| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 6, December 2022, pages 370-378
Acute Kidney Injury After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in a Portuguese Population
Eduarda Cariasa, c, Hugo Ferreirab, Teresa Chuvab, Ana Paivab, Jose Maximinob
aDepartment of Nephrology, Centro Hospitalar e Universitario do Algarve, Faro, Portugal
bDepartment of Nephrology, Instituto Portugues de Oncologia do Porto Francisco Gentil, Porto, Portugal
cCorresponding Author: Eduarda Carias, Department of Nephrology, Centro Hospitalar e Universitario do Algarve, Faro, Portugal
Manuscript submitted October 24, 2022, accepted November 14, 2022, published online December 1, 2022
Short title: Acute Kidney Injury After HIPEC
doi: https://doi.org/10.14740/wjon1540
| Abstract | ▴Top |
Background: Acute kidney injury (AKI) after cytoreductive surgery followed by the infusion of hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) is associated with a higher rate of major complications, resulting in prolonged hospitalization and increased mortality. Our objective was to evaluate the incidence of AKI and further progression to chronic kidney disease (CKD) in patients submitted to this procedure and recognize the associated risk factors.
Methods: This retrospective study collected demographic, tumor-related, intraoperative, and postoperative data from 182 patients who underwent CRS/HIPEC from January 2016 to December 2019. Renal impairment was defined according to Kidney Disease Improving Global Outcomes criteria for AKI. We conducted univariate and multiple logistic regression analyses to assess the association between variables of interest and AKI.
Results: Twenty-three patients (12.6%) developed AKI. In the AKI group, the risk for developing CKD was six times higher (odds ratio (OR) 6.48, confidence interval (CI) 1.601 - 26.255). Multivariate regression identified higher risk of developing AKI in patients who underwent HIPEC with cisplatin (OR 12.21, CI 1.26 - 109.70, P = 0.025), in each additional day spent in the intensive care unit (ICU) (OR 2.42, CI 1.07 - 5.45, P = 0.033), and an association for each unit increase in estimated glomerular filtration rate (eGFR) before HIPEC (OR 0.96, CI 0.94 - 0.98, P = 0.037) and AKI development.
Conclusion: Patients who are at higher risk of AKI after CRS/HIPEC include those who performed cisplatin HIPEC regimen, had poorer preoperative renal function and had longer ICU stays. Early institution of preventive measures and frequent monitoring should be considered to minimize AKI risk and its associated morbidity, such as CKD progression.
Keywords: Acute kidney injury; Intraoperative chemotherapy; Nephrotoxicity; Platinum agents; Chronic kidney disease
| Introduction | ▴Top |
The management of peritoneal metastases has proved to be a challenge due to the complexity and incomplete understanding of the pathobiology of peritoneal carcinomatosis (PC) and lack of studies that prove the efficacy of some therapeutic modalities [1].
Hyperthermic intraperitoneal chemotherapy (HIPEC) with cytoreduction is an increasingly used technique for treating intra-abdominal malignancy, mainly gastrointestinal or gynecological cancers with peritoneal dissemination or primary peritoneal neoplasms [1, 2]. This technique has been shown to improve median survival at 2 years and in 20% patients, survival was shown to be greater than 5 years [3].
This is a complex therapeutic modality, and it includes an aggressive and extensive cytoreductive surgery (CRS), followed by the infusion of HIPEC. In vitro and in vivo studies have demonstrated improved therapeutic index and cytotoxic efficacy of chemotherapeutic drugs when delivered in the setting of HIPEC [1].
The intraperitoneal instillation of the chemotherapeutic agents achieves very high local doses, allowing diffusion into tumor nodules, while minimizing systemic absorption and toxicity [1, 4]. Some studies indicate that malignant cells are selectively destroyed by hyperthermia in the range of 41 to 43 °C. The combination of heat and cytotoxic drugs frequently results in increased drug penetration in tissue, an increased cytotoxicity, and inhibition of cell repair mechanisms [1, 5].
The chemotherapeutic drug chosen should have a well-established activity against the malignancy treated and have a reduced systemic uptake. The most used agents are mitomycin and the platinum-based drugs, and, less commonly, doxorubicin.
