| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 6, December 2023, pages 476-487
Construction and Validation of a Novel Nomogram for Predicting the Risk of Metastasis in a Luminal B Type Invasive Ductal Carcinoma Population
Xu Dong Zhua, b, d, Jia Hui Yuc, Fu Lu Aia, Yue Wanga, Wu Lva, Gui Lin Yua, Xian Kui Caoa, Jie Lina, d
aDepartment of General Surgery, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang 110042, Liaoning Province, China
bDepartment of Oncology, Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning Province, China
cDepartment of Ultrasound, Shengjing Hospital of China Medical University, Shenyang 110004, Liaoning Province, China
dCorresponding Author: Xu Dong Zhu and Jie Lin, Department of General Surgery, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang 110042, Liaoning Province, Chinaand
Manuscript submitted February 4, 2023, accepted October 25, 2023, published online November 3, 2023
Short title: Nomogram for Predicting Breast Cancer Metastasis
doi: https://doi.org/10.14740/wjon1553
| Abstract | ▴Top |
Background: Postoperative distant metastasis is the main cause of death in breast cancer patients. We aimed to construct a nomogram to predict the risk of metastasis of luminal B type invasive ductal carcinoma.
Methods: We applied the data of 364 luminal B type breast cancer patients between 2008 and 2013. Patients were categorized into modeling group and validation group randomly (1:1). The breast cancer metastasis nomogram was developed from the logistic regression model using clinicopathological variables. The area under the receiver-operating characteristic curve (AUC) was calculated in modeling group and validation group to evaluate the predictive accuracy of the nomogram.
Results: The multivariate logistic regression analysis showed that tumor size, No. of the positive level 1 axillary lymph nodes, human epidermal growth factor receptor 2 (HER2) status and Ki67 index were the independent predictors of the breast cancer metastasis. The AUC values of the modeling group and the validation group were 0.855 and 0.818, respectively. The nomogram had a well-fitted calibration curve. The positive and negative predictive values were 49.3% and 92.7% in the modeling group, and 47.9% and 91.0% in the validation group. Patients who had a score of 60 or more were thought to have a high risk of breast cancer metastasis.
Conclusions: The nomogram has a great predictive accuracy of predicting the risk of breast cancer metastasis. If patients had a score of 60 or more, necessary measures, like more standard treatment methods and higher treatment adherence of patients, are needed to take to lower the risk of metastasis and improve the prognosis.
Keywords: Nomogram; Luminal B type breast cancer; Breast cancer metastasis; Prognosis prediction
| Introduction | ▴Top |
Breast cancer is one of the malignant tumors with the highest morbidity, greatly threatening the health of women [1-3]. The Journal of CA has published 250,000 emerging invasive breast cancers and 40,000 deaths owing to breast cancer in American women just in 2017. The risk of breast cancer is 12.4% in a lifetime of American women [4, 5]. Although the development of the molecular subtyping of breast cancer and the improvement of the treatment strategies have ameliorated the prognosis of breast cancer, breast cancer metastasis is still the main cause of the death of breast cancer patients [2, 6-8].
Among the different molecular subtypings, the luminal B type breast cancer has a worse disease-free survival (DFS) and overall survival at 5 or 10 years [9-11]. Luminal B type breast cancer is defined as estrogen receptor (ER) positive, progesterone receptor (PR) negative and the high expression of Ki67 under the condition of human epidermal growth factor receptor 2 (HER2) negative status or ER positive, any status of PR and Ki67 expression under the condition of HER2 positive status [12, 13]. It is the type that most possibly causes the organ-specific metastasis, like bone, brain, lung and other organs [14, 15]. At the 5-year follow-up, the basal-like type has the worst prognosis. But in the case of 10 years, the luminal B type may have a worse survival outcome. In addition, the luminal B type breast cancer represents 30% of all diagnosed breast cancers [16, 17]. Li et al also found a higher proportion of bone metastasis in luminal B breast cancer patients than in non-luminal group breast cancer patients. The risk of breast cancer metastasis in luminal B breast cancer patients is still increased during a 2- to 5-year period and after 5 years, but the risk in non-luminal breast cancer patients is obviously decreased during the same periods [16]. Besides, the chemotherapy and endocrine therapy cannot greatly improve the prognosis of luminal B breast patients at present. Many luminal B breast cancer patients are confronted with drug resistance [18, 19]. Therefore, for improving the outcome of luminal B type breast cancer, breast cancer metastasis should be predicted as early as possible.
Currently, the nomograms for predicting breast cancer metastasis are mainly focused on lymph node metastasis [20, 21]. As for the metastasis of other organs or positions, it is often found the moment the metastasis appears after the operation. A few of researches used the clinicopathological characteristics to predict the breast cancer metastasis [22-24]. In this paper, tumor size, No. of positive level 1 axillary lymph nodes (PL-1-ALNs), HER2 status and Ki67 index were used to construct a nomogram to predict the risk of luminal B type breast cancer metastasis.
Clinically, if patients are diagnosed as having a PL-1-ALN, the common surgical procedure includes axillary lymph node dissection (ALND) [25, 26]. Therefore, to control the influence of different operation strategies on the risk of breast cancer metastasis, all the patients included in the study underwent the original tumor’s excision and level 1 and level 2 ALND. In other words, all the patients’ level 1 axillary lymph nodes included in these two models are positive. By using the nomogram, if certain patient has a high risk of breast cancer metastasis, the therapy strategies can be changed appropriately and reviewed after operation timely. Eventually, the survival time of these patients may be increased and their quality of life may be improved. To our knowledge, this is the first nomogram for predicting the risk of luminal B type breast cancer metastasis.
| Materials and Methods | ▴Top |
Patients
We collected data of 986 breast cancer patients between 2008 and 2013 at the China Medical University. Among them, 405 patients had luminal B type breast cancer. After excluding the patients who did not meet the following criteria, data of 364 patients with invasive ductal carcinomas of no special type were used to construct the nomogram. The median follow-up period of these 364 patients with luminal B type breast cancer was 56.9 months. Because all of these patients included in the research had luminal B type breast cancer with ER positive status, the ER status did not need to be analyzed.
The inclusion criteria were: 1) PL-1-ALN; 2) luminal B type invasive ductal carcinoma; 3) complete immunohistochemistry (IHC) information including ER, PR, HER2, and Ki67; 4) patients combined with local recurrence and distant metastasis. The exclusion criteria were: 1) lacking of data; 2) negative level 1 axillary lymph node; 3) other cancer types except luminal B type invasive ductal cancer; 4) receiving the neoadjuvant chemotherapy. Patients were categorized into modeling group and validation group randomly (1:1). The occurrence of the metastasis was identified by the results of the imaging examinations or the follow-up information that was obtained by telephone or interviewing directly with patients in the outpatient settings. The project was approved by Ethics Committee of China Medical University. The institution review board (IRB) number is 2018PS336K. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Treatment
Rational surgical treatment was executed according to the guidelines of China. Operations included the excision of the original tumor and level 1 and level 2 ALND. The level 1 and level 2 lymph nodes’ histological status and quality were analyzed. For postoperative drug therapy, all these patients with luminal B type breast cancer have received standard treatment options according to the National Comprehensive Cancer Network guidelines of China, including chemotherapy, endocrine therapy and anti-HER2 therapy for HER2-positive patients.
