| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 000, Number 000, April 2024, pages 000-000
Potential Therapeutic Role of Respiratory Muscle Training in Dyspnea Management of Cancer Survivors: A Narrative Review
Baruch Vainshelboima, b , Sagar D. Sardesaia, Dharini Bhammara
aDivision of Medical Oncology, Department of Internal Medicine, The Ohio State University, Columbus, OH 43214, USA
bCorresponding Author: Baruch Vainshelboim, Division of Medical Oncology, Department of Internal Medicine, The Ohio State University, Columbus, OH 43214, USA
Manuscript submitted November 29, 2023, accepted March 4, 2024, published online April 11, 2024
Short title: RMT for Dyspnea in Cancer
doi: https://doi.org/10.14740/wjon1781
- Abstract
- Introduction
- Respiratory Muscle Physiology and Mechanisms of Dyspnea
- RMT
- The Rationale for Integrating RMT in Dyspnea Management of Cancer Survivors
- Methodological Consideration of Dyspnea Evaluation
- CPET
- Future Research Directions and Conclusions
- References
| Abstract | ▴Top |
Dyspnea is a disabling symptom presented in approximately half of all cancer survivors. From a clinical perspective, despite the availability of pharmacotherapies, evidence-based effective treatments are limited for relieving dyspnea in cancer survivors. Preliminary evidence supports the potential of respiratory muscle training to reduce dyspnea in cancer survivors, although large randomized controlled studies are warranted. The aims of this article were to review the relevant scientific literature on the potential therapeutic role of respiratory muscle training in dyspnea management of cancer survivor, and to identify possible mechanisms, strengths and limitations of the evidence as well as important gaps for future research directions.
Keywords: Breathlessness; Inspiratory muscle training; Exercise therapy; Cancer survivorship
| Introduction | ▴Top |
Dyspnea or breathlessness is a common, debilitating symptom among cancer survivors, especially in those with advanced cancers [1, 2]. Dyspnea is defined as “subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity” [3]. Other terms such as “shortness of breath”, “difficulty breathing”, “chest tightness” and “air hunger” are also frequently used in the literature [1, 3]. The prevalence of dyspnea is ranging from 10% to 70% of patients with cancer according to systematic review of over 10,000 patients [4]. A recent systematic review reported an average dyspnea prevalence of 58% (range 10% to 90%) among patients with advanced all-type cancers, and 34.9% among patients with lung cancer, respectively [1]. In particular, 45.5% of cancer survivors experienced exertional dyspnea, a factor that negatively affects activities of daily living and exercise [1]. Despite this high prevalence and tremendous burden, dyspnea is often underestimated by clinicians [1, 5]. For example, while clinicians reported that less than 30% of patients with lung cancer experienced moderate to severe dyspnea, over 50% of their patients reported the occurrence of this symptom [1]. Dyspnea in patients with advanced cancers is clinically significant in particular when presented at rest, which indicates a poor prognosis (typically a survival less than a few months) [2, 6]. Studies in patients with lung, breast and other cancers reported a significantly lower survival rate in those who present dyspnea compared to those who did not [7, 8]. In addition to a high mortality risk, the distressing burden of dyspnea is frequently compounded by fatigue, anxiety and depression, resulting in functional limitations in activity of daily living, avoidance of physical activity and poor quality of life [2, 6].
The cause of dyspnea is multifactorial and many demographic and clinical characteristics have been shown to be associated with dyspnea. These include: age, gender, cancer type and stage, presence of metastatic disease, type of cancer treatments, smoking history, cardiopulmonary comorbidities, physical inactivity, low pulmonary function, body weight loss > 5%, obesity, anemia, anxiety, depression pain and fatigue [1]. While pharmacological therapies are part of dyspnea management in cancer [2], and opioids such as morphine remain the cornerstone for treating dyspnea [5], their efficacy in alleviating dyspnea is limited [2]. Literature review of 17 randomized controlled trials found that opioids and anxiolytics such as benzodiazepines were no more effective than placebo in improving breathlessness [2]. Although supervised exercise therapy/pulmonary rehabilitation has been shown to be effective in improving dyspnea in patients with lung cancer [9], this treatment modality has not been recommended by American Society of Clinical Oncology (ASCO) for dyspnea management in patients with advanced cancer [2]. This is possibly due to insufficient evidence, and the fact that supervised exercise therapy/pulmonary rehabilitation is not a standard of care for patients with cancer [2]. While preliminary findings of respiratory muscle training (RMT) are encouraging with potential benefits in alleviating dyspnea among cancer survivors [10-17], the evidence of RMT efficacy for reducing dyspnea is still premature and large randomized controlled trials of RMT in cancer survivors are warranted. Given the prevalence of dyspnea in cancer survivors, its detrimental effects on physical function and quality of life, its association with poor survival while effective treatments are limited [1, 2, 6], exploring other potentially effective therapies has important clinical implications for symptoms management in cancer survivorship. Herein, the current review paper aimed to explore the potential therapeutic role of RMT for dyspnea management in cancer survivors. The article outlines the evidence on the effectiveness of RMT on respiratory muscle function as potential mechanism to reduce dyspnea. The article summarizes the existing evidence on RMT in health and disease for dyspnea improvement, and systematically reviews the existing evidence of RMT on dyspnea in cancer survivors. Finally, the review proposes a scientific rationale for why and how RMT could potentially treat dyspnea in cancer, a principle that should be empirically tested in future large randomized controlled trials.
| Respiratory Muscle Physiology and Mechanisms of Dyspnea | ▴Top |
The respiratory muscles are responsible for the air flow in (inspiration) and out of (expiration) the lungs [18, 19]. The primary muscle of inspiration is the diaphragm and additional muscles include the external intercostals, as well as accessory muscles including the sternocleidomastoid and the scalene muscles [19]. The primary muscles of expiration are the abdominal muscles: rectus abdominis, internal and external obliques and transversus abdominis, and the internal intercostals as accessory muscles. At rest, inspiration is an active process of the diaphragm and external intercostals muscles contraction, while expiration occurring primarily passively via elastic recoil of the lung and chest wall [19]. Respiratory physiology during physical exercise is quite different from resting state, since both inspiratory and expiratory processes are energy demanding especially at higher exercise intensities. The metabolic cost of breathing at rest is approximately 2% of total body oxygen (VO2) consumption, while during strenuous exercise it can rise up 10-15% of maximal VO2 consumption (VO2max) [19]. In normal healthy individuals, the respiratory system including the respiratory muscles has been traditionally viewed as well capable to meet the physiological demands of exercise even at high intensity, whereas the respiratory muscles are highly resilient to fatigue [19]. However, respiratory muscle function could be impaired due to cancer treatments and their associated toxicities, deconditioning, muscle atrophy, cardiopulmonary conditions, anemia and hypoxemia, conditions that are common in cancer survivors [20-23]. This can lead to reduced respiratory muscle strength and endurance, respiratory muscles fatigue during exercise and dyspnea on exertion [24, 25]. Studies that assessed direct physiological responses in cancer survivors supported this mechanism. Travers et al [26] measured the physiological responses at rest and during cardiopulmonary exercise testing (CPET) between cancer survivors with different cancer types. The study compared cancer survivors with and without dyspnea to normal, age-matched healthy controls. This study showed that at peak exercise and for a given ventilation and oxygen uptake levels, dyspnea intensity was greater in cancer survivors with dyspnea compared to those without and control groups. Peak oxygen uptake (a gold-standard measure of cardiorespiratory fitness (CRF)) was significantly lower among cancer survivors with dyspnea compared to cancer survivors without dyspnea and healthy controls [26], which is a strong predictor of reduced survival [27, 28]. Breathing pattern was more rapid and shallow, and dyspnea was the primary reason for exercise termination among cancer survivors with dyspnea compared to cancer survivors without dyspnea and healthy controls. The authors concluded that in the absence of obstructive and restrictive lung disease in cancer survivors, respiratory muscle weakness is a possible mechanism for dyspnea [26]. Similarly, O’Donnell et al [29] compared breast cancer survivors to healthy age-matched controls. This study demonstrates a 20% lower peak VO2 and > 50% greater dyspnea intensity in the breast cancer group compared to controls. In addition, the results showed that breast cancer survivors had lower respiratory and limb muscle strength and more rapid and shallow breathing pattern. The study concluded that breast cancer survivors have dyspnea and exercise intolerance due to multifactorial reasons including limb and respiratory muscle weakness and deconditioning [29].
