HRR) and ataxia-telangiectasia mutated (ATM) gene (HR 0.43 (0.30 - 0.62); P < 0.05) in 95% confidence interval (CI). Furthermore, similar outcomes were observed in OS analysis (HR 0.81 (0.67 - 0.99); P < 0.05), despite olaparib group disclosed higher AEs rate with insignificant difference in mortality rate.
Conclusion: The efficacy and safety of olaparib-added regimens in mCRPC patients need to be explored more extensively in trials because they are beneficial, particularly among HRR-mutated individuals.
Keywords: Castration-resistant prostate cancer; mCRPC; PARP; Olaparib; HRR mutation
| Introduction | ▴Top |
Anti-hormonal treatment resistance among prostate cancer (PC) had established a considerable challenge in modern urology practice, since treatment resistance might translate into aggressive and fatal cancer characteristics with a substantial effect to prognostic value [1, 2]. As the most prevalent cancer according to the GLOBOCAN 2020 report with a prominent fifth leading cause of malignancy-related mortality, exploration of PC treatment options is rapidly progressing, as represented by the more detailed identification of its cellular aspects over the last few decades [3].
Although the majority of PC patients initially presented with localized disease, advanced PC may develop refractory characteristics toward hormonal-inhibiting agents or procedures, hence termed metastatic or non-metastatic castration-resistant prostate cancer (mCRPC or nmCRPC). Findings of prostatic biochemical recurrences after androgen deprivation therapy (ADT) will eventually translate into limited treatment options [1, 4, 5]. Shifting from castration-sensitive prostate cancer (CSPC) to CRPC created an urgency to explore other approaches by interrupting the cancer cell’s cycle progression through targeted therapy; despite reliance on androgen receptor (AR) activation, this may still be observable to some extent [6-8]. These unmet clinical needs are establishing a prominent clinical question on whether recently introduced drug proved beneficial to improve the prognosis, considering current scenario of chemotherapy agents involved resistance or adverse effects (AEs) issues and recognized global intention to avoid disease progression and prolong patient’s life expectancies [9, 10].
Olaparib, a poly (adenosine diphosphate (ADP)-ribose) polymerase (PARP) inhibitor, had been approved for mCRPC by the US Food and Drug Administration (FDA) in mid-2020, although it was originally aimed at individuals with mutated BRCA1/2 pathway when it was introduced more than a decade ago [11]. A previous meta-analysis on olaparib efficacy to improve the outcomes of other cancers, e.g., ovarian cancer, breast cancer, etc., had confirmed that the therapeutic rationale to incorporate olaparib into routine treatment regimen is justifiable, in which the populations with mutated BRCA gene might possess much favorable outcomes. BRCA gene mutations, particularly BRCA2, can significantly increase the risk of hereditary PC. Individuals with a BRCA2 mutation are generally found to have a lifetime risk of developing PC ranging from 20% to 40%, and in certain cases, this risk may escalate to as much as 60%, according to various research findings [12]. Combination use with new hormonal agent (NHA) has been recommended by NCCN clinical practice, driving the current research question to cooperate PARP inhibitor (PARPi) into mCRPC guideline. Homologous recombinant repair (HRR) is a method for fixing damaged DNA by bringing in an undamaged DNA molecule that closely matches the damaged one. It then uses this undamaged molecule as a guide to rebuild the damaged section, essentially copying from the undamaged template to repair the DNA. The prevalence of HRR mutations can range from 11.8% when examining only germline mutations or 23% when considering solely somatic mutations, to as high as 33% when assessing both germline and somatic mutations collectively, depending on the testing approach and the specific genes under evaluation [13, 14].
Objectives
This meta-analysis aimed to establish the role of olaparib in NHA-based regimen in improving mCRPC therapeutic outcomes and to compare its influence on treatment-modification decision within the currently available evidence to date.
| Materials and Methods | ▴Top |
Study design
Initially, we screened the titles and abstracts according to our prior eligibility criteria-guided study selection. The inclusion criteria are phase II/III or controlled trials, investigating the effect of olaparib-addition on anti-hormonal regimen (e.g., androgen biosynthesis inhibitors (ABIs)) as compared to the latter regimen alone. We performed the literature search from databases, e.g., PubMed, ScienceDirect, and Cochrane Review, to identify eligible studies until October 2022. The Boolean term method and Medical Subject Headings (MeSH) were applied to specify the keywords used on title/abstract section, related to olaparib or PARP inhibitor, as combined to metastatic castration-resistant prostate cancer (mCRPC) and its derivative words. Focusing on mCRPC populations is mandatory regardless of the status of genetic alteration, e.g., BRCA1/2, ATM, or HRR, although our secondary analysis included sub-group evaluation of the latter gene’s mutations along with observable AEs on both arms. We excluded studies if the trial itself published interim-specific outcome (i.e., population-based) analysis. The reported outcomes should be presented in a prognosis-based investigation, e.g., investigator assessed imaging-based progression-free survival (PFS) and/or overall survival (OS) in hazard ratio (HR), with 95% confidence interval (CI). PRISMA reporting tools were utilized as the standard protocol in the preparation of this study, which has been registered on PROSPERO for international database reporting of systematic review under the issued ID of CRD42022376362.