Intraperitoneal chemotherapy agents can cause adverse effects due to absorption of the drugs themselves, and absorption of their carrier solutions which can lead to organ toxicity. To reduce this, doses of chemotherapeutic agents and volume of the carrier are standardized based on body surface area and adjusted to kidney function.
Acute kidney injury (AKI) is one of the most common complications following CRS with HIPEC, with some studies reporting an incidence as high as 20% particularly with administration of cisplatin [6-8].
AKI after CRS with HIPEC is associated with a higher rate of major complications, resulting in prolonged hospitalization and increased mortality [7, 9]. Suggested pathophysiological mechanisms for AKI during HIPEC include hypotension or renal hypoperfusion related to intra-abdominal hypertension, major fluid shifts, nephrotoxic chemotherapy agents, and the inflammatory response [10]. Potential predictors of AKI include older age, worse baseline renal function, higher body mass index (BMI), use of a platinum-based infusion, longer operative time, and large estimated blood loss [6, 7]. Thereby, in patients with these AKI predictors, a nephroprotective bundle approach is usually employed.
Our objective was to evaluate the incidence of AKI and further progression to chronic kidney disease (CKD) in patients submitted to CRS/HIPEC procedure and understand more thoroughly the risk factors associated with this perioperative morbidity in this group of patients.
| Materials and Methods | ▴Top |
Study population and enrollment
From January 2016 to December 2019, a total of 184 patients with cancer peritoneal metastases who received CRS/HIPEC at Instituto Portugues de Oncologia (IPO) do Porto were retrospectively analyzed. The HIPEC procedure was indicated for: curative intent of peritoneal metastases from primary or recurrent ovarian cancer, colorectal cancer, gastric cancer, peritoneal mesothelioma, pseudomyxoma peritonei and other malignancies with peritoneal metastases.
The patients included in the study were younger than 80 years, had Eastern Cooperative Oncology Group performance status (ECOG-PS) of 0, 1 or 2, and had biopsy-proven PC by laparotomy or laparoscopy and a potentially resectable primary or recurrent tumor with no signs of distant metastases on abdominal and thoracic computed tomography (CT) scans.
Surgical technique
All subjects underwent multidetector CT or magnetic resonance imaging (MRI) before surgery. The preoperative CT/MRI peritoneal carcinomatosis index (PCI) was scored by experienced radiologists according to the Sugarbaker classification [2].
PCI was used to score the extent of peritoneal involvement at the time of surgery as reported in the 13-region and lesion size system. Completeness of cytoreduction score (CC) was reported as CC0 for no residual disease, CC1 for microscopic residual disease (< 2.5 mm), CC2 for macroscopic residual disease (> 2.5 mm), and CC3 for residual disease > 2.5 cm. After multidisciplinary team discussion, the patients were scheduled for the CRS/HIPEC procedure.
Our treatment consisted of tumor resection and removal of the involved organs and peritoneum, as deemed safe. HIPEC was performed using the closed abdomen.
The perfusion equipment consisted of an infusion pump, a heat exchanger, and a reservoir with a filter. The solution used for the infusion was 1.5% dextrose peritoneal dialysis solution, with an infusion flow of 1 L/min, and a heat exchanger programmed to ensure a perfusion temperature of 42 °C. The intraperitoneal chemotherapy drugs were drained out after HIPEC had been completed (90 min after).
Intraperitoneal chemotherapy regimens used included mitomycin C (15 mg/m2/2 L) for carcinomatosis of appendicular, colorectal, and gastric origin and cisplatin (50 mg/m2/2 L) for carcinomatosis of ovarian origin, peritoneal sarcomatosis and mesothelioma.
AKI diagnostic criteria, monitoring, study parameters and data collection
Kidney Disease Improving Global Outcomes (KDIGO) criteria for AKI [11] diagnosis were used, so AKI was defined as a rise in serum creatinine (sCr) of ≥ 0.3 mg/dL within 48 h of surgery.