The role of CRF in dyspnea
CRF plays an important role in dyspnea pathophysiology [30]. CRF reflects the integrated physiological capacity of the heart, lungs, and skeletal muscle to supply the required energy during maximal aerobic exercise, commonly termed maximal oxygen uptake (VO2max) [27, 31]. Poor CRF is a strong predictor of morbidity and mortality in many patient populations including cancer survivors [27, 28], and has been demonstrated to be associated with dyspnea [30, 32, 33]. Unfortunately, cancer survivors typically present low CRF, which increases their risk for variety of adverse outcomes and high symptom burden [22, 34]. For instance, a systematic review of 27 studies involving a total of 1,856 breast cancer survivors showed that the weighted mean VO2max in breast cancer survivors prior to adjuvant therapy was 24.6 mL/kg/min (17% lower than healthy sedentary women (29.7 mL/kg/min)). After completion of adjuvant therapy, weighted mean VO2max was 22.2 mL/kg/min, which is a 25% lower than healthy sedentary women [22]. Another systematic review of 28 studies involving patients with breast cancer, lung cancer, lymphoma, hematological cancers, prostate cancer and mixed cancer types reported that VO2max ranged between 16 and 25 mL/kg/min. These levels are 4% to 35% lower VO2max when compared with normal healthy references [34]. The mechanism in which low CRF (VO2max) can provoke dyspnea could be related to the reduced physiological reserves and increased relative intensity of any given physical task [27, 30, 31]. Most activities of daily living such as walking and household work require energy of 2 - 5 metabolic equivalent task (MET; 1 MET = 3.5 mL O2/kg/min), corresponding to 7 to 17.5 mL O2/kg/min [35]. For normal independent function in activities of daily living, a minimum of 18 mL/kg/min for men and 15 mL/kg/min for women of VO2max is necessary [27, 36]. Given the above mentioned low VO2max values among cancer survivors, it could be argued that many activities of daily living and leisure physical activity for these patients would be considered as high intensity effort relative to their low VO2max, remaining them with low physiological reserves. A relative high intensity physical activity causes hyperventilation response including exponential increase in minute ventilation and breathing frequency, which in turn could be perceived as dyspnea [31, 35]. Additionally, due to a low CRF and mitochondrial dysfunction, at any given physical work rate, lactate concentration levels and blood acidity will be higher. As a consequence to this increased metabolic acidosis, an earlier buffering response by bicarbonate will be present, resulting in higher carbon dioxide (CO2) levels, triggering a disproportional increase in ventilation and higher dyspnea perception [30, 31, 35, 37]. Although dyspnea was not assessed as an outcome, and causality should not be drawn, a meta-analysis of 48 randomized controlled studies, involving 3,632 patients with different cancers demonstrated an increased CRF (+2.13 mL O2/kg/min) following supervised exercise training [38]. This powerful finding supports the potential of supervised exercise training for dyspnea improvement during activities of daily living, although interventional studies examining the effect on dyspnea as an outcome are needed.
Obesity and dyspnea
An additional important factor that may contribute to dyspnea among cancer survivors is obesity [39, 40]. Obesity is commonly defined as body mass index ≥ 30 kg/m2 [41], and its prevalence in the general adult population is approximately 40% [42], with projection of reaching half of the US population by 2030 [43]. Considerable body of literature indicates that obese individuals often present impaired respiratory muscle function and dyspnea at rest and during physical exertion [39, 44]. Reduced inspiratory and expiratory muscle strength and inspiratory muscle endurance are documented in subjects with obesity [39, 44]. There are several potential mechanisms that can explain the role of obesity in dyspnea. These include obesity-related alterations in breathing mechanics and obesity-related metabolic alterations of locomotion [31, 39, 40, 44]. Alterations in breathing mechanics could be related to excessive fat mass in the abdominal and chest wall. The extra fat weight in the abdominal and chest areas increases the respiratory muscles load and work of breathing at any given level of ventilation, while requires higher metabolic demand (oxygen cost of breathing) and respiratory muscles effort [39, 40, 44]. In addition, adipose tissue encasing the chest and the abdomen decreases chest wall compliance (more stiffness), while placing the diaphragm and intercoastal muscles at a mechanical disadvantage. These impaired biomechanics further increase the mechanical load by inefficient respiratory muscles contraction, resulting in ventilatory constraints, especially during exercise [31, 40, 44]. The elevated mechanical load on the respiratory muscles also increases a neural ventilatory drive, which can be perceived as dyspnea [40, 44]. Another potential mechanism that could contribute to dyspnea is obesity-related metabolic alterations of locomotion. Compared to people with normal weight, individuals with obesity have a metabolic disadvantage even in non-body weight bearing activities such as cycling on recumbent bicycle. During locomotion, the excessive fat weight in the body and the limbs place an additional metabolic load on the active muscles at any given work-rate, decreasing the mechanical efficiency (oxygen consumption/work rate). As a consequence, there is an earlier onset of metabolic acidosis and increased buffering by bicarbonate, which results in higher CO2 levels, disproportionate increase in ventilation, higher dyspnea perception and early exercise termination [31, 40].
| RMT | ▴Top |
RMT is a form of repetitive breathing exercises performed during inspiration, expiration or both [19, 45]. There are two main types of RMT: 1) The voluntary isocapnic hyperpnea, an endurance type of RMT; and 2) The resistive flow-dependent or pressure threshold loading, a strength type of RMT [19, 24, 45, 46]. The voluntary isocapnic hyperpnea RMT typically involves performing relatively high level of ventilation at rest for up to 30 min, aiming to improve respiratory muscle endurance. This regimen is carried out for 3 - 5 times per week for 3 - 5 weeks at intensity of 50-90% of maximal voluntary ventilation (MVV) [19, 24]. The resistive flow-dependent or pressure threshold loading RMT typically involves breathing exercise performed at rest while using a custom-built or a commercially available device which provides resistance to inhalation (inspiratory muscle training (IMT)) or exhalation (expiratory muscle training (EMT)) or both, aiming to improve respiratory muscle strength [19, 45, 46]. The load is often set at 15% to 70% of maximal inspiratory pressure (MIP) for IMT or maximal expiratory pressure (MEP) for EMT measured at baseline. The training regimen performed for 5 to 20 min per session, 3 - 5 times per week for about 4 weeks [19, 45].