Data extraction
Data were extracted thoroughly to ease the authors in determining similarity of the included studies by identifying first author’s last name and study design, population characteristics or size, administered/compared regimens, detailed patients’ status (age, genetic mutation status, metastasis status and site, prior treatment either failing treatment during hormone-sensitive prostate cancer (HSPC) stage or ongoing approaches, prostate-specific antigen (PSA) levels, etc.) and reported outcomes, preferably the treatment drug efficacy-related parameters. The HR of those statistical outcomes with 95% CI was extracted, concentrating on the olaparib-added arm vs. olaparib-without arm regardless of the genetic mutation proportion which may reflect population variations among mCRPC patients. The reported AEs were also extracted, and subsequently presented as risk ratio (RR) outcomes to determine whether olaparib administration will demonstrate significantly higher unexpected events among the population. This secondary analysis was presented for all grades of reactions, and for severe reactions only (grade ≥ 3).
Risk of bias (RoB) analysis
The quality of each study was mainly assessed by an author (NNF) by using the revised Cochrane RoB tool on RevMan 5.4.1 software, consisting of six variables, i.e., selection bias (random sequence generation and allocation concealment), performance bias, detection bias, attrition bias, reporting bias, and other potential bias. The results were internally discussed between authors on whether there are disagreements of the interpretations; therefore, the quality assessment results will be presented on summarizing figures.
Statistical analysis
All mathematical analyses were performed by using the Review Manager (RevMan) statistical software version 5.4.1., which automatically calculated the log (HR) and standard of error (SE), and interpreted RR calculations on forest plots. The AEs analysis will also be presented in a similar way, though we will also attempt to delineate changes in treatment course during the trial’s progression by systematically presenting the outcomes or AEs-related dose reduction, discontinuation, or even mortality. The heterogeneity of each analysis was determined by I2 results, as > 50% of the value may signify studies’ heterogeneity, hence random-effects model (REM) was adopted, and vice versa for the fixed-effect model (FEM). After confirming the 95% CI analysis on each outcome, the P value of < 0.05 will be considered statistically significant; hence the addition of olaparib may prove either beneficial or disadvantageous to the mCRPC population.
Ethical compliance is reviewed by Ethical Committee for Health Research Universitas Sumatera Utara. Standard protocol is registered in PROSPERO for international database reporting of systematic review under the issued ID of CRD42022376362.
| Results | ▴Top |