Renal impairment was staged according to the serum creatinine levels as follows: grade 1 represented an increase to more than or equal to 1.5- to two-fold from baseline; grade 2 represented an increase to more than two- to three-fold from baseline; and grade 3 represented an increase to more than 3.0 times the baseline or more than or equal to 4 mg/dL (with an increase of at least 0.5 mg/dL) or need for renal replacement therapy (RRT).
CKD was defined by an eGFR < 60 mL/min/1.73 m2 lasting more than 3 months.
Data on the patients’ characteristics, intraoperative conditions, including fluid input/output, medications, blood loss and blood transfusion were evaluated from the anesthesia and internal medicine consultation care records.
Laboratory tests, including hematology and basic chemistry panels, including renal function, and electrolytes were drawn immediately postoperatively and on a daily basis until the day of discharge.
Since our aim was to examine incidence of AKI, the risk factors, and the impact to further progression to CKD, kidney function was also assessed at 3 - 6 months after surgery and 1 year follow-up.
Statistical analysis
In this study, the primary endpoint was to describe the AKI incidence in a single institution’s cohort of CRS and HIPEC, and its correlation with the development of CKD. Our secondary endpoint was the identification of risk factors that would predict the occurrence of AKI and CKD.
Patients who developed post-CRS/HIPEC creatinine increase were defined as the “AKI” group and those who did not were defined as the “non-AKI” group.
Descriptive statistics were reported as mean ± standard deviation (SD), median with minimum and maximum, or frequency with percentage as appropriate. Continuous variables were presented as the means ± SDs and were compared using Student’s t-test, or the non-parametric Mann-Whitney test for two groups or using the analysis of variance (ANOVA) for more than two groups.
The associations between covariates and the occurrence of creatinine increase were examined using univariate analysis with the Chi-square test or Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for continuous variables. For those covariates with P < 0.15 in the univariate analysis, they were then considered in multiple logistic regression. The explanatory variables in the final model were identified manually by dropping the covariates with P-value > 0.05 one at a time until all regression coefficients were significant.
Statistical analyses were performed using SPSS statistical software (IBM SPSS Statistics for Windows, version 22.0). All tests were two-sided, and a P-value < 0.05 was considered as statistically significant.
