| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Short Communication
Volume 15, Number 2, April 2024, pages 319-324
Characterization of Asciminib-Resistant Philadelphia Chromosome-Positive Cells
Seiichi Okabea, b, Mitsuru Moriyamaa, Akihiko Gotoha
aDepartment of Hematology, Tokyo Medical University, Tokyo, Japan
bCorresponding Author: Seiichi Okabe, Department of Hematology, Tokyo Medical University, Tokyo 160-0023, Japan
Manuscript submitted December 16, 2023, accepted March 6, 2024, published online March 21, 2024
Short title: Asciminib-Resistant Ph+ Cells
doi: https://doi.org/10.14740/wjon1818
| Abstract | ▴Top |
Background: Asciminib is approved for treating patients with chronic-phase chronic myeloid leukemia who were previously treated with two or more tyrosine kinase inhibitors or those with T315I mutation. However, the mechanisms underlying asciminib resistance remain unclear.
Methods: In this study, we established a new asciminib-resistant cell line. We examined BCR::ABL1 gene mutation analysis and the effects of conventional chronic myelogenous leukemia inhibitors.
Results: Direct sequencing revealed Y139D and T315I mutations in asciminib-resistant cells. Ponatinib and omacetaxine were effective against asciminib-resistant cells.
Conclusions: Y139D and T315I mutations are extremely resistant to asciminib. Ponatinib and omacetaxine show potential for treating asciminib-resistant chronic myeloid leukemia.
Keywords: Chronic myeloid leukemia; Ponatinib; Omacetaxine; Asciminib; Philadelphia-positive cell
| Introduction | ▴Top |
ABL tyrosine kinase inhibitor (ABL TKI) therapies have improved the prognosis of chronic myeloid leukemia (CML) [1]. Imatinib was the first TKI approved for treating patients with chronic-phase CML (CML-CP). However, some patients with CML experience TKI intolerance or resistance. It has been estimated that more than 25% of patients diagnosed with CML will require a change in TKI therapy at some during their lifetime. This necessity arises from either intolerance or resistance to the initial TKI treatment [2]. Several mechanisms of resistance to ABL TKIs have been identified. ABL TKIs retain effectiveness against the majority of ABL1 kinase domain mutations, including those associated with resistance to ABL inhibitors such as T315I [2]. Clinically, the four commercially available frontline treatment options for CML TKIs are imatinib, dasatinib, nilotinib, and bosutinib [1].
Asciminib is a first-in-class drug that specifically targets the ABL myristoyl pocket (STAMP) inhibitor and suppresses BCR::ABL1 kinase activity through allosteric binding [3]. Asciminib is indicated for the treatment of patients with CML-CP who previously received two TKIs or have a T315I mutation [4]. The ASCEMBL trial, a phase 3 study comparing asciminib to bosutinib in patients with CML-CP who were previously treated with at least two TKIs, demonstrated that larger number of patients in the asciminib group sustained treatment and achieved long-term benefits. These findings suggest that asciminib should be considered as a standard treatment option for patients who have been treated with at least two TKIs [5]. The mechanisms underlying asciminib resistance remain unknown. In this study, we established an asciminib-resistant Ba/F3 cell line (Ba/F3-asciminib-R: Ba/F3 asc-R) and evaluated its drug sensitivity.
| Materials and Methods | ▴Top |
Reagents
Ponatinib, dasatinib, and homoharringtonine were obtained from MedKoo Biosciences (Chapel Hill, NC, USA), LC Laboratories (Woburn, MA, USA), and MedChemExpress (Monmouth Junction, NJ, USA), respectively. Asciminib (ABL001), a selective BCR::ABL inhibitor, was obtained from ActiveBiochem (Maplewood, NJ, USA). Imatinib and nilotinib were supplied by Novartis Pharma AG (Basel, Switzerland). The inhibitors were prepared as stock solutions in dimethyl sulfoxide, and imatinib was dissolved in distilled water, aliquoted, and stored at -20 °C.
Cell line and cell culture
Parental Ba/F3 cells were transduced with the BCR::ABL1 gene and better designated as BCR::ABL-expressing Ba/F3 cells, and Ba/F3 BCR::ABL cells containing the T315I: Ba/F3 T315I point mutation were described previously [6]. The cells were cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum at 37 °C in 5% CO2 and passaged for less than 1 month before replacement from early passage frozen stocks.