From the initially identified 356 studies, we included three trials involving 1,325 individuals with mCRPC (726 patients were allocated to intervention arm), in which the PRISMA identification flow and its characteristics are presented in Figure 1 and Table 1 [15-17], respectively. Those trials combined olaparib (300 mg) per oral (PO) twice a day with abiraterone (1,000 mg) or enzalutamide (160 mg) PO once a day, plus prednisone/prednisolone (5 mg) PO twice a day as well. Therefore, considering the similarity of intervention, the investigated outcomes were also revolved around regimens’ efficacy in prolonging PFS and OS duration and rate among patients with similar baseline characteristics (Table 2) [15-17]. Genetic mutations were reported, in which some subgroup analyses were conducted to determine whether the mutation might influence the primary outcomes, implying certain recognizable mutation will eventually influence the decision to administer olaparib in those populations.
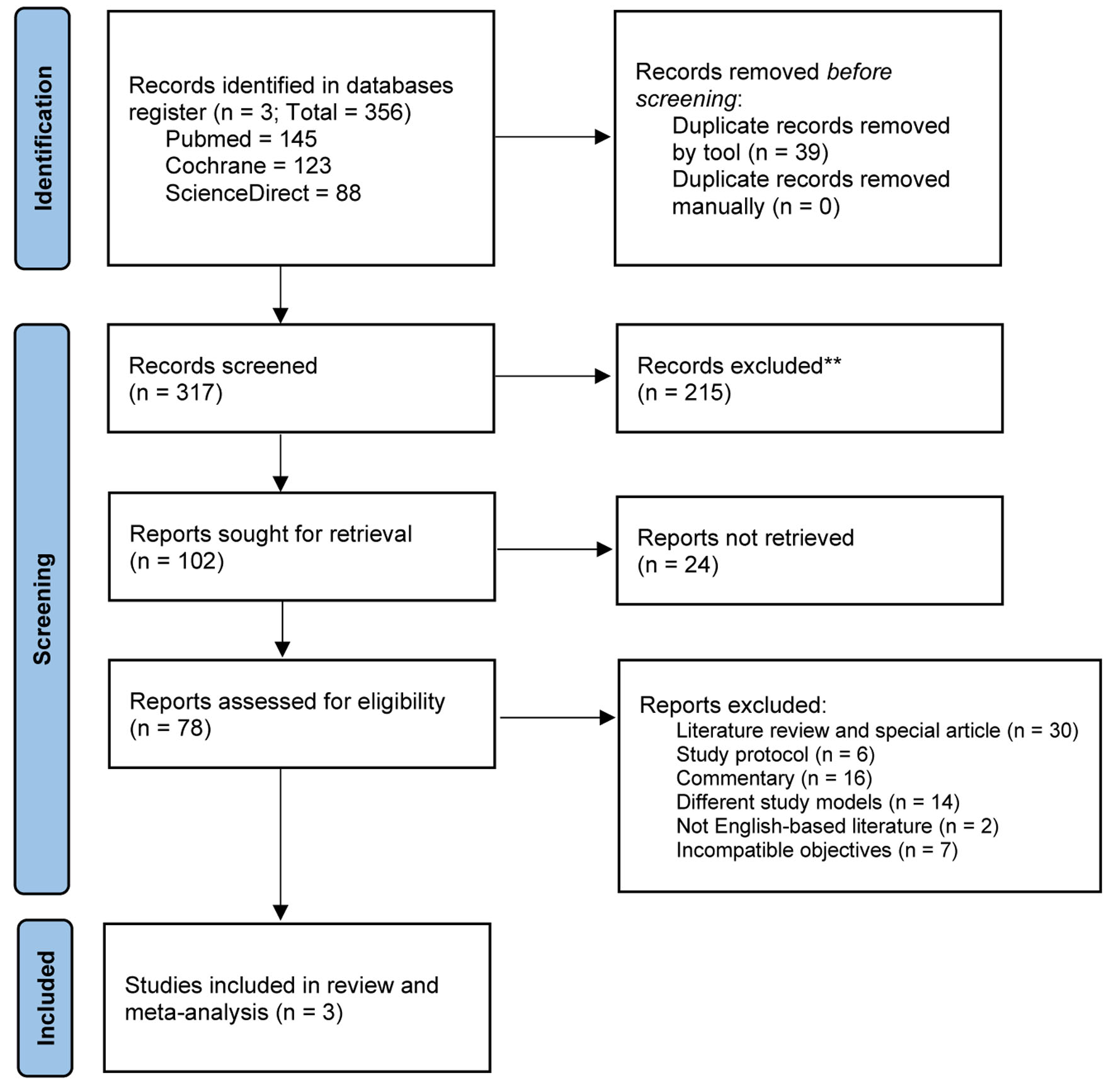 Click for large image | Figure 1. PRISMA reporting diagram to identify eligible trials for review. |
 Click to view | Table 1. Summary and Characteristics of the Included Studies |
 Click to view | Table 2. The Characteristics of Populations Included in Each Trial |
The RoB analysis disclosed “low risk” on almost all variables assessed, which are reasonably accepted considering all of the included trials are preeminent investigations on novel oncology or PC treatment. We qualitatively present our summarized RoB interpretation (Fig. 2) and no statistical confirmation was performed to assess the biases’ magnitude. However, all studies included are some globally recognized trials which are supported by significant pharmaceutical companies as disclosed on the respective studies’ declaration of interest. Therefore, the biases’ variables, e.g., selection bias, etc., had been reported thoroughly on the open-access trial protocols (accessible through each publisher websites) [15-17].
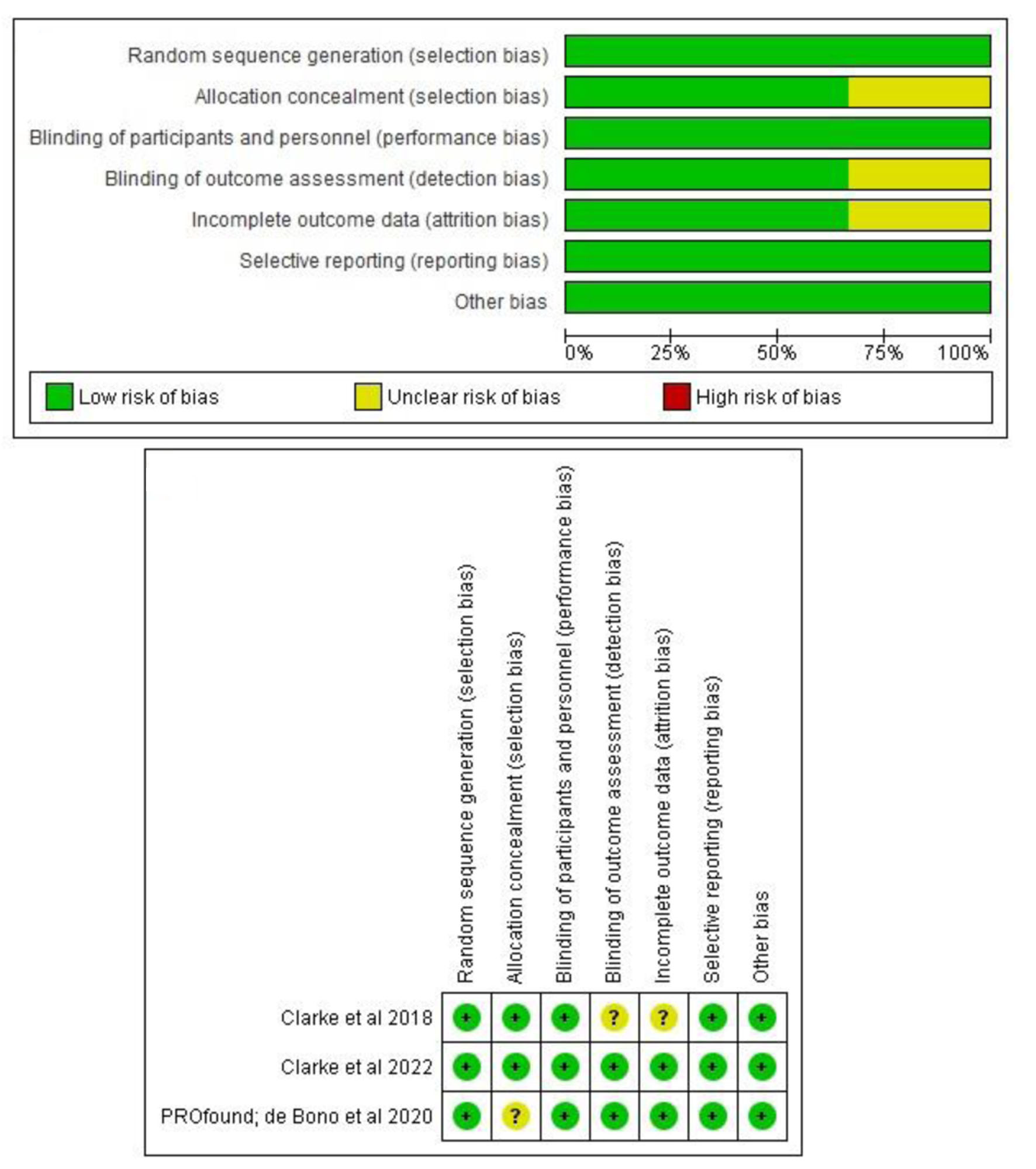 Click for large image | Figure 2. Summary of risk of bias (RoB) assessment of the included studies. All bias variables disclosed as low risk of bias. |
PFS and OS
Our analysis on those three trials demonstrated that olaparib addition into the ABIs plus corticosteroid regimen toward pooled mCRPC patients possessed better PFS rate with the HR of 0.59 (0.48 - 0.73) with P < 0.05 (Mantel-Haenszel test, I2 = 42%), in 95% CI and REM analysis. Subgroup analysis on mutated HRR or ATM gene revealed the superiority of olaparib among those genetic-mutated mCRPC individuals to extend the PFS, with the HR of 0.43 (0.30 - 0.62) with P < 0.05 (Mantel-Haenszel test, I2 = 45%) in REM analysis (Fig. 3a). Similar outcomes were also observed on OS analysis, since we found that olaparib may positively improve the survival rates of the mCRPC patients regardless of a preexisting genetic mutation or else. We calculated the cumulated HR value of 0.81 (0.67 - 0.99) within 95% CI, P value < 0.05 (Mantel-Haenszel test, I2 = 0%) in REM analysis (Fig. 3b).