Effects of RMT in health and disease
Respiratory muscles have the capacity to adapt to repetitive stimuli similarly to other skeletal muscle [19, 45, 46]. In healthy individuals, systematic reviews and meta-analyses have demonstrated that RMT improves respiratory muscle strength and endurance as well as maximal oxygen consumption primarily in less fit subjects [19, 46], providing the opportunity for the utilization among cancer survivors, as these patients present low fitness as well [22, 34]. The effect of RMT on physical performance is not different between endurance and strength type of RMT, while the combined IMT and EMT strength type of RMT showed 12.8% higher improvement than IMT alone [19, 46].
In patients with chronic obstructive pulmonary disease (COPD), a meta-analysis of 32 randomized controlled trials showed significant improvement in inspiratory muscle strength (MIP; +13 cm H2O), endurance time (+261 s), 6-min walking distance (+32 m) and quality of life (+3.8 units), while dyspnea was significantly reduced (Borg score -0.9 point; Transitional Dyspnea Index +2.8 units) after IMT interventions compared to control [47]. More recent meta-analysis of 14 randomized controlled trials in patients with COPD showed that RMT improved both daily dyspnea level (Medical Research Council Dyspnea Scale -0.38 units) and dyspnea during exercise (Borg Dyspnea Score -0.72 point) [48].
In patients with asthma, a meta-analysis of 11 randomized controlled studies (total of 270 participants) reported that IMT significantly improved inspiratory muscle strength (MIP +21.95 cm H2O, 95% confidence interval (CI): 15.05 - 28.85) [49]. While a quantitative meta-analysis for dyspnea outcomes was not possible, a qualitative analysis of five studies showed improvement in dyspnea based on Borg and Medical Research Council Scales. Three of the five studies showed significant association between increase in MIP and decrease in dyspnea, supporting the mechanism of increasing inspiratory muscles strength for reducing dyspnea as well as the potential role of IMT in dyspnea management. In addition, the analysis revealed a significant reduction in fatigue following the IMT intervention [49], a symptom that is most common among cancer survivors [50], and could be potentially improved with IMT.
A recent meta-analysis in patients with heart failure including 13 randomized controlled studies (n = 342) (10 IMT alone and three IMT combined with other interventions) demonstrated a significant improvement in inspiratory muscle strength (MIP +25.12 cm H2O, 95% CI: 15.29 - 34.95) [51]. Although only few of these studies assessed the effect of IMT on dyspnea, one study of combined IMT and aerobic exercise has demonstrated a significant reduction in dyspnea after the intervention. Despite this limited evidence on the beneficial effect of IMT on dyspnea, this meta-analysis robustly demonstrated an improvement in CRF, functional capacity and quality of life, suggesting clinical improvement using IMT for this patient group [51].
In patients undergoing thoracic surgery, a network meta-analysis of 25 studies, involving 2,070 patients, compared RMT to aerobic exercise training on post-operation outcomes [52]. The study showed RMT resulted in lower odds ratio of post-operation complications (0.33), respiratory failure (0.22) and reduction in length of hospitalization (-1.69 days) compared to control. The effect of RMT was similar to aerobic exercise training for these outcomes [52], providing an opportunity for safe and effective low-cost, low resources intervention. Additionally, considering the fact that most patients diagnosed with solid tumors will undergo some surgical procedure [50], RMT could be integrated during the pre-operative waiting window to optimize post-operative outcome.
In patients with lung cancer undergoing curative lung resection surgery, a meta-analysis of 10 studies (eight included both IMT and aerobic exercise) showed that preoperative IMT compared with controls decreased the length of hospitalization (-3.44 days) and the effect was further enhanced when IMT was combined with aerobic exercise [53]. Preoperative IMT reduced the likelihood of developing pneumonia and post-operation complications by 63% and increased functional capacity in 6-min walking distance (+20.2 m) [53].
Efficacy of RMT on dyspnea in cancer survivors
A systematic literature search of three electronic databases (MEDLINE/PubMed, Web of Science and EBSCO) was conducted according to Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines through February 4, 2024 [54]. The search strategy was created considering terms related to the effect of intervention (RMT) and the outcome of interest (dyspnea) in cancer survivors. Utilizing the following keywords: “respiratory muscle training” OR “inspiratory muscle training” OR “expiratory muscle training” AND “cancer” OR “cancer survivors” OR “patients with cancer” AND “dyspnea” OR “breathless” OR “shortness of breath”, relevant publications were extracted. Quality of the studies was assessed using the National Heart, Lung, and Blood Institute’s tailored quality assessment tools [55, 56]. A total of 76 articles were identified through initial literature search. After reviewing titles/abstracts and full-text studies, 66 articles were excluded due to not meeting the eligibility criteria. Ten studies (randomized controlled trials (n = 7), controlled but not randomized controlled trial (n = 1) and single arm studies (n = 2)) assessing the effect of RMT on dyspnea in patients with cancer were included in the current review (Fig. 1). Although dyspnea was a secondary outcome in these studies, a risk of bias was relatively low and most studies had a good quality (Supplementary Material 1, www.wjon.org).