Data extraction
The following variables were used in this research: age, menopausal status, tumor size, No. of PL-1-ALN, level 2 axillary lymph node status, histological grade, PR status, HER2 status, and Ki67 index.
Pathological evaluation
The guidelines of China were applied to extract the surgical tumors, including the histological grade, PR status, HER2 status and the Ki67 index. ER and PR were graded 0 if there was no expression, 1+ if the expression was 0-10%, 2+ if the expression was 10-50%, and 3+ if the expression was ≥ 50%. Grades 2+ and 3+ were considered positive and 1+ or 0 was considered negative [27]. Similar standards were also used for HER2. HER2 positivity was defined as IHC score of 3+ or fluorescence in situ hybridization (FISH) 2+ with amplification [28]. The cutoff value of Ki67 expression was 20%. The expression ratio > 20% was regarded as Ki67 high expression, and the expression ratio ≤ 20% was regarded as Ki67 low expression [29, 30]. The typical pictures are shown in Figure 1.
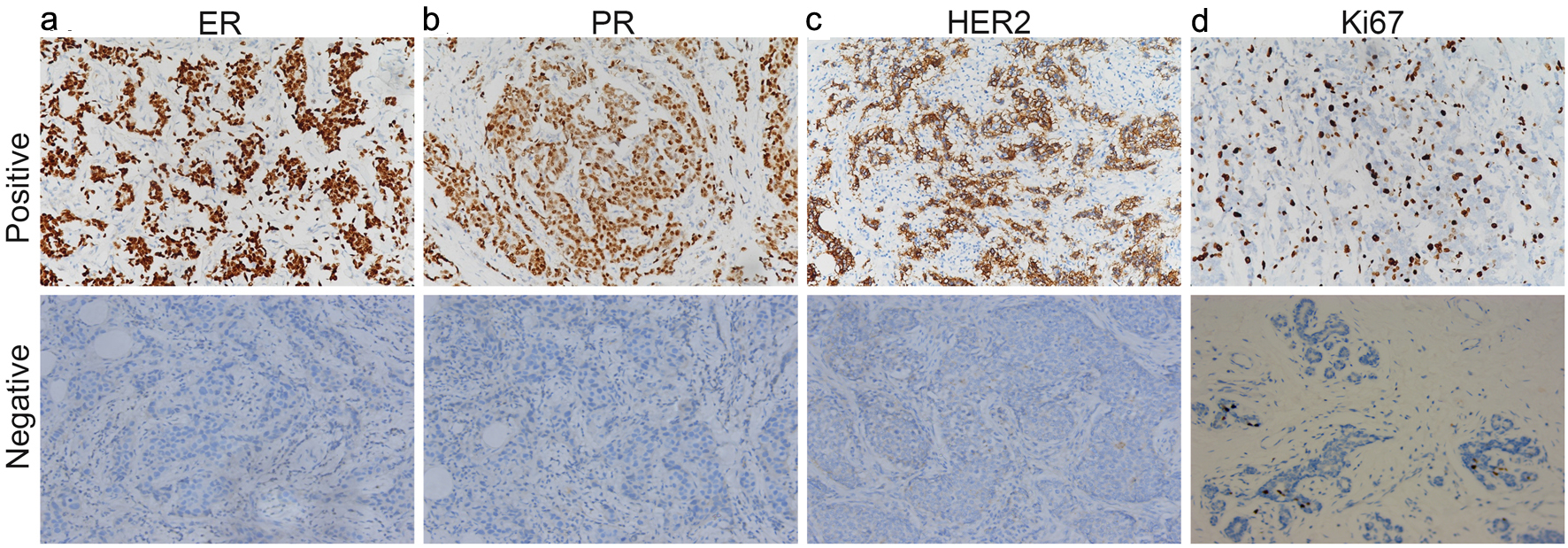 Click for large image | Figure 1. ER (a), PR (b), HER2 (c) and Ki67 (d) were found positive expression and negative expression in breast cancer tissues. ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2. |
Statistical analysis
For categorical data, Chi-square test was applied. As for independent samples’ between-group comparisons, independent sample t-test was used. For construction of the nomogram, univariate logistic regression analysis of the nine factors in the modeling group described above was applied to find factors that were associated with breast cancer metastasis. Variables which were statistically significant after univariate logistic regression were incorporated into multivariate logistic regression. Then independent predictors which were statistically significant in the multivariate logistic regression analysis were obtained using a forward selection procedure. These independent predictors were utilized to construct the nomogram of predicting breast cancer metastasis. Then the receiver-operating characteristic (ROC) curve was obtained and the accuracy of the nomogram was appraised by area under the ROC curve (AUC). According to the overall rules, a model whose AUC was 0.7 - 0.9 was accepted. The goodness of fit was appraised by Hosmer-Lemeshow test. It showed that the nomogram was well suited if P > 0.05. A calibration plot was also drawn to describe the connection between actual probability and predicted probability [31, 32].
For clinical use of the nomogram, the ROC curve was applied to determine the optimal cutoff values calculated by the Youden index (sensitivity + specificity - 1) [33]. And the optimal cutoff value’s accuracy was evaluated by the sensitivity, specificity, positive predictive values, negative predictive values, positive likelihood ratios and negative likelihood ratios. For constructing the nomograms which could predict the probability of breast cancer metastasis at specific stage, Cox regression analysis was also used. All the P values were two-sided. P < 0.05 was believed statistically significant. Statistical analyses were calculated by SPSS 17.0 and R software (version 3.1.0).
| Results | ▴Top |
Clinicopathological characteristics
The research included 364 breast cancer patients. Their clinicopathological characteristics are shown in Table 1. Because these patients have luminal B type breast cancer, all of their ER status is positive. Therefore, the ER positive status is not shown in Table 1. The breast cancer metastasis rate of both groups is 24.17% (44/182). In these patients with metastasis, the most common organ is bone. However, there is still metastasis of other organs, e.g., lung, liver and so on. The variables in the two groups did not differ significantly (P > 0.05). The consequence of univariate logistic regression analysis of the modeling group is depicted in Table 2. After the univariate logistic regression analysis, the tumor size, No. of PL-1-ALN, HER2 status and Ki67 index were then included in the multivariate logistic regression analysis. The results of this analysis showed that the tumor size, No. of PL-1-ALN, HER2 status and Ki67 index were independently predictors used to construct the nomogram. The results of the multivariate logistic regression analysis are shown in Table 3.