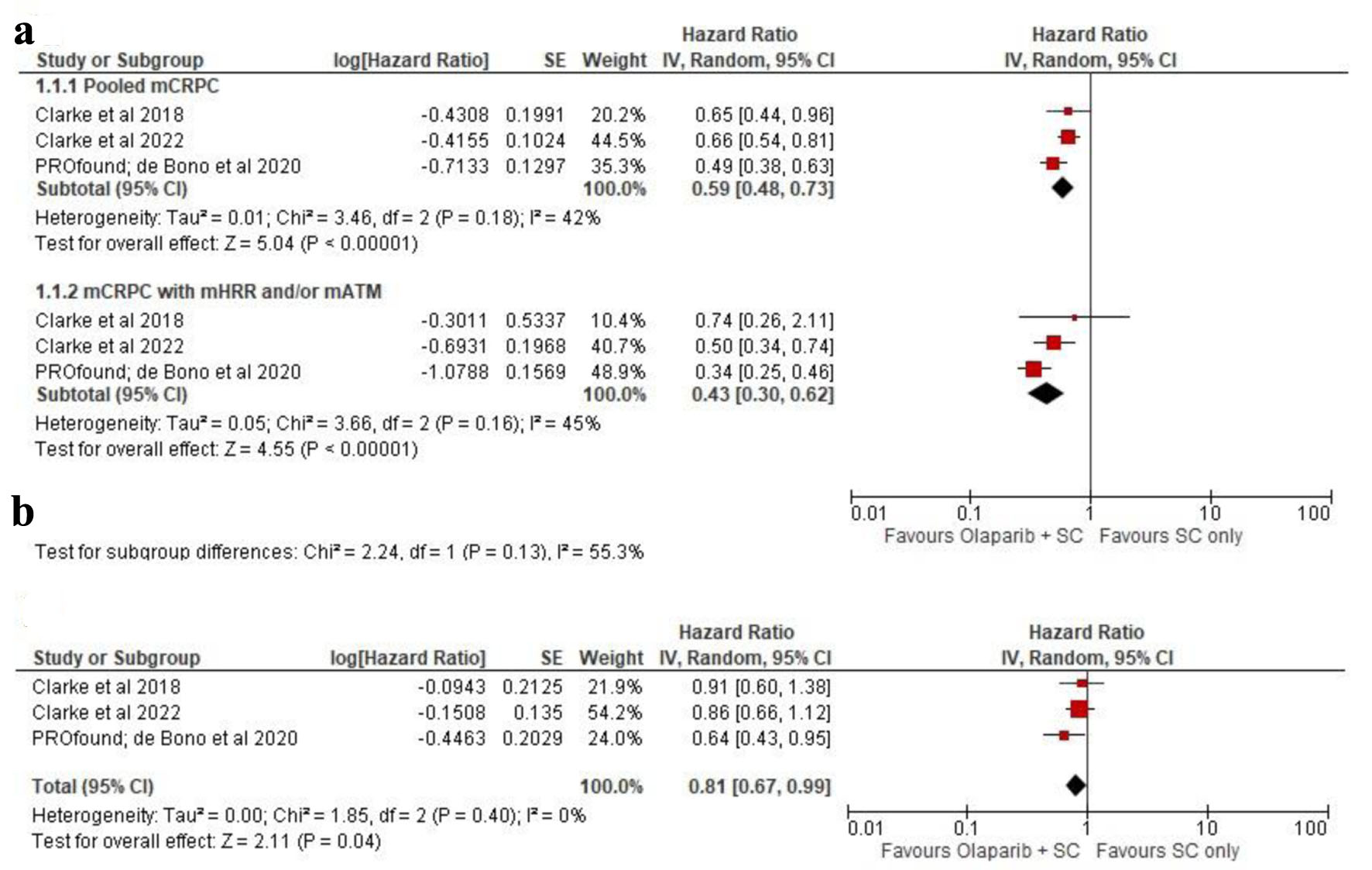 Click for large image | Figure 3. (a) Progression-free survival (PFS) as presented in pooled metastatic castration-resistant prostate cancer (mCRPC) and mutated homologous recombinant repair (mHRR)/ataxia-telangiectasia mutated (mATM) populations. (b) Pooled overall survival (OS) analysis of the included study. |
Administered-regimen-related AEs
The AEs were classified into two different sections, i.e., all grades and severe (grade ≥ 3) AEs, to systematically measure the unexpected consequences of both regimens (Table 3). Overall, addition of olaparib into the regimen dramatically increased the RR value of the reported symptoms as seen on both hematological and non-hematological reactions. For instance, RR for anemia in all grades was 3.16 (2.06 - 4.84), compared to grade ≥ 3 with 4.64 (2.96 - 7.28) and both outcomes appeared to be statistically significant (P < 0.05) on 95% CI. Nausea, decrease of appetite, and diarrhea also revealed to be the most in-risk reactions after olaparib administration with the RR value > 2.0 on all grades (all P < 0.05), although the similar calculations were not shown on severe grade AEs. Among the grade ≥ 3 reactions, vomiting became the symptom with the highest RR value of 5.92 (1.77 - 19.82), interestingly higher than any documented reactions. Quantitatively, the all grades AEs rates among both arms were similar; however, the number of significantly different or meaningful RRs was reduced on grade ≥ 3 reactions, placing olaparib-included regimen on relatively safe zone regarding this outcome. All P values presented in AEs outcomes are presented from Mantel-Haenszel test to estimate RR based on proportional-odds analysis model. We implemented the REM analysis to all AEs’ estimation in concordant with the approximated I2 value which is > 50.0%.