Ethics approval
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Patients
The 184 patients analyzed received CRS/HIPEC procedures in our unit during the period specified above. The patient enrollment flowchart is shown in Figure 1. From a total of 184 patients, 182 patients were included in the study (in two patients, there were no data on renal function during hospitalization).
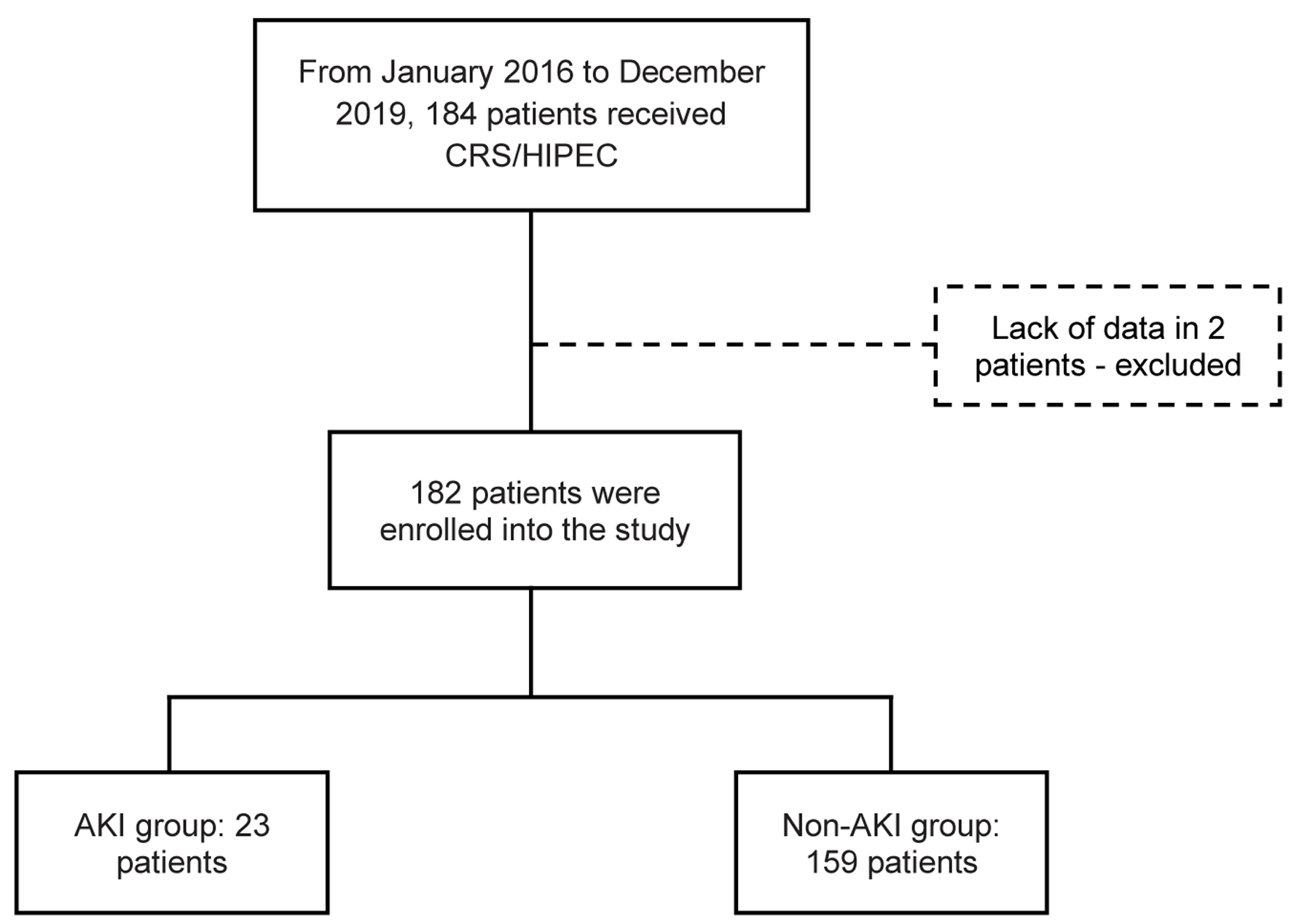 Click for large image | Figure 1. Flowchart of patient enrollment and study population. CRS/HIPEC: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. |
The mean age ± SD was 58 ± 12.1, and of the 182 patients, 23 (12.6%) were identified as having postoperative creatinine increased - AKI group, as defined by the previous criteria. Fifty-nine patients (32.4%) had preoperative chemotherapy, with most of them (55.9%) receiving a platinum agent. There was a median of 283 mL (0 - 2,000) of blood loss intraoperatively, 35.7% of the patients lost > 200 mL during procedure.
The distribution of cancer types in these two groups was different, and the AKI group had a higher rate of mucinous appendix cancer (P = 0.018), ovarian cancer (P = 0.025), and mesothelioma (P = 0.026). The mean duration of hospital stay was higher in the AKI group (22.1 ± 17.8 vs. 13.1 ± 7.9 days for the AKI group and non-AKI group respectively, P = 0.025). Other demographic variables were comparable between the two groups.
Characteristics of AKI group of patients
As shown in Table 1, before CRS/HIPEC regimens, the AKI group had a higher proportion of patients with CKD than the non-AKI group (17.4% vs. 3.1%, P = 0.016). In patients who developed renal impairment, most (73.9%) developed renal impairment of stage 1 and stage 2 (17.4%) that settled with conservative management. Two (8.7%) developed grade 3 AKI: one of them died during hospitalization, and the other one required RRT transiently, during hospitalization. This same patient (who had baseline CKD-3a) progressed to end-stage renal disease and started hemodialysis (HD) 24.5 months after HIPEC.
 Click to view | Table 1. Demographic and Clinical Data of the Patients Who Underwent CRS/HIPEC |
When the AKI population was studied, it was shown that the risk for developing CKD in the future was six times higher in this group of patients (odds ratio (OR) 6.48, 95% confidence interval (CI) 1.601 - 26.255).
Risk factors of AKI
We analyzed all the possible risk factors to develop AKI. The presence of CKD history before HIPEC procedure (P = 0.016), the length of stay (P = 0.025) and the use of antibiotics during hospitalization (P = 0.025) were risk factors associated with the development of AKI post-HIPEC.