 Click to view | Table 1. Comparison of Clinicopathological Characteristics of Modeling and Validation Groups for the Breast Cancer Metastasis Nomogram |
 Click to view | Table 2. Univariate Logistic Regression Analysis of Different Variables Predicting Breast Cancer Metastasis in the Modeling Group |
 Click to view | Table 3. Results of Multivariate Logistic Regression Testing the Association of Each Variable With the Breast Cancer Metastasis |
Construction of the breast cancer metastasis nomogram
To evaluate the risk of breast cancer metastasis, based on the results of Table 3, the equation was constructed: ln(p/1 - p) = 1.101 × A + 0.793 × B + 1.823 × C + 1.279 × D - 6.383. The “p” represents the risk of metastasis [34, 35], “A” represents the No. of PL-1-ALN (1 if 1 or 2 PL-1-ALNs, 2 if 3 or 4 PL-1-ALNs, 3 if ≥ 5 PL-1-ALNs), “B” represents the tumor size, “C” represents HER2 status, and “D” represents Ki67 index. The nomogram is established by the consequences of multivariate logistic regression (Fig. 2).
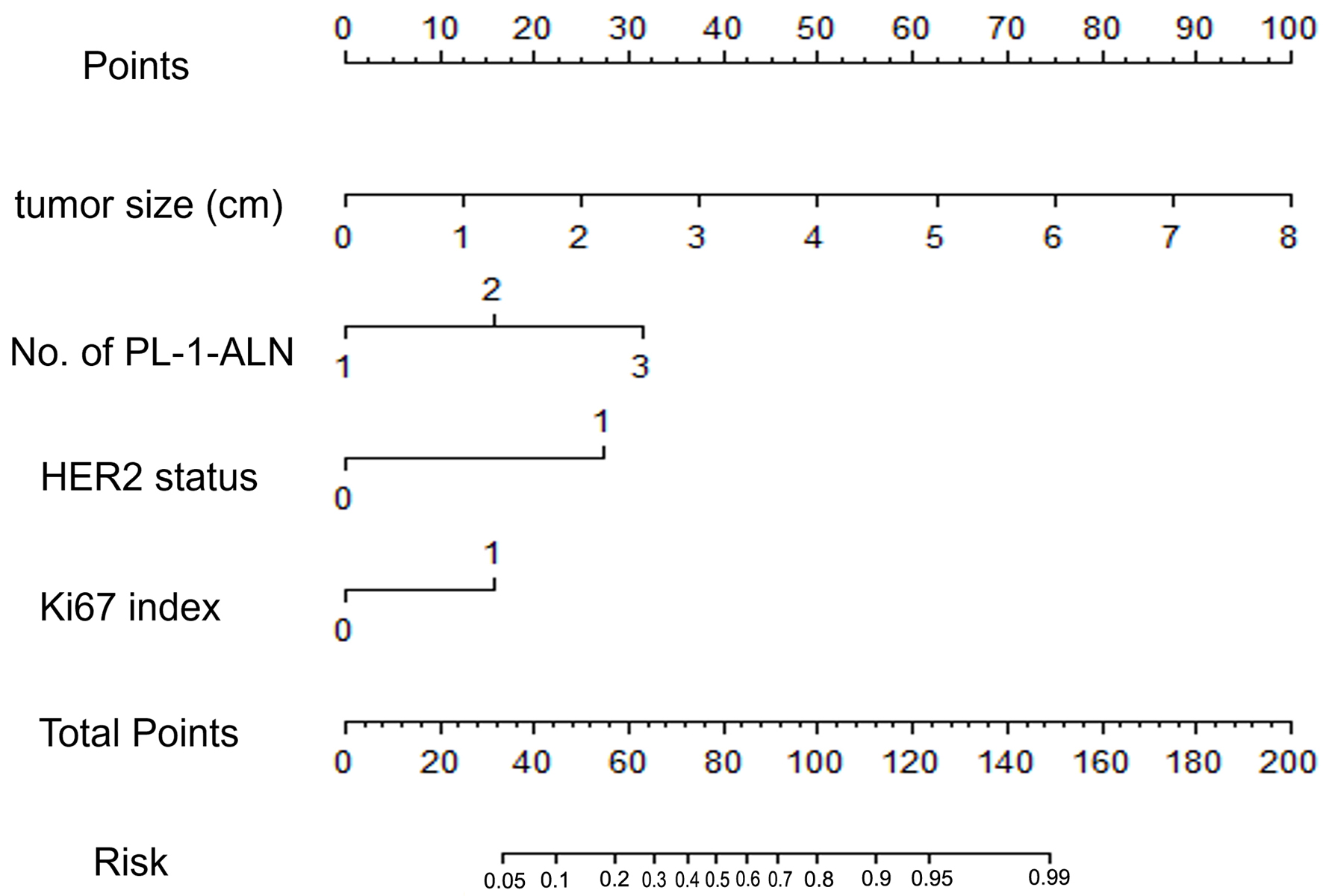 Click for large image | Figure 2. A nomogram for predicting the metastasis of breast cancer in a luminal B type invasive ductal carcinoma population. The nomogram is composed by seven rows. The first one is the point assignment for every variable. For a patient, every variable is assigned a value depending on the variables by painting a vertical line between the exact variable value and points line. As a consequence, the total points can be gained by pulsing all of the points for the four variables. Finally, the predictive probability of the luminal B type breast cancer metastasis can be gained by painting a vertical line between total points and risk (the final row). About the risk, from left to right, it refers to 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 0.95, and 0.99. |
Validation of the breast cancer metastasis nomogram
The ROC curves of the modeling group (Fig. 3a) and the validation group (Fig. 3b) were utilized to evaluate the accuracy of the nomogram. In the modeling group, the AUC of the ROC curve was 0.855, which represented good accuracy in predicting the risk of breast cancer metastasis. The result of Hosmer-Lemeshow test showed that the nomogram fitted well (P = 0.147). Calibration plots graphically (Fig. 3c) indicated that the accuracy of the nomogram is excellent. In the validation group, the AUC of the ROC curve was 0.818, which also represented good accuracy.