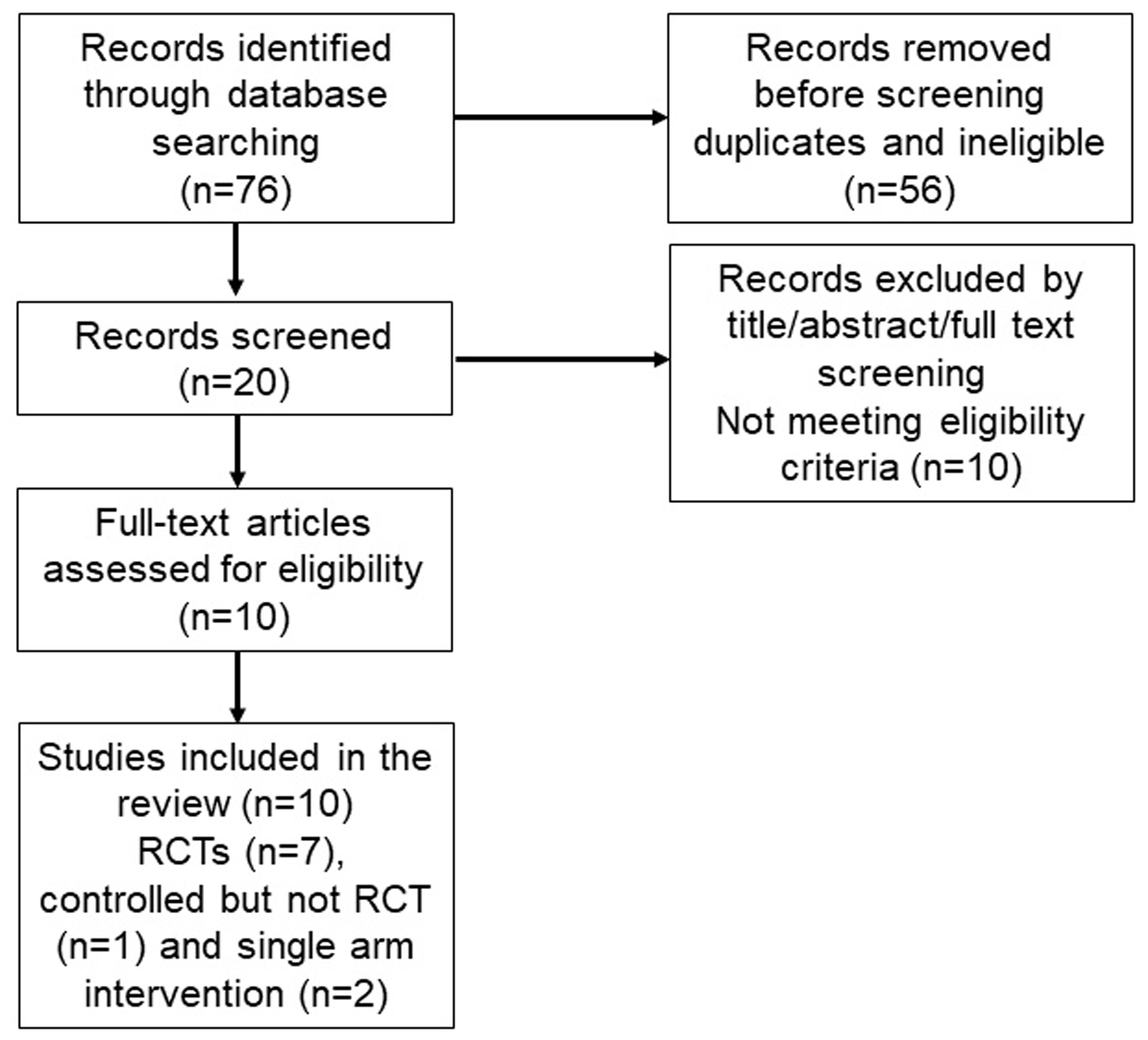 Click for large image | Figure 1. PRISMA flow diagram of literature search and studies selection. |
While strong evidence for the beneficial effect of RMT on dyspnea in cancer survivors is scarce, preliminary data support its potential therapeutic value (Table 1) [10-17, 57, 58]. A small pilot, single group study has demonstrated a beneficial effect of 4-week RMT on dyspnea in cancer survivors (lung, n = 5 and breast, n = 5) [10]. Both Baseline Dyspnea Index (+1.2 units) and Transitional Dyspnea Index (+5.1 units) were significantly improved following the RMT intervention [10]. A small randomized controlled pilot study has shown that an addition of RMT to standard 12-week exercise training improved dyspnea in daily life (Transitional Dyspnea Index +2.9 points) among 19 breast cancer survivors [11]. A randomized controlled study of 40 patients with non-small-cell lung cancer undergoing lobectomy has shown significant improvement in dyspnea following 3 weeks of preoperative pulmonary rehabilitation combined with breathing exercises but not specifically RMT [12]. Patients in the rehabilitation group reduced their dyspnea after the intervention (Borg dyspnea -0.7 units) and remained with significantly lower post-operation scores compared to the usual care control group (Borg dyspnea 2 versus 3.1 points) [12]. A pilot randomized controlled trial of 46 patients with clinically stable lung cancer has shown dyspnea improvement after IMT intervention [13]. The experimental group performed five IMT sessions weekly for 12 weeks, while the control group continued with usual care. At 12-week time point, a significant difference (-0.8 points) was found between the groups in modified Borg Dyspnea Scale, suggesting the efficacy of IMT for reducing dyspnea [13].
 Click to view | Table 1. Studies on Respiratory Muscle Training in Cancer Survivors |
A pilot investigation (single group) evaluated the efficacy of 4 weeks EMT among six patients with head and neck cancers who underwent laryngectomy. This study showed 38% reduction in dyspnea (-2.3 units in dyspnea index) after the intervention [16]. Recent, large controlled (not randomized) study (66 hospitalized stable patients with lung cancer) demonstrated a significant reduction in dyspnea on exertion after 2 weeks intervention. The intervention group participated in IMT + exercise therapy (30 breathes twice daily at 30-40% of MIP + 20 - 40 min exercise therapy 5 days/week for 2 weeks), while the control group received exercise therapy alone. The mean difference between the groups following the intervention was -1 unit on modified Borg Dyspnea Scale, suggesting beneficial therapeutic effect of IMT [17]. However, a virtual telemedicine pilot randomized controlled study found no improvement in dyspnea among 22 patients with lung cancer. Patients randomly received either IMT + walking or educational intervention. The IMT + walking group participated in six video conferences (every 2 weeks for 12 weeks) instructing to perform 10 - 15 min of IMT twice daily for 5 days/week + to walk four times a week. The control group participated in video conferences with general education on the benefits of exercise. After both 6 and 12 weeks, no difference in dyspnea score was observed between the groups [57].
Three randomized controlled trials evaluated the effect of RMT on dyspnea in patients undergoing hematopoietic stem cell transplantation [14, 15, 58]. While two studies demonstrated positive effect of IMT + rehabilitation exercise versus exercise alone on dyspnea reduction [14, 15], one study found only a trend towards dyspnea reduction with IMT but did not reach statistically significant difference between the groups [58]. A possible explanation could be that in the latter study, patients were hospitalized with more severe acute condition, where complementary aerobic exercise was performed at lower volume and intensity [58].
Given the importance of dyspnea in cancer survivorship, ongoing studies including from our group are currently in the process. These include “Inspiratory muscle training and behavioral support to alleviate dyspnea and promote walking in lung cancer survivors: a pilot study” (NCT05059132), “Respiratory muscle training before surgery in preventing lung complications in patients with stage I-IIIB lung cancer” (NCT04067830) and “Inspiratory muscle training in obese breast cancer survivors” (NCT05193149) (ClinicalTrials.gov).