The univariate analysis (Table 2) showed that patients who had a history of CKD before HIPEC (OR 0.15, CI, 0.38 - 1.62, P = 0.009), and in patients with lower eGFR before HIPEC (OR 1.02, CI, 1.00 - 1.04, P = 0.029), were at a higher risk of creatinine increase, thus, for every 1 mL/min decrease in eGFR prior to HIPEC, there is a 2% risk of worsening renal function.
 Click to view | Table 2. Analysis of Risk Factors for the Occurrence of AKI Using Univariate Logistic Regression Models |
Kidney function decline was also associated with antibiotic use (OR 2.68, CI 1.1 - 6.51, P = 0.029), platinum-based chemotherapy (OR 3.76, CI 1.52 - 9.31, P = 0.040), and longer hospital and intensive care unit (ICU) stay (OR 1.06, CI 0.02 - 0.11, P = 0.010 and OR 2.06, CI 1.11 - 3.82, P = 0.022, respectively); the risk increased for each extra day of hospitalization. In contrast, diabetes mellitus (DM) was not related to the development of creatinine increase.
Furthermore, after significant variables in the univariate analysis were considered simultaneously, the multivariable logistic regression model analysis (Table 3) revealed that patients who underwent HIPEC with cisplatin had 12.2 times greater risk of AKI than patients who received mitomycin (OR 12.21, CI 1.26 - 109.70, P = 0.025); for each additional day spent in the ICU, the risk of developing AKI increased by 2.4 times (OR 2.42, CI 1.07 - 5.45, P = 0.033); and for each unit increase in eGFR before HIPEC (CKD-EPI mL/min/1.73 m2), the risk of having AKI decreased by 4% (OR 0.96, CI 0.94 - 0.98, P = 0.037).
 Click to view | Table 3. Analysis of Risk Factors for the Occurrence of AKI Using Multivariable Logistic Regression Models |
| Discussion | ▴Top |
This study aimed to identify the incidence and risk factors for the development of AKI, and to assess the progression to CKD in this group of patients.
To date, this was the first study that evaluated this comorbidity in a Portuguese population. In this large sample of patients (182), 12.6% developed AKI stage 1 to 3, according to KDIGO classification after CRS/HIPEC; only one required RRT.
CRS with HIPEC is associated with perioperative mortality of 0-4% and major complications can be as high as 40% [12-15]. Risk factors for perioperative morbidity and mortality after HIPEC include poor performance status, older age, smoking history, cardiovascular disease, and diabetes [16-18].
There is also a wide range of incidence rates of AKI reported in the literature. In previous studies, rates of AKI after CRS-HIPEC ranged between 2% and 22% [6, 19-22]. Of the patients described by Kusamura et al [20], 4% developed renal toxicity grade 3 or higher when using cisplatin ± doxorubicin or mitomycin, while Bakrin et al observed nephrotoxicity in 8% of patients (with 2% developing chronic renal insufficiency and 1% requiring long-term dialysis) [23]. Schmidt et al reported only one case of nephrotoxicity among 67 patients when using cisplatin [24].
A justification for this discrepancy in values may be the absence of a clear definition (and standardization) of AKI by the authors, which could explain why some groups described very low incidences [25]. In this study, we chose to define the AKI event according to KDIGO 2017 AKI definition.
As known, chemotherapeutic agents have been associated with AKI after HIPEC. For example, cisplatin, oxaliplatin, mitomycin and doxorubicin are commonly used in HIPEC, and they have been associated with chemotherapy-induced renal toxicity and electrolyte metabolic disturbance [8].
In our population, there was a 12.2 times greater risk of developing AKI with platinum-based protocols. While this finding is similar to what has been described in previous studies [6, 8, 19, 25], the extent to which this association may be wholly ascribed to the direct nephrotoxic effects of the platinum agents has been the subject of controversy [6, 10, 22].
It has been proposed that other factors such as hypoperfusion, as a result of intra-abdominal hypertension or systemic hypotension may also play a role in the development of AKI [6, 10].