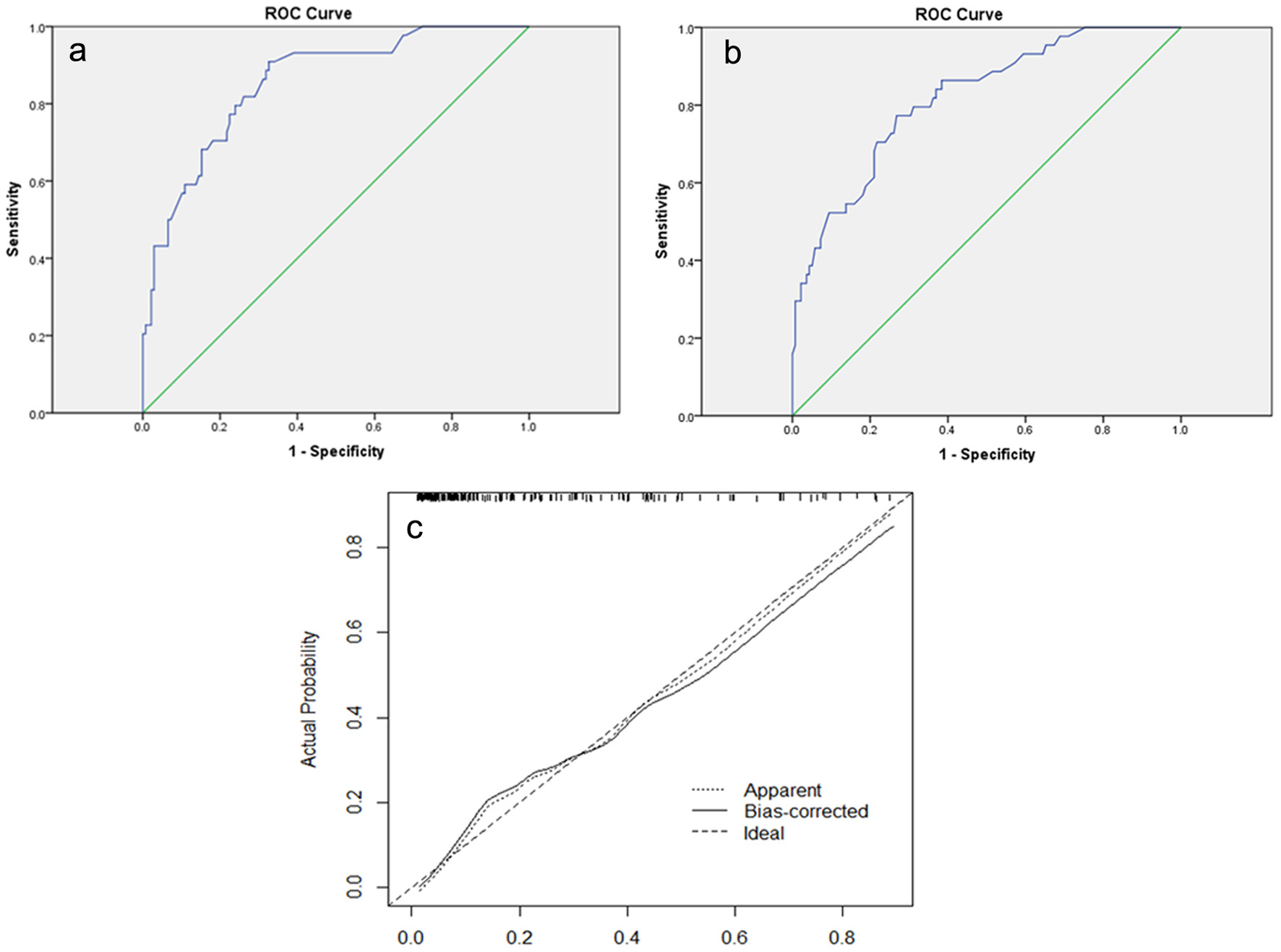 Click for large image | Figure 3. (a) The ROC curve of the modeling group. The AUC is 0.855 (95% CI: 0.793 - 0.917). (b) The ROC curve of the validation group. The AUC is 0.818 (95% CI: 0.747 - 0.888). (c) Calibration plot of the nomogram for predicting the probability of breast cancer metastasis. ROC: receiver-operating characteristic; AUC: area under the ROC curve; CI: confidence interval. |
Predictive values of the breast cancer metastasis nomogram at the optimal cutoff value
We calculated every score of these 364 patients with breast cancer. After that, according to the Youden index, the optimal cutoff value was determined to be 60 in the modeling group and the validation group. The sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, positive predictive value and negative predictive value when used in predicting the breast cancer metastasis were 81.8%, 73.9%, 3.1, 0.25, 50.0% and 92.7% in the modeling group, and 77.3%, 73.2%, 2.9, 0.31, 47.9% and 91.0% in the validation group (Table 4).
 Click to view | Table 4. Predictive Values of the Breast Cancer Metastasis Nomogram at the Optimal Cutoff Value in the Modeling Group and the Validation Group |
Construction and validation of the nomograms which can predict the probability of breast cancer metastasis at specific stage
To further clarify whether these 364 included patients recurred at an early stage or late stage, we reconstructed nomograms by Cox regression analysis based on these four clinicopathological characteristics and DFS. DFS was defined from the date of the operation to the date of recurrence/metastases. The survival probability in the nomogram represented the probability of recurrence/metastases.
For the early stage, a nomogram was constructed to predict the 1-, 3-, and 5-year metastasis probability of patients with luminal B type breast cancer using tumor size, No. of PL-1-ALN, HER2 status and Ki67 index (Fig. 4a). Calibration curves were created to predict the metastasis probability for the 1-, 3-, and 5-year periods with reasonable accuracy (Fig. 4b). The predictions made by the nomogram were close to the actual outcomes. Subsequently, the ROC curves and AUC values were calculated (Fig. 4c). The ROC curves are significantly above the diagonal dashed lines, with AUC values above 0.7. The above results showed that this nomogram had significant high diagnosis ability for the early metastasis probability of patients with luminal B type breast cancer. For the late stage, a nomogram was constructed to predict the 6- and 7-year metastasis probability of patients with luminal B type breast cancer using tumor size, No. of PL-1-ALN, HER2 status and Ki67 index (Fig. 4d). Calibration curves were created to predict the metastasis probability for the 6- and 7-year periods with reasonable accuracy (Fig. 4e). The predictions made by the nomogram were close to the actual outcomes. Subsequently, the ROC curves and AUC values were calculated (Fig. 4f). The ROC curves are significantly above the diagonal dashed lines, with AUC values even above 0.8. The above results showed that this nomogram had significant high diagnosis ability for the late metastasis probability of patients with luminal B type breast cancer.