Taken together, the existing evidence from randomized controlled trials, controlled study and single arm interventional studies supports the potential efficacy of RMT on dyspnea reduction in cancer survivors. In particular, the efficacy was pronounced when RMT was combined with other modalities of rehabilitative exercise and delivered in a supervised, in-person fashion. However, given the variation and some inconsistency in the study outcomes, large randomized controlled trials assessing dyspnea as primary outcome in survivors with different cancer types are warranted to establish the therapeutic efficacy of RMT on dyspnea in cancer survivors.
| The Rationale for Integrating RMT in Dyspnea Management of Cancer Survivors | ▴Top |
As previously stated, dyspnea is highly prevalent among cancer survivors, it is associated with increased mortality risk, causing functional limitations, compromising mental health, and resulting in avoidance of physical activity and poor quality of life [1, 2, 4]. Effective therapeutic options are limited, and pharmacological interventions have unclear benefits [2]. There are multifactorial reasons for dyspnea in cancer survivors [2, 4, 6, 7]; however, physiological objective data indicate that weakness and fatigue of respiratory muscles could play a mechanistic role in dyspnea among cancer survivors [6, 24-26, 29]. Moreover, obesity, low CRF and physical inactivity are highly prevalent among cancer survivors [59], factors that further exacerbate dyspnea [6]. While only preliminary data exist on the efficacy of RMT among cancer survivors [10-13], robust evidence in healthy and diseased populations has demonstrated the efficacy of RMT to increase inspiratory muscle strength and reduce dyspnea [19, 45-47, 49, 51]. Achieving higher strength and endurance of respiratory muscles with RMT can improve the fatigability resilience of these muscles, decrease the relative mechanical effort of breathing and reduce the perception of dyspnea (Fig. 2) [19, 45-47, 49, 51]. Since RMT has an excellent safety profile along with a potential mechanism of reducing dyspnea by enhancing respiratory muscle function, integrating this training modality in cancer survivorship and cancer rehabilitation could be clinically valuable for patients with cancer and dyspnea complaints [19, 45-47, 49, 51]. In addition, most RMT devices are easy to use, have low costs and can be utilized in home-based/limited supervision interventions, which further support their feasibility, practical application and integration in clinical and research settings for cancer survivors.
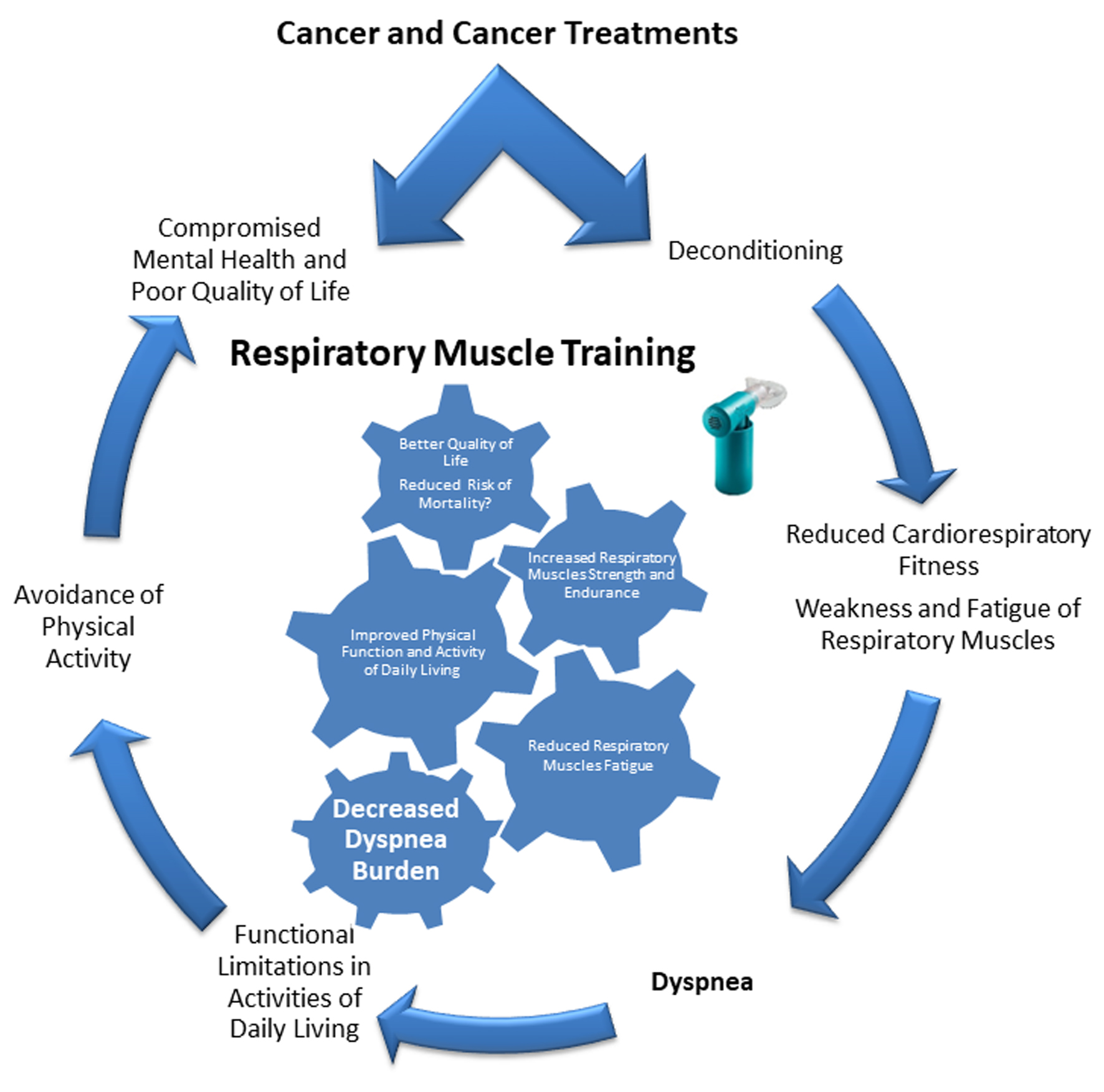 Click for large image | Figure 2. Mechanisms of dyspnea in cancer and the potential therapeutic role of respiratory muscles training for dyspnea management. |
| Methodological Consideration of Dyspnea Evaluation | ▴Top |
A comprehensive dyspnea evaluation is beneficial for RMT intervention efficacy assessment prior to integration in cancer survivorship [60, 61]. Dyspnea is a complexed multidimensional symptom that warrants evaluation of sensory-perceptual experience (subjective report of the breathing), affective distress (unpleasantness of breathing), and impact or burden on functional ability or activity of daily living and quality of life) as recommended by American Thoracic Society [1, 3]. Dyspnea is moderately linked to pain, and commonly co-occurs with fatigue, anxiety and depression, encompassing physiological, psychological and emotional factors, which further challenge its evaluation [1, 61]. Although many instruments and scales are available for dyspnea evaluation, only few have been studied in patients with cancer to comprehensively assess the sensory-perceptual experience, affective distress and impact [3, 60, 61]. An important consideration for utilizing any instrument is its ability to diagnose/characterize dyspnea versus its responsiveness of detecting change over time with intervention [60, 61]. While, Baseline Dyspnea Index, Transitional Dyspnea Index and Borg Dyspnea Scale showed responsiveness to RMT interventions [10-13], their validity in cancer survivor has yet to be established [60, 61].