 Click to view | Table 3. Meta-Analysis of Adverse Effects in Hematological and Non-Hematological System of This Study (All Studies Included) |
Changes of interventions were observed on both arms, as the AEs might interrupt, reduce, discontinue, or cause death in some instances. Olaparib in addition to the regimen will theoretically have higher rate of treatment modification as seen on RR of therapeutic interruption (2.03 (1.43 - 2.90), Mantel-Haenszel test in REM (I2 = 66%); P < 0.05), received dose reduction decision (4.30 (1.51 - 12.20), Mantel-Haenszel test in REM (I2 = 71%); P < 0.05), and therapeutic discontinuation probably to higher AEs’ grade occurrence on the individuals (1.84 (1.14 - 2.98), Mantel-Haenszel test in REM (I2 = 57%); P < 0.05) as presented in Figure 4. However, significant difference on AEs-related mortality was not observed in this study (RR, 0.99 (0.57 - 1.73), Mantel-Haenszel test in REM (I2 = 0%); P = 0.98), thus presenting a premise that olaparib addition into the regimen might not negatively influence the mortality rate even in its AEs-related complications.
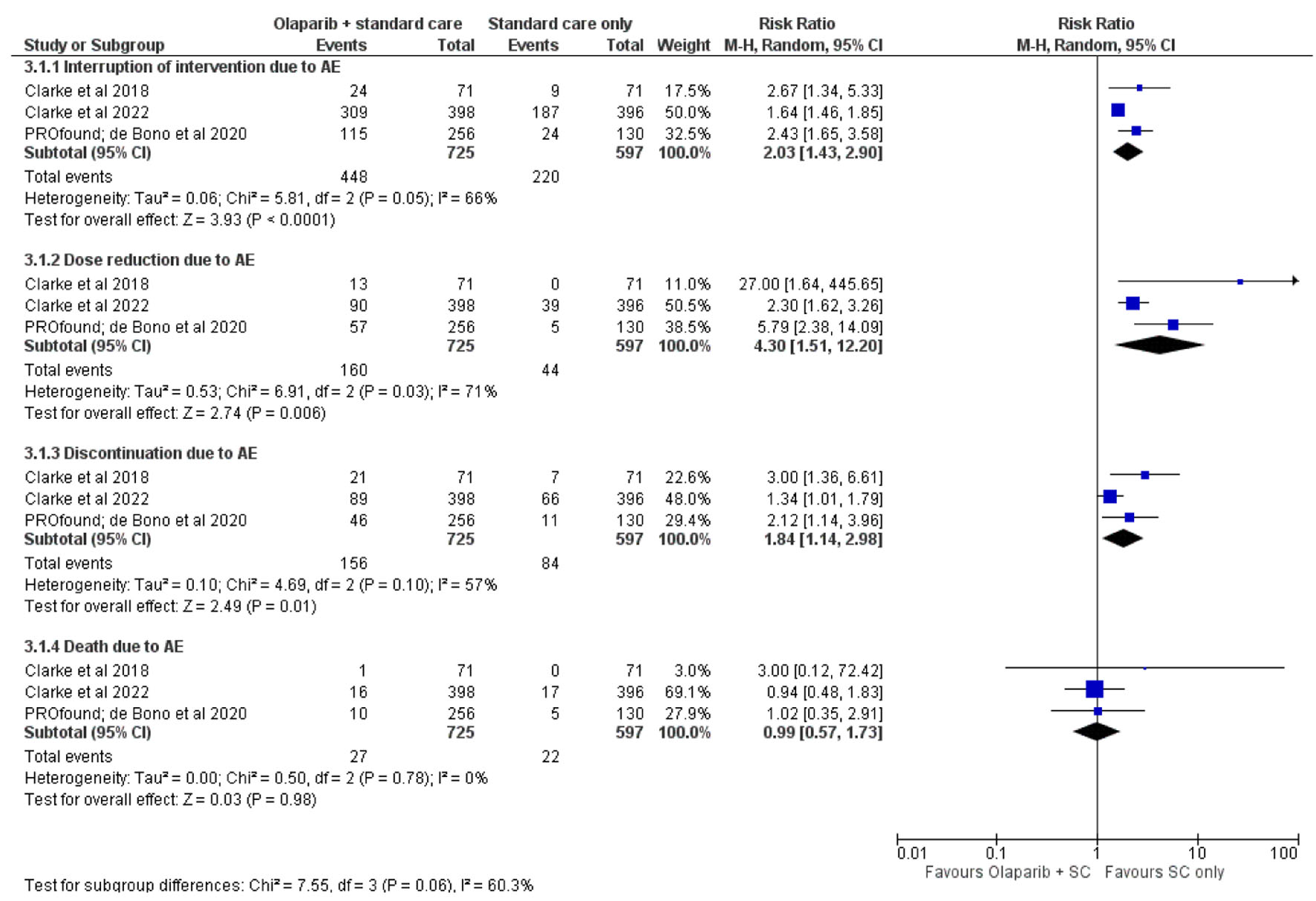 Click for large image | Figure 4. Change of intervention course due to adverse effects (AEs). |
| Discussion | ▴Top |
Clinical utility of PARP inhibitors (PARPi) or targeted therapy in general has been associated with the precision medicine revolution, as current global research direction is aimed to identify the most optimal patient-drug relationship hence termed personalized approach, rather than solely focusing on the disease itself. Due to its specific nature in disrupting cancerous DNA repair by inhibiting PARP pathway, designating an appropriate setting of PARPi, i.e., olaparib administration should consider the status of DNA damage repair (DDR) mutation. Individuals with mutated HRR gene, e.g., BRCA1/2 (mBRCA1/2 or mutated HRR (mHRR)), are at a higher risk to develop PC at their lifetime regardless of whether there were germline or somatic changes [11, 14, 18]. Therefore, the current NCCN guideline recommends conducting HRR gene testing prior to PARPi administration; considering its mechanism of action interferes with “alternative” DNA repair mechanisms in case of double-strand breaks (DSB) after PARP is trapped by its inhibitor drug, leading to accumulations of genetic abnormalities [13, 18].
Identifying the most suitable groups of patients is ultimately the main objective of the popular modern oncologic approach; however, therapeutic rationale to utilize specific molecular pathway inhibition in case of option’s unavailability as evident on mCRPC should be considered as well. Therefore, even though genetic counseling is highly recommended for PARPi’s administration, unmet favorable outcomes among metastatic PC patients are urging olaparib use in practice, regardless of the pathologic genetic testing status, since it theoretically benefits prognostic estimation [11, 13, 14, 19-20]. Our study demonstrated that olaparib inclusion into NHA regimen, e.g., abiraterone or enzalutamide plus glucocorticoid agents, may positively influence the PFS and OS. Individuals with mHRR genes might in fact have better outcomes after those regimens compared to unaltered populations, hence partially explaining the reasoning of pre-PARPi mHRR detection, which is supposed to increase the probability of prolonged PFS/OS [15-17].