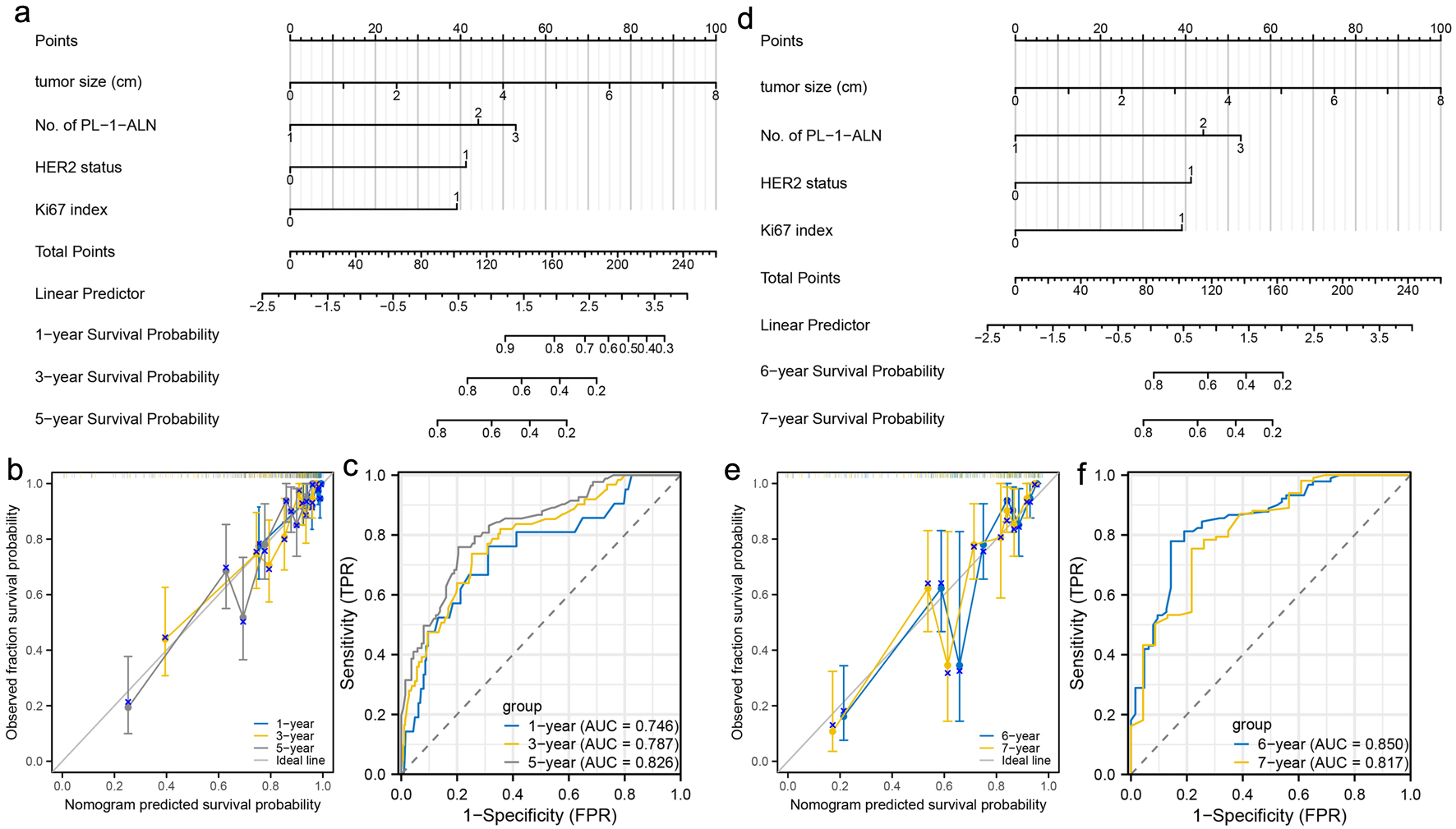 Click for large image | Figure 4. Construction and validation of nomograms which can predict the probability of breast cancer metastasis at specific stage. (a) Prognostic nomogram for the 1-, 3-, and 5-year metastasis probability of patients with luminal B type breast cancer. (b) Calibration curves of the nomogram for predicting 1-, 3-, and 5-year metastasis probability of patients with luminal B type breast cancer. (c) ROC curves and AUC values of the nomogram for predicting 1-, 3-, and 5-year metastasis probability of patients with luminal B type breast cancer. (d) Prognostic nomogram for the 6- and 7-year metastasis probability of patients with luminal B type breast cancer. (e) Calibration curves of the nomogram for predicting 6- and 7-year metastasis probability of patients with luminal B type breast cancer. (f) ROC curves and AUC values of the nomogram for predicting 6- and 7-year metastasis probability of patients with luminal B type breast cancer. ROC: receiver-operating characteristic; AUC: area under the ROC curve. |
For patients with luminal B type breast cancer, hormonal environments are different depending on the menopausal status [36]. Therefore, we have separated the pre-menopausal patients (180/364) and post-menopausal patients (184/364) in these 364 cases. Among different subgroups based on menopausal status, we constructed nomograms which could predict the probability of breast cancer metastasis at specific stage.
For the pre-menopausal patients, a nomogram was constructed to predict the 1-, 3-, and 5-year metastasis probability of patients using tumor size, No. of PL-1-ALN, HER2 status and Ki67 index for the early stage (Fig. 5a). Calibration curves were created to predict the metastasis probability for the 1-, 3-, and 5-year periods with reasonable accuracy (Fig. 5b). The predictions made by the nomogram were close to the actual outcomes. Subsequently, the ROC curves and AUC values were calculated (Fig. 5c). The ROC curves are significantly above the diagonal dashed lines, with AUC values above 0.7. The above results showed that this nomogram had significant high diagnosis ability for the early metastasis probability of patients. For the late stage, a nomogram was constructed to predict the 6- and 7-year metastasis probability of patients using tumor size, No. of PL-1-ALN, HER2 status and Ki67 index (Fig. 5d). Calibration curves were created to predict the metastasis probability for the 6- and 7-year periods with reasonable accuracy (Fig. 5e). The predictions made by the nomogram were close to the actual outcomes. Subsequently, the ROC curves and AUC values were calculated (Fig. 5f). The ROC curves are significantly above the diagonal dashed lines, with AUC values even above 0.8. The above results showed that this nomogram had significant high diagnosis ability for the late metastasis probability of patients.