Several dyspnea instruments have been validated for patients with cancer [61]: Cancer Dyspnea Scale (CDS), the Dyspnea-12 (D-12) and Total Dyspnea Scale for Cancer (TDSC) [61]. CDS is a 12-item patient-reported outcome measure of perceived breathlessness. The CDS was studied among lung cancer patients and was validated for other cancers as well. The scale is divided into three factors related to “sense of anxiety”, “sense of effort” and “sense of discomfort”. The scale was cross-culturally validated and showed good to excellent internal consistency and good test-rest reliability. The CDS showed moderate to high correlation with Visual Analogue Scale-Dyspnea (VAS-D) and modified Borg Dyspnea Scale, although responsiveness to interventions is yet to be appraised [61]. The D-12 is a 12-item patient-reported outcome scale, developed to assess dyspnea severity in cardiopulmonary and cancer diseases. D-12 is divided into two factors called “physical” and “affective” which describes physical and mental breathlessness components [61]. D-12 scale was cross-culturally validated, has demonstrated good to excellent internal consistency and moderately to strongly correlated with dyspnea severity (modified Borg Dyspnea Scale) and (modified Medical Research Council Dyspnea Scale). In addition, a moderate correlation was found between D-12 and measures related to physical status, psychological status, quality of life, and activities of daily living. However, responsiveness to interventions is very low [61]. TDSC is an 11-item patient-reported outcome for cancer patients. The scale is a comprehensive measure of dyspnea and its impact on activities of daily living. TDSC is composed of two factors (effects on activities of daily living and psychology, and effects on social activity). The scale has a good to excellent internal consistency, has a strong correlation with CDS and a moderate correlation with quality of life and psychological status. Responsiveness to interventions of TDSC is unknown [61]. The minimal clinically important difference (MCID) for interventions was established for the D-12 scale only and included patients with cardiorespiratory diseases but not with cancer. The MCID for D-12 is 2.8 points for interventions ranging from 2 to 6 months [62, 63].
| CPET | ▴Top |
CPET is a gold-standard tool for evaluation of unexplained exertional dyspnea and direct measure of CRF (VO2max), a strong predictor of survival including in patients with cancer [27, 28, 30, 31, 64]. Directly measured variables from CPET enable to distinguish between cardiopulmonary and muscles limitations to exercise and assist to identify the cause of exertional dyspnea. Despite the overwhelming evidence on the utility of CPET in diagnosis, prognosis and treatment efficacy evaluation, CPET is underutilized in oncology and cancer survivorship settings and is not a standard of care [2, 30, 31, 64]. Thus, referring cancer survivors who complain on dyspnea to CPET combined with pulmonary function test and respiratory muscle strength testing (MIP and MEP) could be valuable for identifying the cause of dyspnea to inform and optimize the treatment [30, 31, 64].
| Future Research Directions and Conclusions | ▴Top |
In patients with cardiopulmonary diseases, the scientific clinical literature on the efficacy of RMT in reducing dyspnea is compelling [19, 45-47, 49, 51]. However, in patients with cancer, the evidence for RMT efficacy is preliminary, insufficient and with many gaps [10-13, 65]. Randomized controlled trials of RMT interventions are warranted in patients with different types of cancer and dyspnea. Evidence on the efficacy of RMT in reducing dyspnea in cancer survivors with and without respiratory muscle weakness seems to be an important topic for investigation, since RMT may be therapeutically beneficial for dyspnea even in patients with normal respiratory muscle function. While evaluation instruments of dyspnea have been developed and studied in patients with cancer, establishing MCID for these tools in cancer survivors is an important clinical research goal. In addition, studying different RMT protocols such as IMT vs. IMT + EMT, varying frequencies and intensities of RMT and combination of strength and endurance RMT could provide more insights for optimization of this intervention to reduce dyspnea. Finally, given the mechanisms of dyspnea, the robust evidence on the health benefits of supervised exercise training in patients with cancer [23, 66-68], the supportive evidence for superior outcomes in combining exercise with RMT [11, 12, 51], rigorously studying this combination in cancer survivors is an important future research direction for cancer care.
In summary, dyspnea is a prevalent and debilitating symptom among cancer survivors, associated with increased mortality risk, mental health compromises, functional limitations in activity of daily living and poor quality of life. Effective evidence-based, therapeutic options are limited. RMT is a well-established form of training having strong evidence for improving respiratory muscle function and alleviating dyspnea primarily in patients with pulmonary diseases. Physiological mechanistic studies and preliminary interventional evidence support the potential therapeutic effects of RMT in reducing dyspnea for cancer survivors. However, large randomized controlled trials examining the effect of RMT alone and in combination with supervised exercise training are necessary for evidence-based recommendations to integrate RMT in dyspnea management for cancer survivors.
| Supplementary Material | ▴Top |
Suppl 1. NHLBI Study Quality Assessment Tools 2021.
Acknowledgments
None to declare.
Financial Disclosure
The authors declare that they do not have a financial relationship with any commercial entity that has an interest in the subject of this manuscript. This study received no external funding.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Author Contributions
All authors participated in review. They were involved in writing and revising the article prior to submission. Baruch Vainshelboim contributed to conceptualization, methodology, visualization, drafting, writing and revising the submitted manuscript. Sagar D. Sardesai and Dharini Bhammar contributed to conceptualization, critical review and revision of the article for important intellectual content. All authors read and approved the final version of the manuscript and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CDS: Cancer Dyspnea Scale; CPET: cardiopulmonary exercise testing; CRF: cardiorespiratory fitness; EMT: expiratory muscle training; IMT: inspiratory muscle training; MIP: maximum inspiratory pressure; MEP: maximum expiratory pressure; RMT: respiratory muscle training; TDSC: Total Dyspnea Scale for Cancer; VO2max: maximal oxygen consumption
| References | ▴Top |
- Shin J, Kober K, Wong ML, Yates P, Miaskowski C. Systematic review of the literature on the occurrence and characteristics of dyspnea in oncology patients. Crit Rev Oncol Hematol. 2023;181:103870.
doi pubmed - Hui D, Bohlke K, Bao T, Campbell TC, Coyne PJ, Currow DC, Gupta A, et al. Management of dyspnea in advanced cancer: ASCO guideline. J Clin Oncol. 2021;39(12):1389-1411.
doi pubmed - Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, Calverley PM, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185(4):435-452.
doi pubmed pmc - Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31(1):58-69.
doi pubmed - Meriggi F. Dyspnea in cancer patients: a well-known and neglected symptom. Rev Recent Clin Trials. 2018;13(2):84-88.
doi pubmed - Koelwyn GJ, Jones LW, Hornsby W, Eves ND. Exercise therapy in the management of dyspnea in patients with cancer. Curr Opin Support Palliat Care. 2012;6(2):129-137.