In settings of unknown HRR mutation status, synergy between olaparib and its regimen of NHA/prednisolone is significantly improving the PFS and OS, thus the mCRPC diagnosis alone may justify the inclusion of olaparib into the treatment plan. As the previous study done by Matsumoto et al, the HR of radiographic progression-free survival (rPFS) in the olaparib arm was 0.41 (95% CI, 0.13 - 1.39) in BRCA1-mutated subgroup and 0.21 (95% CI: 0.13 - 0.32) in BRCA2-mutated subgroup. While the trial showed a median OS of 19.1 months with olaparib [14]. Nevertheless, the impact of mHRR and/or mATM findings had been statistically proved by HR value in this analysis (0.43 (0.30 - 0.62) vs. 0.59 (0.48 - 0.73) in 95% CI on sub-group differences), furtherly confirming the NCCN guideline on genetic testing recommendation. Progress of incorporating PARPi into advanced solid cancer treatment had been continuously referred to germline mutation of HRR specifically BRCA gene since it was first approved by FDA for ovarian cancer. Hence, extending its approval to non-mutated HRR mCRPC or at least including somatic mutation should be an alternative option to increase the prognostication rather than relying on chemotherapy or NHA alone. Molecular evidence to administer PARPi toward mHRR is centered on its failure to repair DSB after accumulation of single-strand DNA breaks (SSB) by PARPi earlier, resulting in higher rate of cell death since cancerous cell is unable to conduct repair mechanism in HRR-deficient settings [21-24].
Providing an agent that might hinder a cancer cell’s genetic defect is also a reasonable strategy to utilize PARPi as a radiosensitizer. Based on the research done by Angel et al, the combination therapy significantly prolongs survival in patient populations with favorable prognosis, but not in patients with unfavorable prognosis. In this study, the safety of olaparib combined with radionuclide Ra-223 resulted in good tolerance for the patients and thus showed a 6-month rPFS. Thus, the advantage and prospect of PARPi is not limited as a single-use agent or medication-based approach alone, but might in fact possess a potential to amplify the other treatment modality [25]. Compared to previous study involving olaparib administration among population with advanced cancer, it also revealed that its inclusion into the treatment regimen significantly improved the outcomes. A meta-analysis by Liu et al demonstrated that its intervention arms had significantly lower HR in treating metastatic triple-negative breast cancer (mTNBC) notably among BRCA-mutated patients [26]. Another extensive review by Maiorano et al which evaluated the efficacy of olaparib in ovarian cancer patients also concluded similar results with the HR of 0.54 among the studied populations and quantitatively higher HR in young age population [27].