BCR::ABL1 kinase domain mutation analysis
Semi-nested reverse transcription polymerase chain reaction (PCR) was performed to amplify BCR::ABL1 fusion transcripts, and then the transcripts of the ABL1 kinase domain were amplified in a second round of PCR. Total RNA was extracted from the cells using a QIAamp RNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Semi-nested PCR was performed using Platinum Taq DNA Polymerase (Thermo Fisher Scientific, Waltham, MA, USA) and PrimeScript RT Master Mix (Perfect Real Time) from Takara Bio (Shiga, Japan) for DNA amplification. The following PCR primers were used in semi-nested PCR and sequencing reactions: NM_minor forward 5′-GGAGTACCAGCCCTACCAGA-3′ and reverse 5′-AGAAGGCGCTCATCTTCATT-3′; NM_ABL_3-6 forward 5′-ACTTTGAGCCTCAGGGTCTG-3′ and reverse 5′-ACTTTGAGCCTCAGGGTCTG-3′, NM_ABL_6-9 forward 5′-ACACCATGGAGGTGGAAGAG-3′ and reverse 5′-AGAAGGCGCTCATCTTCATT-3′; NM2_ABL1_253-359 forward 5′-TACGACAAGTGGGAGATGGA-3′ and reverse 5′-CAATACTCCAAATGCCCAGA-3′. Sequencing was performed using a BigDye Terminator v1.1 Cycle Sequencing Kit (Thermo Fisher Scientific), and DNA was sequenced on an ABI PRISM 3130xl DNA Analyzer (Thermo Fisher Scientific). The optimal PCR conditions were not disclosed by SRL Medisearch. We compared these sequences to the wild-type ABL sequence of a normal control human sample (GenBank accession number X16416.1).
Cell proliferation assay
CML cells were treated with the indicated concentrations of chemicals for 72 h, after which cell proliferation was analyzed using Cell Counting Kit-8 (Dojindo Laboratories, Mashikimachi, Kumamoto, Japan). Absorbance was measured at 450 nm using an EnSpire Multimode Plate Reader (PerkinElmer, Waltham, MA, USA).
Caspase 3/7 activity
To examine caspase activity, we utilized a Caspase Glo® 3/7 assay kit from Promega (Madison, WI, USA), following the manufacturer’s instructions. The luminescence of each sample was quantified using an EnSpire Multimode Plate Reader after 48-h incubation with the specified concentrations of chemicals.
Cytotoxicity assay
Cytotoxicity towards CML cells exposed to the specified concentrations of chemicals for 48 h was assessed. Lactate dehydrogenase (LDH) release from cells was used as an indicator for cytotoxicity evaluation, utilizing the Cytotoxicity LDH Assay Kit-WST (Dojindo Laboratories). The EnSpire Multimode Plate Reader was used to measure the absorbance at 490 nm, which represented the quantity of LDH released from dead cells.
Statistical analyses
GraphPad Prism version 10 software (GraphPad, Inc., San Diego, CA, USA) was used to analyze all data. Two-tailed Student’s t-tests were used to evaluate statistical significance. If one of the groups in the study is considered the control group, data were analyzed using Dunnett’s test as the post-hoc test following analysis of variance. When comparing three or more samples, data were examined using one-way analysis of variance with Turkey post-hoc comparison tests with alpha = 0.05 (n ≥ 3). Significance was expressed as *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
| Results | ▴Top |
Establishment of asciminib-resistant cells and sequence of BCR::ABL1
We generated cells resistant to asciminib to examine the resistance mechanism. Ba/F3 T315I mutant cells were cultured in medium containing low concentrations of asciminib, which was changed twice per week while adjusting the concentration of asciminib every 2 weeks. Asciminib concentrations were higher than 1 µM and we established resistant strains after 2 months. We established the asciminib-resistant Ba/F3 cell line (Ba/F3 asc-R). Direct sequencing is commonly performed to identify point mutations in oncogenic BCR::ABL1. Direct sequencing of BCR::ABL1 revealed Y139D and T315I mutations in Ba/F3 asc-R cells (Fig. 1). Cells with Y139D and T315I mutations were resistant to asciminib.