doi pubmed pmc - Cheville AL, Novotny PJ, Sloan JA, Basford JR, Wampfler JA, Garces YI, Jatoi A, et al. The value of a symptom cluster of fatigue, dyspnea, and cough in predicting clinical outcomes in lung cancer survivors. J Pain Symptom Manage. 2011;42(2):213-221.
doi pubmed pmc - Escalante CP, Martin CG, Elting LS, Cantor SB, Harle TS, Price KJ, Kish SK, et al. Dyspnea in cancer patients. Etiology, resource utilization, and survival-implications in a managed care world. Cancer. 1996;78(6):1314-1319.
doi pubmed - Zhou W, Woo S, Larson JL. Effects of perioperative exercise interventions on lung cancer patients: An overview of systematic reviews. J Clin Nurs. 2020;29(23-24):4482-4504.
doi pubmed - Ray AD, Mahoney MC. Respiratory muscle training improves exercise performance and quality of life in cancer survivors: A pilot study. Rehabilitation Oncology. 2017;35:81-89.
- Dahhak A, Devoogdt N, Langer D. Adjunctive inspiratory muscle training during a rehabilitation program in patients with breast cancer: an exploratory double-blind, randomized, controlled pilot study. Arch Rehabil Res Clin Transl. 2022;4(2):100196.
doi pubmed pmc - Stefanelli F, Meoli I, Cobuccio R, Curcio C, Amore D, Casazza D, Tracey M, et al. High-intensity training and cardiopulmonary exercise testing in patients with chronic obstructive pulmonary disease and non-small-cell lung cancer undergoing lobectomy. Eur J Cardiothorac Surg. 2013;44(4):e260-265.
doi pubmed - Molassiotis A, Charalambous A, Taylor P, Stamataki Z, Summers Y. The effect of resistance inspiratory muscle training in the management of breathlessness in patients with thoracic malignancies: a feasibility randomised trial. Support Care Cancer. 2015;23(6):1637-1645.
doi pubmed - Bargi G, Guclu MB, Aribas Z, Aki SZ, Sucak GT. Inspiratory muscle training in allogeneic hematopoietic stem cell transplantation recipients: a randomized controlled trial. Support Care Cancer. 2016;24(2):647-659.
doi pubmed - Bayram S, Bargi G, Celik Z, Bosnak Guclu M. Effects of pulmonary rehabilitation in hematopoietic stem cell transplantation recipients: a randomized controlled study. Support Care Cancer. 2023;32(1):72.
doi pubmed - Palmer AD, Bolognone RK, Thomsen S, Britton D, Schindler J, Graville DJ. The safety and efficacy of expiratory muscle strength training for rehabilitation after supracricoid partial laryngectomy: a pilot investigation. Ann Otol Rhinol Laryngol. 2019;128(3):169-176.
doi pubmed - Sakai Y, Yamaga T, Yamamoto S, Matsumori K, Ichiyama T, Hanaoka M, Ikegami S, et al. Effects and usefulness of inspiratory muscle training load in patients with advanced lung cancer with dyspnea. J Clin Med. 2023;12(10):3396.
doi pubmed pmc - Cirino C, Marostegan AB, Hartz CS, Moreno MA, Gobatto CA, Manchado-Gobatto FB. Effects of inspiratory muscle warm-up on physical exercise: a systematic review. Biology (Basel). 2023;12(2):333.
doi pubmed pmc - Sheel AW. Respiratory muscle training in healthy individuals: physiological rationale and implications for exercise performance. Sports Med. 2002;32(9):567-581.
doi pubmed - Leduc C, Antoni D, Charloux A, Falcoz PE, Quoix E. Comorbidities in the management of patients with lung cancer. Eur Respir J. 2017;49(3):1601721.
doi pubmed - Ng HS, Vitry A, Koczwara B, Roder D, McBride ML. Patterns of comorbidities in women with breast cancer: a Canadian population-based study. Cancer Causes Control. 2019;30(9):931-941.
doi pubmed - Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc. 2014;3(1):e000432.
doi pubmed pmc - Campbell KL, Winters-Stone KM, Wiskemann J, May AM, Schwartz AL, Courneya KS, Zucker DS, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375-2390.
doi pubmed pmc - Bhammar DM, Jones HN, Lang JE. Inspiratory muscle rehabilitation training in pediatrics: what is the evidence? Can Respir J. 2022;2022:5680311.
doi pubmed pmc - Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, Dube BP, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53(6):1801214.
doi pubmed - Travers J, Dudgeon DJ, Amjadi K, McBride I, Dillon K, Laveneziana P, Ofir D, et al. Mechanisms of exertional dyspnea in patients with cancer. J Appl Physiol (1985). 2008;104(1):57-66.
doi pubmed - Ross R, Blair SN, Arena R, Church TS, Despres JP, Franklin BA, Haskell WL, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653-e699.
doi pubmed - Ezzatvar Y, Ramirez-Velez R, Saez de Asteasu ML, Martinez-Velilla N, Zambom-Ferraresi F, Lobelo F, Izquierdo M, et al. Cardiorespiratory fitness and all-cause mortality in adults diagnosed with cancer systematic review and meta-analysis. Scand J Med Sci Sports. 2021;31(9):1745-1752.
doi pubmed - O'Donnell DE, Webb KA, Langer D, Elbehairy AF, Neder JA, Dudgeon DJ. Respiratory factors contributing to exercise intolerance in breast cancer survivors: a case-control study. J Pain Symptom Manage. 2016;52(1):54-63.
doi pubmed - Guazzi M, Adams V, Conraads V, Halle M, Mezzani A, Vanhees L, Arena R, et al. EACPR/AHA Scientific Statement. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126(18):2261-2274.
doi pubmed pmc - American Thoracic Society, American College of Chest Physicians. ATS/ACCP Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167(2):211-277.
doi pubmed - Liew ZH, Kalyanasundaram G, Ong TH, Loo CM, Koh MS. Cardiopulmonary exercise testing for evaluating patients with unexplained exertional dyspnoea: potential role in risk stratification? Ann Acad Med Singap. 2018;47(4):169-171.
pubmed - Schaeffer MR, Cowan J, Milne KM, Puyat JH, Voduc N, Corrales-Medina V, Lavoie KL, et al. Cardiorespiratory physiology, exertional symptoms, and psychological burden in post-COVID-19 fatigue. Respir Physiol Neurobiol. 2022;302:103898.
doi pubmed pmc - Steins Bisschop CN, Velthuis MJ, Wittink H, Kuiper K, Takken T, van der Meulen WJ, Lindeman E, et al. Cardiopulmonary exercise testing in cancer rehabilitation: a systematic review. Sports Med. 2012;42(5):367-379.
doi pubmed - American College of Sports Medicine. Acsm's guidelines for exercise testing and prescription. Tenth ed.; Wolters Kluwer/Lippincott Williams & Wilkins Health: 2018.