Our study disclosed that the addition of olaparib might change the intervention’s course to some extent, as reported by AEs-related treatment interruption, dose reduction, or even discontinuation, with a significantly higher rate in the combination arm. However, we believe this finding is concordant with the basic pharmacology theory, which states that more drugs will translate into more reactions, hence unexpected events will eventually be more common among the olaparib-added group compared to olaparib-without group. The mortality rate due to AEs among intervened populations does not favor either regimen, as the RR value was shown to be 0.99 (0.57 - 1.73), hence placing olaparib into a relatively safe drug in terms of its life-threatening potential. Reaction-specific analysis of the AEs rate revealed most of the pooled AEs’ rate, regardless of its severity, favors the non-olaparib-added group, especially anemia (RR 3.16 (2.06 - 4.84); P < 0.05 in 95% CI).
Concordant to the previously mentioned expectation of adding an agent, it is reasonable to expect a higher event rate among the olaparib-added group. Moreover, analysis of grade ≥ 3 reactions confirmed that the RR difference among both arms is comparable and statistically insignificant except for anemia and vomiting, with those reactions’ occurrences valued at 4.64 (2.96 - 7.28) and 5.92 (2.77 - 19.82) in a similar analysis method. Therefore, this study attempted to mathematically define the rate of unexpected reactions rate, which definitely interfered with an individual’s quality of life. It is also generally accepted that minimizing those reactions is one of the main objectives in onco-pharmacological science, hence, apart from its favorable influence on a patient’s prognosis, we may expect nearly similar yet predictable AEs among mCRPC individuals after olaparib administration.
The estimated HRs of both PFS and OS outcomes were differed with previous meta-analysis conducted and predicted to be closely related with each populations’ cancer type. Yang et al in its meta-analysis focusing on ovarian cancer demonstrated an insignificant HR of 0.90 (0.75 - 1.08) in 95% CI (P = 0.56) for OS but significant PFS outcome (HR, 0.49 (0.36 - 0.68) in 95% CI (P < 0.05)). Though they also found that olaparib will significantly increase the side effects’ occurrences probability [28]. Another meta-analysis by Liu et al revealed similar outcomes since TNBC patients might benefit well from olaparib administration, with considerable increase in mutated BRCA gene population. This premise had also been confirmed by Guo et al, in which mutation of the BRCA gene will significantly improve the HR of both PFS and OS outcomes [12, 26].
Limitations
This review might hold some limitations that appear to be admissible considering the current research progress on PARPi and mCRPC. The most significant and distinctive factor is the number of eligible trials that fulfill our PICO model, as we were only able to identify three major investigations, justifying our objective to conduct a meta-analysis with clinical-centered narration within the limited available studies. Combining PARPi with NHA or even the chemotherapy regimens also prevailed as an issue among uro-oncology physicians, considering the current state of unmet clinical demand for better mCRPC prognosis and focusing on the most effective regimen, which can be acquired through in-depth analysis of the recently performed trials. Nevertheless, this study is expected to become one of the cornerstones for PARPi inclusion in the updated guidelines for PC management, and might provide some perspectives on the urgency for conducting pathologic genetic testing prior to targeted therapy administration. Future investigations should be focused on fully integrating olaparib into the PC therapeutic strategy (either by combining olaparib with chemotherapy or another medication) and exploring the importance of genetic testing prior to PARPi administration.
Conclusion
The addition of olaparib to an established mCRPC treatment modality which consisted of NHA as reviewed in this study is confirmed to significantly improve the PFS and OS by 41% and 19%, respectively. Furthermore, higher but acceptable AEs rates among olaparib arms were also observed without any significant influence on the mortality rate due to unexpected events caused by olaparib administration.
Acknowledgments
The authors acknowledged Ignatius Ivan Putrantyo, MD, from Department of Urology, Faculty of Medicine, Universitas Indonesia - Haji Adam Malik General Hospital, Medan, Indonesia who is also an alumnus of Master of Science Program in Nanotechnology and Regenerative Medicine in University College London for his suggestion during this work preparation, especially in conducting its methodological reasoning and statistical interpretation.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
For this study, informed consent is not applicable.
Author Contributions
Conceptualization: SMW, AJV. Data curation: SMW, AJV, NNF. Formal analysis: SMW, AJV, NNF, ZZT. Investigation: SMW, AJV, NNF, ZZT. Methodology: SMW, ZZT. Project administration: AJV, NNF. Resources: SMW, ZZT. Software: SMW, NNF. Supervision: SMW, ZZT. Validation: SMW. Visualization: AJV, NNF, ZZT. Writing - original draft: SMW, AJV. Writing - review and editing: SMW, AJV, NNF, ZZT.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Moreira DM, Howard LE, Sourbeer KN, Amarasekara HS, Chow LC, Cockrell DC, Pratson CL, et al. Predicting time from metastasis to overall survival in castration-resistant prostate cancer: results from SEARCH. Clin Genitourin Cancer. 2017;15(1):60-66.e62.
doi pubmed pmc - Figueiredo A, Costa L, Mauricio MJ, Figueira L, Ramos R, Martins-da-Silva C. Nonmetastatic castration-resistant prostate cancer: current challenges and trends. Clin Drug Investig. 2022;42(8):631-642.
doi pubmed pmc - Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Artibani W, Porcaro AB, De Marco V, Cerruto MA, Siracusano S. Management of biochemical recurrence after primary curative treatment for prostate cancer: a review. Urol Int. 2018;100(3):251-262.
doi pubmed - Turco F, Gillessen S, Cathomas R, Buttigliero C, Vogl UM. Treatment landscape for patients with castration-resistant prostate cancer: patient selection and unmet clinical needs. Res Rep Urol. 2022;14:339-350.