As Ceresoli et al described, the hyperthermic phase mimics a hyperdynamic state concomitant to an acute abdominal hypertension [10]. This state is characterized by the musculocutaneous vasodilatation secondary to the hyperthermia with splanchnic vasodilatation, the filling of the abdominal cavity and a massive cytokine production and release. These situations implicate major changes in blood volume which may result in severe depletion of intravascular volume and organ hypoperfusion.
For these reasons, a similarity between CRS/HIPEC and septic state (or even hepato-renal syndrome) has been proposed. In light of these considerations, it could be hypothesized that part of the renal impairment is caused by a hypoperfusion (like in the septic shock) rather than a drug-induced toxicity [10]; on the other hand, this will happen with both mitomycin and cisplatin administration.
Some reviews showed that hydration remains the most effective strategy to prevent nephrotoxicity in patients treated with cisplatin [26, 27]. We did not find an association between the development of AKI and intraoperative blood loss, or crystalloid reposition during procedure.
The choice of chemotherapy regimen for HIPEC is related to the type of cancer. The AKI group had a higher rate of mucinous appendix cancer (P = 0.018), ovarian cancer (P = 0.025), and mesothelioma (P = 0.026). Conversely, preoperative cisplatin use had no impact on the risk of postoperative AKI (P = 0.326). However, only 33 (18.1%) patients received preoperative cisplatin, and that number might be too small to pick up low incidences of cisplatin-induced renal impairment. Sin et al showed that both a high number of preoperative cycles of carboplatin and a short time interval between preoperative chemotherapy and hyperthermic intra-peritoneal chemotherapy were risk factors for kidney injury [25].
Among patients in the ICU, the incidence of AKI may be 50% [28]. A higher risk (> 2.4 times) of developing AKI was also demonstrated for each additional day of stay in the ICU. This may be associated with the presence of sepsis, cardiogenic shock and other major complications in these patients.
Sodium thiosulfate has also been used to prevent cisplatin nephrotoxicity during HIPEC procedure [26]. However, since this prophylaxis was not performed in our population, we have no data on this.
In a retrospective review of 935 patients (91 with diabetes) who underwent CRS with HIPEC, diabetes was a strong independent predictor for major complications, including renal insufficiency [16]. However, this was not confirmed in our study population.
Lower baseline glomerular filtration rate before surgery and lower preoperative albumin levels have been found to be risk factors of nephrotoxicity [26]. We also demonstrated that for each unit increase in eGFR before HIPEC (CKD-EPI mL/min/1.73 m2), the risk of having AKI decreases by 4%.
Identification of patients at risk preoperatively, such as those with higher baseline creatinine/lower eGFR, would help us adopt suitable preventive measures. We suggest that the renal function of patients should be carefully monitored for 7 days after the operation, for early detection of AKI after HIPEC, especially in patients with previous changes in renal function, and that the use of nephrotoxic antibiotics, such as non-steroidal anti-inflammatory drugs (NSAIDs) and radiocontrast agents, be avoided in these patients.
As is known, AKI events and the severity of creatinine raise are related to long-term renal prognosis. In this population, when patients with kidney impairment were studied, it was shown that the risk for developing CKD in the future was six times higher in this group of patients (OR 6.48, CI 1.601 - 26.255).
There are several strengths to this study. First, this study presented a good population sample, managing to include more than 180 patients. Also, all the patients were from one single teaching hospital, and the CRS/HIPEC procedure was uniform.
Second, there are not many studies focusing on the development of AKI after CRS/HIPEC in the Portuguese population. This study further analyzed the risk of CKD development after this complication.
However, our study has limitations related to its retrospective design. Significant selection, lack of laboratory information limits the interpretation of our results.
Also, in our institution, more frequent serum creatinine determinations are typically made in patients at risk for AKI, which is a source of detection bias.
In conclusion, patients, who are at higher risk of AKI after CRS and HIPEC, include those who performed the cisplatin HIPEC regimen, and had poorer preoperative renal function and longer ICU stays.
Early institution of preventive measures and frequent monitoring should be considered to minimize AKI risk and its associated morbidity, such as CKD progression.