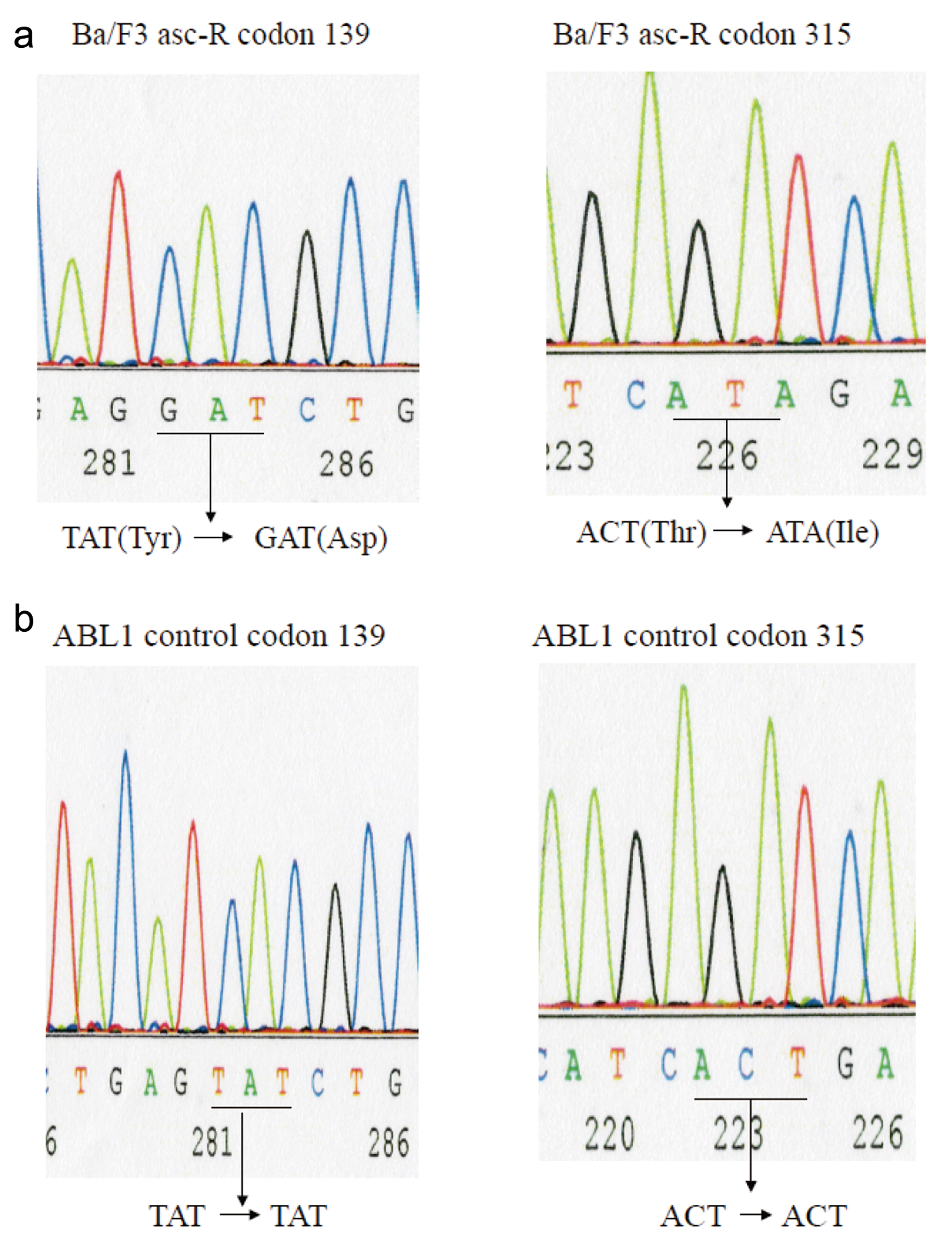 Click for large image | Figure 1. BCR-ABL kinase domain mutation analysis. Mutation screening via sequence analysis was performed to identify the ABL kinase domain in Ba/F3-asciminib-R (Ba/3 asc-R) cells (a) and normal control samples (b). Direct sequencing to detect mutations in the ABL kinase domain in the cell lines revealed Y139D and T315I mutations in Ba/F3 asc-R cells. |
Drug sensitivity of asciminib-resistant cells
Ba/F3 asc-R cells were resistant to high doses of asciminib, with an IC50 of up to 10 µM (Fig. 2a). In contrast, Ba/F3 T315I cells were sensitive to lower concentrations of asciminib. Ba/F3 T315I cells were sensitive to asciminib, indicating that this cell line is a single asciminib-resistant clone. Ponatinib is used to treat specific types of leukemia, such as CML and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) [7]. As a third-generation kinase inhibitor, ponatinib was developed to overcome the gatekeeper T315I mutation [8]. Ponatinib was effective against Ba/F3 T315I cells, and a high dose of ponatinib was effective against Ba/F3 asc-R cells (Fig. 2a).