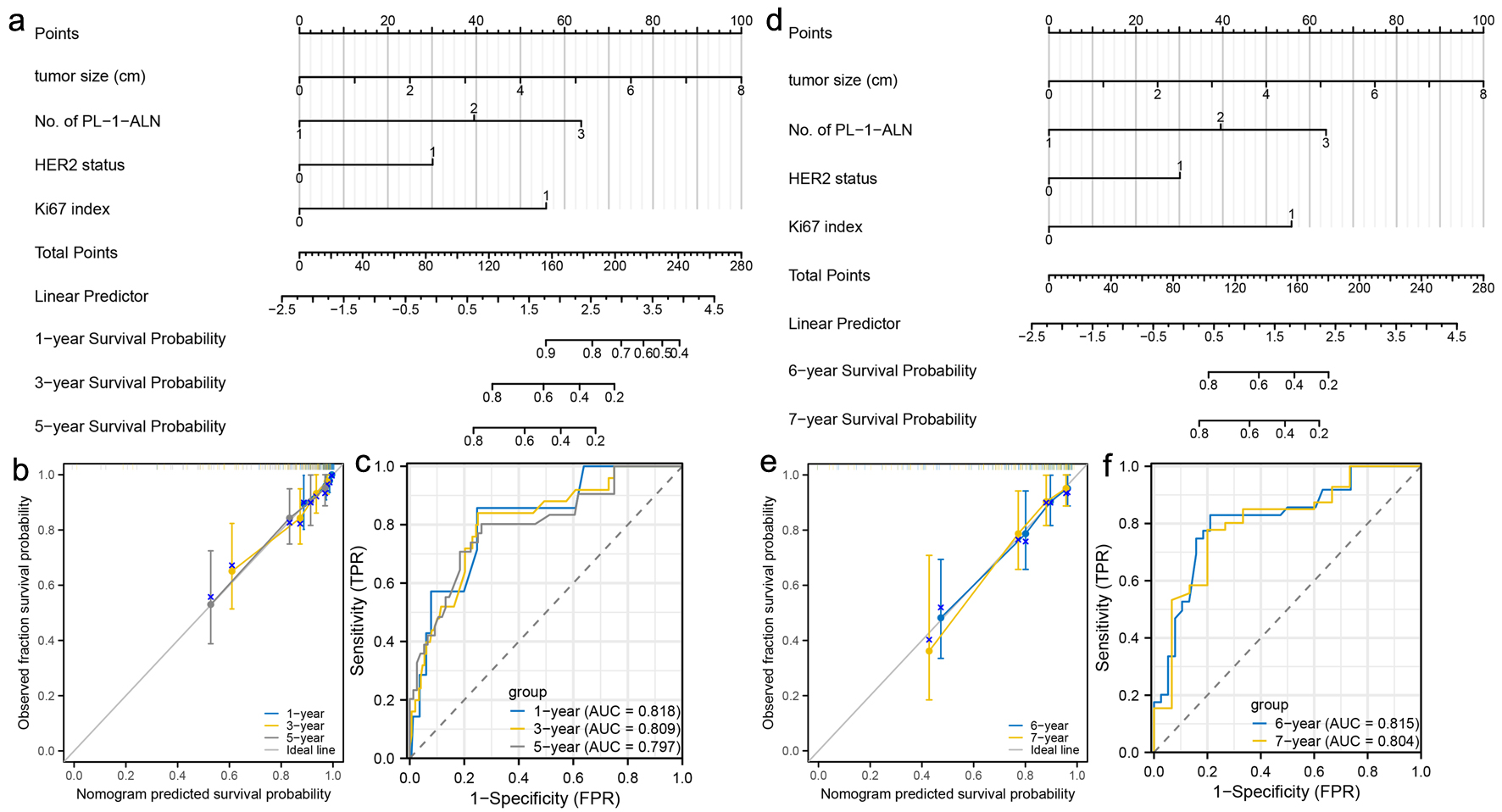 Click for large image | Figure 5. Construction and validation of nomograms which can predict the probability of breast cancer metastasis in pre-menopausal patients. (a) Prognostic nomogram for the 1-, 3-, and 5-year metastasis probability of pre-menopausal patients. (b) Calibration curves of the nomogram for predicting 1-, 3-, and 5-year metastasis probability of pre-menopausal patients. (c) ROC curves and AUC values of the nomogram for predicting 1-, 3-, and 5-year metastasis probability of pre-menopausal patients. (d) Prognostic nomogram for the 6- and 7-year metastasis probability of pre-menopausal patients. (e) Calibration curves of the nomogram for predicting 6- and 7-year metastasis probability of pre-menopausal patients. (f) ROC curves and AUC values of the nomogram for predicting 6- and 7-year metastasis probability of pre-menopausal patients. ROC: receiver-operating characteristic; AUC: area under the ROC curve. |
For the post-menopausal patients, a nomogram was constructed to predict the 1-, 3-, and 5-year metastasis probability of patients using tumor size, No. of PL-1-ALN, HER2 status and Ki67 index for the early stage (Fig. 6a). Calibration curves were created to predict the metastasis probability for the 1-, 3-, and 5-year periods with reasonable accuracy (Fig. 6b). The predictions made by the nomogram were close to the actual outcomes. Subsequently, the ROC curves and AUC values were calculated (Fig. 6c). The ROC curves are significantly above the diagonal dashed lines, with AUC values above 0.7. The above results showed that this nomogram had significant high diagnosis ability for the early metastasis probability of patients. For the late stage, a nomogram was constructed to predict the 6- and 7-year metastasis probability of patients using tumor size, No. of PL-1-ALN, HER2 status and Ki67 index (Fig. 6d). Calibration curves were created to predict the metastasis probability for the 6- and 7-year periods with reasonable accuracy (Fig. 6e). The predictions made by the nomogram were close to the actual outcomes. Subsequently, the ROC curves and AUC values were calculated (Fig. 6f). The ROC curves are significantly above the diagonal dashed lines, with AUC values even above 0.8. The above results showed that this nomogram had significant high diagnosis ability for the late metastasis probability of patients.
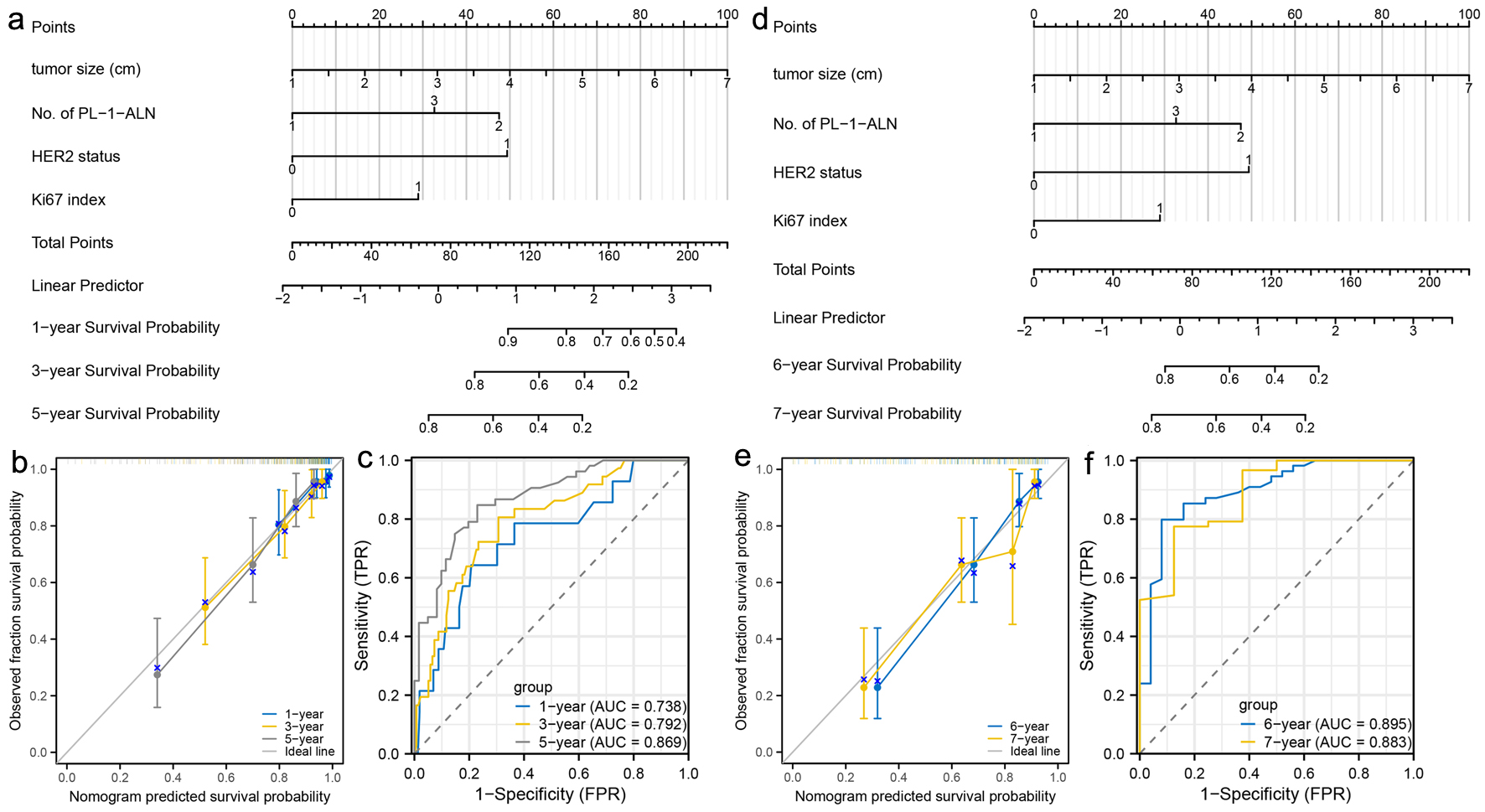 Click for large image | Figure 6. Construction and validation of nomograms which can predict the probability of breast cancer metastasis in post-menopausal patients. (a) Prognostic nomogram for the 1-, 3-, and 5-year metastasis probability of post-menopausal patients. (b) Calibration curves of the nomogram for predicting 1-, 3-, and 5-year metastasis probability of post-menopausal patients. (c) ROC curves and AUC values of the nomogram for predicting 1-, 3-, and 5-year metastasis probability of post-menopausal patients. (d) Prognostic nomogram for the 6- and 7-year metastasis probability of post-menopausal patients. (e) Calibration curves of the nomogram for predicting 6- and 7-year metastasis probability of post-menopausal patients. (f) ROC curves and AUC values of the nomogram for predicting 6- and 7-year metastasis probability of post-menopausal patients. ROC: receiver-operating characteristic; AUC: area under the ROC curve. |
| Discussion | ▴Top |
Breast cancer is a kind of heterogeneous diseases. Different molecular classification of breast cancer has different biological behavior and prognosis [37]. Although the treatment of breast cancer has made great progress, there still is 20-30% metastasis developed in breast cancer patients [38, 39]. Luminal B type breast cancer particularly with HER2 positive was obviously connected with poor DFS and disease-specific survival regardless of the treatment strategy [11, 40]. Tumor size, lymph node status, histological stage, PR status, HER2 status and others are all predictors of breast cancer metastasis [41-44]. But there are a few models that can predict the exact risk of breast cancer metastasis using these clinicopathological characteristics before we found the metastasis after the surgery.