Patient stratification based on these risk factors should be considered in further prospective studies. Furthermore, designing a prospective study to validate the new finding of the influence of the peritoneal dialysis solution is the future research aspect. The development and implementation of tools to detect AKI at an early stage remains an unmet medical need, as well as the subsequent evaluation of patients for detection of late CKD.
Acknowledgments
We would like to thank the Statistics Department of the IPO of Porto, for their help in the statistical analysis of this study.
Financial Disclosure
No funding or support to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
EC conceived the manuscript and undertook the literature searches; EC and HF drafted the manuscript. HF, TC, AP and JM read and revised the manuscript. All authors have read and approved the content of the manuscript and confirmed the accuracy or integrity of any part of the work.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
AKI: acute kidney injury; BMI: body mass index; CC score: completeness of cytoreduction score; CKD: chronic kidney disease; CRS: cytoreductive surgery; ECOG-PS: Eastern Cooperative Oncology Group performance status; eGFR: estimated glomerular filtration rate; HIPEC: hyperthermic intraperitoneal chemotherapy; ICU: intensive care unit; IPO: Instituto Portugues de Oncologia; IQR: interquartile range; KDIGO: Kidney Disease Improving Global Outcomes; NSAID: non-steroidal anti-inflammatory drug; PCI: peritoneal cancer index; RAS: renin-angiotensin system; RRT: renal replacement therapy; SD: standard deviation
| References | ▴Top |
- MacArthur KM, Nicholl MB. Principles and innovations in peritoneal surface malignancy treatment. World J Oncol. 2013;4(3):129-136.
doi pubmed - Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: Progress toward a new standard of care. Cancer Treat Rev. 2016;48:42-49.
doi pubmed - Abreu J, Serralva M, et al. Citorreducao seguida de quimioperfusao intraperitoneal hipertermica no tratamento da doenca peritoneal maligna: Estudo de fase II com reduzida toxidade e morbilidade. Revista portuguesa de Cirurgia. Serie II, n°4 Marco. 2008.
- Gonzalez-Moreno S, Gonzalez-Bayon LA, Ortega-Perez G. Hyperthermic intraperitoneal chemotherapy: Rationale and technique. World J Gastrointest Oncol. 2010;2(2):68-75.
doi pubmed - de Bree E, Tsiftsis DD. Principles of perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis. Recent Results Cancer Res. 2007;169:39-51.
doi pubmed - Cata JP, Zavala AM, Van Meter A, Williams UU, Soliz J, Hernandez M, Owusu-Agyemang P. Identification of risk factors associated with postoperative acute kidney injury after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: a retrospective study. Int J Hyperthermia. 2018;34(5):538-544.
doi pubmed - Naffouje SA, Tulla KA, Chorley R, Armstrong N, Salti GI. Acute kidney injury increases the rate of major morbidities in cytoreductive surgery and HIPEC. Ann Med Surg (Lond). 2018;35:163-168.
doi pubmed - Chen CY, Chang HY, Lu CH, Chen MC, Huang TH, Lee LW, Liao YS, et al. Risk factors of acute renal impairment after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia. 2020;37(1):1279-1286.
doi pubmed - Arjona-Sanchez A, Cadenas-Febres A, Cabrera-Bermon J, Munoz-Casares FC, Casado-Adam A, Sanchez-Hidalgo JM, Lopez-Andreu M, et al. "Assessment of RIFLE and AKIN criteria to define acute renal dysfunction for HIPEC procedures for ovarian and non ovarian peritoneal malignances". Eur J Surg Oncol. 2016;42(6):869-876.
doi pubmed - Ceresoli M, Coccolini F, Ansaloni L. HIPEC and nephrotoxicity: A cisplatin induced effect? Eur J Surg Oncol. 2016;42(6):909-910.
doi pubmed - Kellum JA, Lameire N, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012 Mar;2(1):1-138.
doi - Hendrix RJ, Damle A, Williams C, Harris A, Spanakis S, Lambert DH, Lambert LA. Restrictive intraoperative fluid therapy is associated with decreased morbidity and length of stay following hyperthermic intraperitoneal chemoperfusion. Ann Surg Oncol. 2019;26(2):490-496.
doi pubmed - Chawla A, Zhu CC, Backer G, O'Gara J, Fong ZV, Deng H, Bao X, et al. Perioperative management of patients undergoing CRS and HIPEC. Clin Surg. 2020;5:2788.