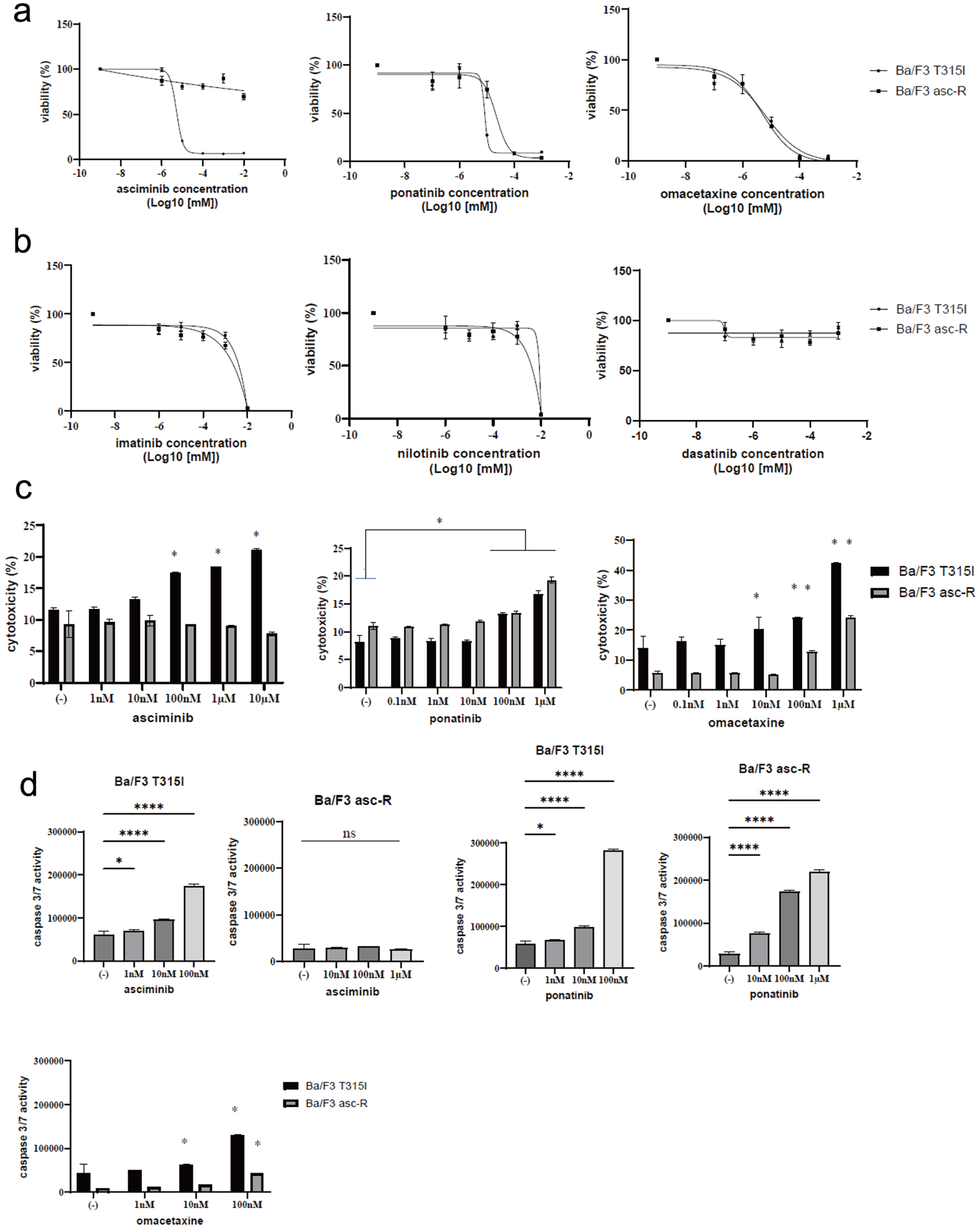 Click for large image | Figure 2. Activity of ABL inhibitors in Ba/F3-asciminib-R (Ba/F3 asc-R) and Ba/F3 T315I cells. (a) Ba/F3 asc-R and Ba/F3 T315I cell lines were cultured with the indicated concentrations of asciminib, ponatinib, or omacetaxine for 72 h. Cell growth was evaluated using Cell Counting Kit-8. (b) Ba/F3 asc-R and Ba/F3 T315I cell lines were cultured with the indicated concentrations of imatinib, nilotinib, or dasatinib for 72 h. Cell growth was evaluated using Cell Counting Kit-8. (c) Ba/F3 asc-R and Ba/F3 T315I cell lines were cultured with the indicated concentrations of asciminib, ponatinib, or omacetaxine for 72 h. Cell death was evaluated using a Cytotoxicity LDH Assay Kit. *P < 0.05 compared to control. (d) Ba/F3 asc-R and Ba/F3 T315I cell lines were cultured with the indicated concentrations of asciminib, ponatinib, or omacetaxine for 48 h. Caspase 3/7 activity was evaluated. *P < 0.05, **P < 0.01, ****P < 0.0001 compared to control. ns: not significant. |
Omacetaxine mepesuccinate (homoharringtonine) has been approved for the treatment of adult patients with CML-CP and accelerated-phase CML who are resistant to two or more TKIs such as imatinib, dasatinib, or nilotinib [9]. At lower doses, omacetaxine inhibited the development of Ba/F3 asc-R cells (Fig. 2a). Other ABL TKIs (imatinib, nilotinib, and dasatinib) had no effect on Ba/F3 T315I or Ba/F3 asc-R cells (Fig. 2b). Asciminib was cytotoxic towards Ba/F3 T315I cells because not Ba/F3 asc-R cells (Fig. 2C). Additionally, ponatinib and omacetaxine induced dose-dependent cytotoxicity in Ba/F3 T315I and Ba/F3 asc-R cells (Fig. 2c). Asciminib increased caspase 3/7 activity in Ba/F3 T315I cells but not in Ba/F3 asc-R cells (Fig. 2d). High concentrations of ponatinib enhanced caspase 3/7 activity in Ba/F3 asc-R cells (Fig. 2d). Omacetaxine also enhanced caspase 3/7 activity in Ba/F3 T315I and Ba/F3 asc-R cells (Fig. 2d).
| Discussion | ▴Top |