- Paterson DH, Cunningham DA, Koval JJ, St Croix CM. Aerobic fitness in a population of independently living men and women aged 55-86 years. Med Sci Sports Exerc. 1999;31(12):1813-1820.
doi pubmed - Kenney WL, Wilmore JH, Costill DL. Physiology of sport and exercise. 5th ed. Human Kinetics: Champaign, IL. 2012:621.
- Scott JM, Zabor EC, Schwitzer E, Koelwyn GJ, Adams SC, Nilsen TS, Moskowitz CS, et al. Efficacy of exercise therapy on cardiorespiratory fitness in patients with cancer: a systematic review and meta-analysis. J Clin Oncol. 2018;36(22):2297-2305.
doi pubmed pmc - Arena R, Cahalin LP. Evaluation of cardiorespiratory fitness and respiratory muscle function in the obese population. Prog Cardiovasc Dis. 2014;56(4):457-464.
doi pubmed - Bernhardt V, Babb TG. Exertional dyspnoea in obesity. Eur Respir Rev. 2016;25(142):487-495.
doi pubmed pmc - Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1-452.
pubmed - Sung H, Siegel RL, Torre LA, Pearson-Stuttard J, Islami F, Fedewa SA, Goding Sauer A, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69(2):88-112.
doi pubmed - Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440-2450.
doi pubmed - Scano G, Stendardi L, Bruni GI. The respiratory muscles in eucapnic obesity: their role in dyspnea. Respir Med. 2009;103(9):1276-1285.
doi pubmed - Cacciante L, Turolla A, Pregnolato G, Federico S, Baldan F, Rutkowska A, Rutkowski S. The use of respiratory muscle training in patients with pulmonary dysfunction, internal diseases or central nervous system disorders: a systematic review with meta-analysis. Qual Life Res. 2023;32(1):1-26.
doi pubmed pmc - Illi SK, Held U, Frank I, Spengler CM. Effect of respiratory muscle training on exercise performance in healthy individuals: a systematic review and meta-analysis. Sports Med. 2012;42(8):707-724.
doi pubmed - Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J. 2011;37(2):416-425.
doi pubmed - Zhang F, Zhong Y, Qin Z, Li X, Wang W. Effect of muscle training on dyspnea in patients with chronic obstructive pulmonary disease: A meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021;100(9):e24930.
doi pubmed pmc - Lista-Paz A, Bouza Cousillas L, Jacome C, Fregonezi G, Labata-Lezaun N, Llurda-Almuzara L, Perez-Bellmunt A. Effect of respiratory muscle training in asthma: A systematic review and meta-analysis. Ann Phys Rehabil Med. 2023;66(3):101691.
doi pubmed - Society AC. Cancer treatment & survivorship facts & figures. 2019-2021. American Cancer Society; Atlanta, GA. 2019.
- Azambuja ACM, de Oliveira LZ, Sbruzzi G. Inspiratory muscle training in patients with heart failure: what is new? Systematic review and meta-analysis. Phys Ther. 2020;100(12):2099-2109.
doi pubmed - Kunadharaju R, Saradna A, Ray A, Yu H, Ji W, Zafron M, Mador MJ. Post-operative outcomes of pre-thoracic surgery respiratory muscle training vs aerobic exercise training: a systematic review and network meta-analysis. Arch Phys Med Rehabil. 2023;104(5):790-798.
doi pubmed - Pu CY, Batarseh H, Zafron ML, Mador MJ, Yendamuri S, Ray AD. Effects of preoperative breathing exercise on postoperative outcomes for patients with lung cancer undergoing curative intent lung resection: a meta-analysis. Arch Phys Med Rehabil. 2021;102(12):2416-2427.e2414.
doi pubmed pmc - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed pmc - National Heart L. and Blood Institute (NHLBI). Study quality assessment tools. NHLBI. 2021.
- Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):7.
doi pubmed pmc - Ha DM, Comer A, Dollar B, Bedoy R, Ford M, Gozansky WS, Zeng C, et al. Telemedicine-based inspiratory muscle training and walking promotion with lung cancer survivors following curative intent therapy: a parallel-group pilot randomized trial. Support Care Cancer. 2023;31(9):546.
doi pubmed pmc - de Almeida LB, Trevizan PF, Laterza MC, Hallack Neto AE, Perrone A, Martinez DG. Safety and feasibility of inspiratory muscle training for hospitalized patients undergoing hematopoietic stem cell transplantation: a randomized controlled study. Support Care Cancer. 2020;28(8):3627-3635.
doi pubmed - Arem H, Mama SK, Duan X, Rowland JH, Bellizzi KM, Ehlers DK. Prevalence of healthy behaviors among cancer survivors in the United States: how far have we come? Cancer Epidemiol Biomarkers Prev. 2020;29(6):1179-1187.
doi pubmed pmc - Mancini I, Body JJ. Assessment of dyspnea in advanced cancer patients. Support Care Cancer. 1999;7(4):229-232.
doi pubmed - Tinti S, Parati M, De Maria B, Urbano N, Sardo V, Falcone G, Terzoni S, et al. Multi-dimensional dyspnea-related scales validated in individuals with cardio-respiratory and cancer diseases. a systematic review of psychometric properties. J Pain Symptom Manage. 2022;63(1):e46-e58.
doi pubmed - Williams MT, Lewthwaite H, Paquet C, Johnston K, Olsson M, Belo LF, Pitta F, et al. Dyspnoea-12 and multidimensional dyspnea profile: systematic review of use and properties. J Pain Symptom Manage. 2022;63(1):e75-e87.
doi pubmed - Ekstrom M, Bornefalk H, Skold CM, Janson C, Blomberg A, Sandberg J, Bornefalk-Hermansson A, et al. Minimal clinically important differences for Dyspnea-12 and MDP scores are similar at 2 weeks and 6 months: follow-up of a longitudinal clinical study. Eur Respir J. 2021;57(3):2002823.
doi pubmed - Patel NB, Balady GJ. The rewards of good behavior. Circulation. 2010;121(6):733-735.
doi pubmed - Tortola-Navarro A, Gallardo-Gomez D, Alvarez-Barbosa F, Salazar-Martinez E. Cancer survivor inspiratory muscle training: systematic review and Bayesian meta-analysis. BMJ Support Palliat Care. 2023;13:e561-e569.
doi pubmed - Hayes SC, Newton RU, Spence RR, Galvao DA. The Exercise and Sports Science Australia position statement: Exercise medicine in cancer management. J Sci Med Sport. 2019;22(11):1175-1199.
doi pubmed - Stout NL, Baima J, Swisher AK, Winters-Stone KM, Welsh J. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature (2005-2017). PM R. 2017;9(9S2):S347-S384.
doi pubmed pmc - Fuller JT, Hartland MC, Maloney LT, Davison K. Therapeutic effects of aerobic and resistance exercises for cancer survivors: a systematic review of meta-analyses of clinical trials. Br J Sports Med. 2018;52(20):1311.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.