Jin et al have made a nomogram for predicting the risks of distant failures in patients with invasive breast cancer [21]. In the nomogram, they found age, molecular subtypes, T stage and N stage were predictors of distant failure. But they did not make full use of all the clinicopathological characteristics and further validate the accuracy of the nomogram like using the ROC curve or setting a validation group. Besides this model, we did not find any other nomograms predicting the metastasis.
In our nomogram, we found four independent predictors to predict the metastasis of luminal B type invasive ductal carcinoma. It is the first model to use the clinicopathological variables to predict the risk of breast cancer metastasis. With the larger tumor size, greater No. of PL-1-ALN, positive HER2 status and the higher Ki67 index, patients are more likely to develop breast cancer metastasis. The AUC of the ROC curve is 0.855 in the modeling group and 0.818 in the validation group. Generally, the AUC of the ROC curve is accepted between 0.7 and 0.9. And the four predictors are easily available before and after the surgery. The consequence of the research showed that our nomogram was useful and reliable in a Chinese breast cancer population. Indeed, there are still some researches constructing nomograms to predict the survival percent of breast cancer patients in 1, 3, 5 or 10 years after the operation [45-47]. However, the main goal of our study was only to predict the risk of breast cancer metastasis occurring after surgery. The key point is not when it will occur. And this is the main difference between our study and others.
For the clinical significance of the nomogram, according to the Youden index, sensitivity, specificity, positive predictive value and negative predictive value, 60 was determined as the cutoff value. The breast cancer patients with the score of 60 or more are thought to have a high risk of metastasis. In the nomogram, score of 60 corresponds to that the risk of distant metastasis is 25% which is almost the normal metastasis risk reported by most studies [38, 48, 49]. Based on these clinicopathological variables, the nomogram can be a useful tool to predict the probability of metastasis in these luminal B type breast cancer patients; if the score is over 60, the doctor needs to tell the patient to reduce the interval between periodic reviews and prolong the time of endocrine therapy appropriately.
The reduction of the risk of breast cancer metastasis may have a great improvement in survival outcomes of breast cancer patients [50-52]. If we can get the probability of breast cancer metastasis before it occurs and take some measures to deal with it, the patients may have a better survival outcome and a high quality of life. This nomogram provides a new way for predicting the probability of breast cancer metastasis and the accuracy of predicting the metastasis would be increased by using this nomogram.
The nomogram also has some limitations. First, our nomogram was constructed by a retrospective, single-institution research. The nomogram needs to be further validated in other centers. Second, only these patients with invasive ductal carcinoma were included in the research. It reduced the scope of the nomogram’s application. Prospective research with larger samples is needed to further validate the nomogram. Third, although the nomogram has a great predictive accuracy, with a cutoff score of 60, the false-negative rate and false-positive rate are 18.2% and 26.8% in the modeling group and 22.7% and 26.8% in the validation group for predicting the risk of breast cancer metastasis. Therefore, it also needs a prospective study with larger samples to improve the predictive value of the nomogram. Finally, there must be other factors including the specific treatment options which can also affect the survival outcomes of patients with breast cancer. This point is also an important limitation of our study. Therefore, in the future, we will focus on the impact of different treatment options on the survival outcomes of patients with same molecular subtype and screen other important factors to construct a more comprehensive nomogram.
In short, in this study, we constructed a nomogram which had a great predictive accuracy of predicting the risk of luminal B type breast cancer patients’ metastasis. If the patient had a score of 60 or more, necessary measures, like more standard treatment methods and higher treatment adherence of patients, are needed to take to lower the risk of breast cancer metastasis and improve the prognosis.