Asciminib is an investigational BCR::ABL1 TKI that is currently being studied for its potential for treating CML. This drug was designed to target BCR::ABL1 protein in a unique manner compared with traditional TKIs such as imatinib, dasatinib, nilotinib, or ponatinib by specifically targeting the myristoyl pocket of ABL1 [10]. Protein kinase activity is regulated by various molecular pathways, and its disruption is a typical cause of oncogenesis. ABL inhibitors that bind to regulatory regions reduce kinase activity [3]. As compared with ATP-competitive inhibitors, allosteric inhibitors are highly selective for ABL kinases. Asciminib has high affinity for myristoyl-binding sites [3]. The NH2 terminus of ABL contains three SRC homology domains (SH1-SH3) [11]. The SH1 domain functions as a tyrosine kinase, whereas the SH2 and SH3 domains enable protein interactions. The inactive and active forms of ABL kinases are reportedly regulated by dynamic intramolecular interactions that modulate ABL kinase activity [12]. The SH3 domain binds to the linker sequence that connects the SH2 and kinase (SH1) domains, and the SH2 domain interacts with the C-terminal lobe of the kinase domain to form an SH3-SH2 clamp structure that locks the kinase in an inactive state [13]. Myristoyl pocket mutations have either been clinically documented (A337T, P465S, and V468F) or predicted using in vitro models (A344P) [10]. Several ABL-activating mutations (PP, K51A, W99A, and Y139D) failed to confer imatinib resistance to BCR::ABL. The SH2 domain mutation, Y139, activates ABL1 by disrupting the SH2 kinase domain connection, which may be the basis for myristoyl-mediated autoinhibition. Previous research showed that BCR::ABL1 compound mutations (Y253H/T315I) are resistant to asciminib [14]. Other BCR::ABL1 mutations within or near the myristoyl-binding pocket confer asciminib resistance (e.g., A337V, P465S, and V468F) [15]. The T315I mutation confers resistance to all currently approved TKIS except for ponatinib, which is a third-generation drug [16].