- Chua TC, Yan TD, Saxena A, Morris DL. Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure? A systematic review of morbidity and mortality. Ann Surg. 2009;249(6):900-907.
doi pubmed - Simkens GA, van Oudheusden TR, Braam HJ, Luyer MD, Wiezer MJ, van Ramshorst B, Nienhuijs SW, et al. Treatment-related mortality after cytoreductive surgery and HIPEC in patients with colorectal peritoneal carcinomatosis is underestimated by conventional parameters. Ann Surg Oncol. 2016;23(1):99-105.
doi pubmed - Randle RW, Ahmed S, Levine EA, Fino NF, Swett KR, Stewart JH, Shen P, et al. Significance of diabetes on morbidity and mortality following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Surg Oncol. 2015;111(6):740-745.
doi pubmed - Turgeon MK, Gamboa AC, Lee RM, Zaidi MY, Kimbrough C, Grotz T, Fournier K, et al. The intersection of age and tumor biology with postoperative outcomes in patients after cytoreductive surgery and HIPEC. Ann Surg Oncol. 2020;27(13):4894-4907.
doi pubmed - Simkens GA, van Oudheusden TR, Luyer MD, Nienhuijs SW, Nieuwenhuijzen GA, Rutten HJ, de Hingh IH. Predictors of severe morbidity after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis. Ann Surg Oncol. 2016;23(3):833-841.
doi pubmed - Hakeam H, Ayman A, Waleed AT, Amen T. Systemic complications of the bidirectional intraoperative chemotherapy with intravenous ifosfamide and hyperthermic intraperitoneal chemotherapy (HIPEC) using cisplatin plus doxorubicin. Pleura Peritoneum. 2019;4(4):20190025.
doi pubmed - Kusamura S, Baratti D, Younan R, Laterza B, Oliva GD, Costanzo P, Favaro M, et al. Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol. 2007;14(9):2550-2558.
doi pubmed - Glehen O, Osinsky D, Cotte E, Kwiatkowski F, Freyer G, Isaac S, Trillet-Lenoir V, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10(8):863-869.
doi pubmed - Bouhadjari N, Gabato W, Calabrese D, Msika S, Keita H. Hyperthermic intraperitoneal chemotherapy with cisplatin: Amifostine prevents acute severe renal impairment. Eur J Surg Oncol. 2016;42(2):219-223.
doi pubmed - Bakrin N, Bereder JM, Decullier E, Classe JM, Msika S, Lorimier G, Abboud K, et al. Peritoneal carcinomatosis treated with cytoreductive surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol. 2013;39(12):1435-1443.
doi pubmed - Schmidt U, Dahlke MH, Klempnauer J, Schlitt HJ, Piso P. Perioperative morbidity and quality of life in long-term survivors following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2005;31(1):53-58.
doi pubmed - Sin EI, Chia CS, Tan GHC, Soo KC, Teo MC. Acute kidney injury in ovarian cancer patients undergoing cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. Int J Hyperthermia. 2017;33(6):690-695.
doi pubmed - Angeles MA, Quenet F, Vieille P, Gladieff L, Ruiz J, Picard M, Migliorelli F, et al. Predictive risk factors of acute kidney injury after cytoreductive surgery and cisplatin-based hyperthermic intra-peritoneal chemotherapy for ovarian peritoneal carcinomatosis. Int J Gynecol Cancer. 2019;29(2):382-391.
doi pubmed - Owusu-Agyemang P, Arunkumar R, Green H, Hurst D, Landoski K, Hayes-Jordan A. Anesthetic management and renal function in pediatric patients undergoing cytoreductive surgery with continuous hyperthermic intraperitoneal chemotherapy (HIPEC) with cisplatin. Ann Surg Oncol. 2012;19(8):2652-2656.
doi pubmed - Sheridan AM. Epidemiology of acute kidney injury (AKI). Nephrol Self Assess Program. 2022;21(1):6-11.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