doi pubmed pmc - Beatson EL, Chau CH, Price DK, Figg WD. PARP inhibitors on the move in prostate cancer: spotlight on Niraparib & update on PARP inhibitor combination trials. Am J Clin Exp Urol. 2022;10(4):252-257.
pubmed pmc - Henriquez I, Roach M, 3rd, Morgan TM, Bossi A, Gomez JA, Abuchaibe O, Counago F. Current and emerging therapies for metastatic castration-resistant prostate cancer (mCRPC). Biomedicines. 2021;9(9):1-13.
doi pubmed pmc - Franza A, Claps M, Procopio G. PARP inhibitors and metastatic castration-resistant prostate cancer: uture directions and pitfalls. Transl Oncol. 2022;15(1):101263.
doi pubmed pmc - Bumbaca B, Li W. Taxane resistance in castration-resistant prostate cancer: mechanisms and therapeutic strategies. Acta Pharm Sin B. 2018;8(4):518-529.
doi pubmed pmc - Lohiya V, Aragon-Ching JB, Sonpavde G. Role of chemotherapy and mechanisms of resistance to chemotherapy in metastatic castration-resistant prostate cancer. Clin Med Insights Oncol. 2016;10(Suppl 1):57-66.
doi pubmed pmc - LeVee A, Lin CY, Posadas E, Figlin R, Bhowmick NA, Di Vizio D, Ellis L, et al. Clinical utility of olaparib in the treatment of metastatic castration-resistant prostate cancer: a review of current evidence and patient selection. Onco Targets Ther. 2021;14:4819-4832.
doi pubmed pmc - Guo XX, Wu HL, Shi HY, Su L, Zhang X. The efficacy and safety of olaparib in the treatment of cancers: a meta-analysis of randomized controlled trials. Cancer Manag Res. 2018;10:2553-2562.
doi pubmed pmc - National Comprehensive Cancer Network. Prostate Cancer Guidelines version 1.2023 [Internet]. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). 2022 [cited 2022 Dec 10]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- Scott RJ, Mehta A, Macedo GS, Borisov PS, Kanesvaran R, El Metnawy W. Genetic testing for homologous recombination repair (HRR) in metastatic castration-resistant prostate cancer (mCRPC): challenges and solutions. Oncotarget. 2021;12(16):1600-1614.
doi pubmed pmc - Heidegger I, Pircher A. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;383(9):890-891.
doi pubmed - Clarke N, Wiechno P, Alekseev B, Sala N, Jones R, Kocak I, Chiuri VE, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19(7):975-986.
doi pubmed - Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M, Shore N, Loredo E, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid [Internet]. 2022;1(9):EVIDoa2200043.
doi - Carr TH, Adelman C, Barnicle A, Kozarewa I, Luke S, Lai Z, Hollis S, et al. Homologous recombination repair gene mutation characterization by liquid biopsy: a phase II trial of Olaparib and abiraterone in metastatic castrate-resistant prostate cancer. Cancers (Basel). 2021;13(22):5830.
doi pubmed pmc - Matsumoto T, Shiota M, Blas L, Eto M. Role of olaparib in the management of metastatic castration-resistant prostate cancer: a Japanese Clinician's perspective. Cancer Manag Res. 2022;14:2389-2397.
doi pubmed pmc - Neviere Z, De La Motte Rouge T, Floquet A, Johnson A, Berthet P, Joly F. How and when to refer patients for oncogenetic counseling in the era of PARP inhibitors. Ther Adv Med Oncol. 2020;12:1758835919897530.
doi pubmed pmc - Rose M, Burgess JT, O'Byrne K, Richard DJ, Bolderson E. PARP inhibitors: clinical relevance, mechanisms of action and tumor resistance. Front Cell Dev Biol. 2020;8:564601.
doi pubmed pmc - Teyssonneau D, Margot H, Cabart M, Anonnay M, Sargos P, Vuong NS, Soubeyran I, et al. Prostate cancer and PARP inhibitors: progress and challenges. J Hematol Oncol. 2021;14(1):51.
doi pubmed pmc - Ricks TK, Chiu HJ, Ison G, Kim G, McKee AE, Kluetz P, Pazdur R. Successes and challenges of PARP inhibitors in cancer therapy. Front Oncol. 2015;5:222.
doi pubmed pmc - Congregado B, Rivero I, Osman I, Saez C, Medina Lopez R. PARP inhibitors: a new horizon for patients with prostate cancer. Biomedicines. 2022;10(6):1-5.
doi pubmed pmc - Angel M, Zarba M, Sade JP. PARP inhibitors as a radiosensitizer: a future promising approach in prostate cancer? Ecancermedicalscience. 2021;15:ed118.
doi pubmed pmc - Liu X, Wu K, Zheng D, Luo C, Fan Y, Zhong X, Zheng H. Efficacy and safety of PARP inhibitors in advanced or metastatic triple-negative breast cancer: a systematic review and meta-analysis. Front Oncol. 2021;11:742139.
doi pubmed pmc - Maiorano BA, Maiorano MFP, Lorusso D, Di Maio M, Maiello E. Efficacy and safety of PARP inhibitors in elderly patients with advanced ovarian cancer: a systematic review and meta-analysis. Int J Gynecol Cancer. 2022;32(11):1410-1418.
doi pubmed pmc - Yang Y, Yang X, Li H, Tong X, Zhu X. Efficacy and safety of olaparib in advanced ovarian cancer: a meta-analysis. J Obstet Gynaecol. 2023;43(1):2151883.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