A phase 1 study enrolled 141 patients with CML-CP and nine patients with accelerated-phase CML who had developed resistance to or experienced unacceptable side effects from at least two previous ATP-competitive TKIs. Among the patients, 12 (28%) achieved or maintained a major molecular response by the 12-month mark, with five patients showing a T315I mutation at the start of the study. Patients with the T315I mutation achieved complete cytogenetic and major molecular responses, with most receiving asciminib doses of more than 150 mg twice daily, which was higher than the required dose for patients without the T315I mutation [17]. Asciminib was effective in patients with CML who had not responded to ponatinib and those with the T315I mutation [17]. Another study showed that 42% of the 45 patients with the T315I mutation achieved a major molecular response by 24 weeks of asciminib treatment [18]. In ABL TKI resistance, the H-RAS T81C polymorphism was found to be associated with CML risk and prognosis [19].
Ponatinib is a third-generation kinase inhibitor developed for treating patients with the T315I gatekeeper mutation, which is a common resistance mutation in cancer cells. This drug targets specific kinases involved in the growth and proliferation of cancer cells and has shown promising results in clinical trials for treating various types of cancer. Ponatinib exhibits inhibitory activity against native BCR::ABL1 kinase and several ABL1 mutations [7, 8]. Ponatinib is currently approved for treating CML in patients who are resistant and/or intolerant to dasatinib and nilotinib, as well as in those who can no longer take imatinib or have the T315I mutation [7, 8]. Omacetaxine is a distinct inhibitor of protein synthesis that does not overlap with kinase inhibition. Numerous studies demonstrated that omacetaxine can yield responses in patients who have been extensively treated for either CML-CP or accelerated-phase CML, regardless of whether they possess tyrosine kinase domain mutations [9]. Ponatinib and omacetaxine mepesuccinate may be effective against asciminib-resistant strains, resulting in clinical improvement. To the best of our knowledge, this is the first study to show that BCR::ABL1 Y139D and T315I is an asciminib-resistant mutant. Because we were unable to obtain cells bearing the BCR::ABL1 Y139D mutation, a Y139D mutant cell line must be generated to explore the impact of asciminib. Asciminib is currently used in clinical practice; however, resistance to asciminib may develop. In such cases, alternative medications such as ponatinib or omacetaxine may be effective for treating patients with asciminib resistance. We determined the mechanisms of ABL TKI resistance and demonstrated the clinical potential of ponatinib and omacetaxine mepesuccinate for treating patients with asciminib-resistant CML.
Acknowledgments
None to declare.
Financial Disclosure
The Japanese Ministry of Education, Culture, Sports, Science and Technology provided financial support for this study (20K07644). AG received research funding from Eisai Co., Ltd., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Chugai Pharmaceutical Co., Ltd., MSD K.K., Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Nippon Shinyaku Co., Ltd., Bayer Yakuhin, Ltd., Daiichi Sankyo Co., Ltd., and Nihon Pharmaceutical Co., Ltd. AG received honoraria from Novartis Pharma K.K., Alexion Pharmaceuticals, Inc., Eisai Co., Ltd., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Nihon Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Janssen Pharmaceutical K.K., Pfizer Japan Inc., and Sanofi K.K. AG received consulting fees from PharmaEssentia Japan K.K., Chugai Pharmaceutical Co., Ltd., and Alexion Pharmaceuticals, Inc. In addition, AG participated in the data safety monitoring board or advisory board of PharmaEssentia Japan K.K., Chugai Pharmaceutical Co., Ltd., and Alexion Pharmaceuticals, Inc. SO and MM report no conflict of interest.
Conflict of Interest
AG received research funding from Eisai Co., Ltd., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Chugai Pharmaceutical Co., Ltd., MSD K.K., Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Nippon Shinyaku Co., Ltd., Bayer Yakuhin, Ltd., Daiichi Sankyo Co., Ltd., and Nihon Pharmaceutical Co., Ltd. AG received honoraria from Novartis Pharma K.K., Alexion Pharmaceuticals, Inc., Eisai Co., Ltd., Ono Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., Chugai Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Daiichi Sankyo Co., Ltd., Nihon Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., Janssen Pharmaceutical K.K., Pfizer Japan Inc., and Sanofi K.K. AG received consulting fees from PharmaEssentia Japan K.K., Chugai Pharmaceutical Co., Ltd., and Alexion Pharmaceuticals, Inc. In addition, AG participated in the data safety monitoring board or advisory board of PharmaEssentia Japan K.K., Chugai Pharmaceutical Co., Ltd., and Alexion Pharmaceuticals, Inc. SO and MM report no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
SO and MM designed the study. SO and AG wrote the manuscript. SO performed the experiments and assisted in creating figures. The final manuscript was read and approved by all authors.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Abbreviations
ABL TKI: ABL tyrosine kinase inhibitor; CML: chronic myeloid leukemia; CML-CP: chronic-phase CML; PCR: polymerase chain reaction; SH: SRC homology domains; STAMP: specifically targets the ABL myristoyl pocket
| References | ▴Top |
- Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2020 update on diagnosis, therapy and monitoring. Am J Hematol. 2020;95(6):691-709.
doi pubmed - Patel AB, O'Hare T, Deininger MW. Mechanisms of resistance to ABL kinase inhibition in chronic myeloid leukemia and the development of next generation ABL kinase inhibitors. Hematol Oncol Clin North Am. 2017;31(4):589-612.
doi pubmed pmc - Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, Turkina A, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood. 2021;138(21):2031-2041.
doi pubmed pmc - Deeks ED. Asciminib: First Approval. Drugs. 2022;82(2):219-226.
doi pubmed - Hochhaus A, Rea D, Boquimpani C, Minami Y, Cortes JE, Hughes TP, Apperley JF, et al. Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: longer-term follow-up of ASCEMBL. Leukemia. 2023;37(3):617-626.
doi pubmed pmc - Kimura S, Naito H, Segawa H, Kuroda J, Yuasa T, Sato K, Yokota A, et al. NS-187, a potent and selective dual Bcr-Abl/Lyn tyrosine kinase inhibitor, is a novel agent for imatinib-resistant leukemia. Blood. 2005;106(12):3948-3954.
doi pubmed - Massaro F, Molica M, Breccia M. Ponatinib: a review of efficacy and safety. Curr Cancer Drug Targets. 2018;18(9):847-856.
doi pubmed - Hoy SM. Ponatinib: a review of its use in adults with chronic myeloid leukaemia or Philadelphia chromosome-positive acute lymphoblastic leukaemia. Drugs. 2014;74(7):793-806.
doi pubmed - Winer ES, DeAngelo DJ. A review of omacetaxine: a chronic myeloid leukemia treatment resurrected. Oncol Ther. 2018;6(1):9-20.
doi pubmed pmc - Hijiya N, Mauro MJ. Asciminib in the treatment of philadelphia chromosome-positive chronic myeloid leukemia: focus on patient selection and outcomes. Cancer Manag Res. 2023;15:873-891.
doi pubmed pmc - Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96(10):3343-3356.
pubmed - Luttman JH, Colemon A, Mayro B, Pendergast AM. Role of the ABL tyrosine kinases in the epithelial-mesenchymal transition and the metastatic cascade. Cell Commun Signal. 2021;19(1):59.
doi pubmed pmc - Greuber EK, Smith-Pearson P, Wang J, Pendergast AM. Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nat Rev Cancer. 2013;13(8):559-571.
doi pubmed pmc - Yeung DT, Shanmuganathan N, Hughes TP. Asciminib: a new therapeutic option in chronic-phase CML with treatment failure. Blood. 2022;139(24):3474-3479.
doi pubmed - Qiang W, Antelope O, Zabriskie MS, Pomicter AD, Vellore NA, Szankasi P, Rea D, et al. Mechanisms of resistance to the BCR-ABL1 allosteric inhibitor asciminib. Leukemia. 2017;31(12):2844-2847.
doi pubmed pmc - Cortes J, Lang F. Third-line therapy for chronic myeloid leukemia: current status and future directions. J Hematol Oncol. 2021;14(1):44.
doi pubmed pmc - Hughes TP, Mauro MJ, Cortes JE, Minami H, Rea D, DeAngelo DJ, Breccia M, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med. 2019;381(24):2315-2326.
doi pubmed pmc - Nicolini FE, Huguet F, Huynh L, Xu C, Bouvier C, Yocolly A, Etienne G. A multicenter retrospective chart review study of treatment and disease patterns and clinical outcomes of patients with chronic-phase chronic myeloid leukemia in third-line treatment or with T315I mutation. Cancers (Basel). 2023;15(16):4161.
doi pubmed pmc - Mir R, Ah I, Javid J, Zuberi M, Guru S, Mirza M, Farooq S, et al. Polymorphism T81C in H-RAS oncogene is associated with disease progression in imatinib (TKI) treated chronic myeloid leukemia patients. World J Oncol. 2015;6(2):321-328.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