Acknowledgments
Thanks for the help of Prof. Cai Gang Liu from Shengjing Hospital of China Medical University in completing the study and preparing the manuscript.
Financial Disclosure
This research was supported by the Natural Science Foundation of Liaoning Province (2020-MS-178).
Conflict of Interest
The authors declare that they do not have any competing interests.
Informed Consent
Not applicable.
Author Contributions
Xu Dong Zhu and Jie Lin designed this research. Xu Dong Zhu, Jia Hui Yu, Fu Lu Ai, Yue Wang, Wu Lv, Gui Lin Yu, and Xian Kui Cao analyzed the data and constructed this nomogram. Xu Dong Zhu and Jie Lin wrote this paper. All authors have read and approved this final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30.
doi pubmed - Woolston C. Breast cancer. Nature. 2015;527(7578):S101.
doi pubmed - Tichy JR, Lim E, Anders CK. Breast cancer in adolescents and young adults: a review with a focus on biology. J Natl Compr Canc Netw. 2013;11(9):1060-1069.
doi pubmed - DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439-448.
doi pubmed - Jokar N, Velikyan I, Ahmadzadehfar H, Rekabpour SJ, Jafari E, Ting HH, Biersack HJ, et al. Theranostic approach in breast cancer: a treasured tailor for future oncology. Clin Nucl Med. 2021;46(8):e410-e420.
doi pubmed - Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747-752.
doi pubmed - Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin Cancer Biol. 2020;60:14-27.
doi pubmed - Redfern A, Martin H. Breast cancer metastasis: mapping long term outcomes in Australia. Med J Aust. 2022;217(8):398-399.
doi pubmed - Micalizzi DS, Maheswaran S. On the trail of invasive cells in breast cancer. Nature. 2018;554(7692):308-309.
doi pubmed - Ades F, Zardavas D, Bozovic-Spasojevic I, Pugliano L, Fumagalli D, de Azambuja E, Viale G, et al. Luminal B breast cancer: molecular characterization, clinical management, and future perspectives. J Clin Oncol. 2014;32(25):2794-2803.
doi pubmed - Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736-750.
doi pubmed pmc - Prat A, Pineda E, Adamo B, Galvan P, Fernandez A, Gaba L, Diez M, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24(Suppl 2):S26-35.
doi pubmed - Pellegrino B, Hlavata Z, Migali C, De Silva P, Aiello M, Willard-Gallo K, Musolino A, et al. Luminal breast cancer: risk of recurrence and tumor-associated immune suppression. Mol Diagn Ther. 2021;25(4):409-424.
doi pubmed pmc - Dowsett M, Sestak I, Lopez-Knowles E, Sidhu K, Dunbier AK, Cowens JW, Ferree S, et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after endocrine therapy. J Clin Oncol. 2013;31(22):2783-2790.
doi pubmed - He L, Zhao S, Liu M, Su Z, Ren Y, Song Y. The reciprocal influences of prognosis between two types of surgical interventions and early breast cancer patients with diverse luminal subtypes: A meta-analysis. Medicine (Baltimore). 2019;98(11):e14912.
doi pubmed pmc - Li ZH, Hu PH, Tu JH, Yu NS. Luminal B breast cancer: patterns of recurrence and clinical outcome. Oncotarget. 2016;7(40):65024-65033.
doi pubmed pmc - Gong G, Kwon MJ, Han J, Lee HJ, Lee SK, Lee JE, Lee SH, et al. A new molecular prognostic score for predicting the risk of distant metastasis in patients with HR+/HER2- early breast cancer. Sci Rep. 2017;7:45554.
doi pubmed pmc - Choi W, Porten S, Kim S, Willis D, Plimack ER, Hoffman-Censits J, Roth B, et al. Identification of distinct basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25(2):152-165.
doi pubmed pmc - Bi M, Zhang Z, Jiang YZ, Xue P, Wang H, Lai Z, Fu X, et al. Enhancer reprogramming driven by high-order assemblies of transcription factors promotes phenotypic plasticity and breast cancer endocrine resistance. Nat Cell Biol. 2020;22(6):701-715.
doi pubmed pmc - Wang W, Wang X, Liu J, Zhu Q, Wang X, Wang P. Nomogram for predicting axillary lymph node pathological response in node-positive breast cancer patients after neoadjuvant chemotherapy. Chin Med J (Engl). 2021;135(3):333-340.
doi pubmed pmc - Jin X, Jiang YZ, Chen S, Shao ZM, Di GH. A Nomogram for Predicting the Pathological Response of Axillary Lymph Node Metastasis in Breast Cancer Patients. Sci Rep. 2016;6:32585.
doi pubmed pmc - Lv M, Li J, Guo H, Wang C, Tian P, Ma Y, Chen X, et al. Impact of ipsilateral supraclavicular lymph node dissection (ISLND) for breast cancer patients and a nomogram for predicting ipsilateral supraclavicular pathological complete response (ispCR). Ann Surg Oncol. 2021;28(9):5098-5109.
doi pubmed - Li S, Zhao J, Zhu L, Su F, Chen K. Development and validation of a nomogram predicting the overall survival of stage IV breast cancer patients. Cancer Med. 2017;6(11):2586-2594.
doi pubmed pmc - Lim YJ, Lee SW, Choi N, Kwon J, Eom KY, Kang E, Kim EK, et al. A novel prognostic nomogram for predicting risks of distant failure in patients with invasive breast cancer following postoperative adjuvant radiotherapy. Cancer Res Treat. 2018;50(4):1140-1148.
doi pubmed pmc - Wu SG, Li FY, Chen Y, Sun JY, Lin HX, Lin Q, He ZY. Therapeutic role of axillary lymph node dissection in patients with stage IV breast cancer: a population-based analysis. J Cancer Res Clin Oncol. 2017;143(3):467-474.
doi pubmed - Wiznia LE, Lannin DR, Evans SB, Hofstatter EW, Horowitz NR, Killelea BK, Tsangaris TN, et al. The number of lymph nodes dissected in breast cancer patients influences the accuracy of prognosis. Ann Surg Oncol. 2014;21(2):389-394.
doi pubmed - Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, et al. Tailoring therapies—improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26(8):1533-1546.
doi pubmed pmc - Perez EA, Dueck AC, McCullough AE, Reinholz MM, Tenner KS, Davidson NE, Gralow J, et al. Predictability of adjuvant trastuzumab benefit in N9831 patients using the ASCO/CAP HER2-positivity criteria. J Natl Cancer Inst. 2012;104(2):159-162.
doi pubmed pmc - Harbeck N, Rastogi P, Martin M, Tolaney SM, Shao ZM, Fasching PA, Huang CS, et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32(12):1571-1581.
doi pubmed - Zhu X, Xue J, Gu X, Chen G, Cao F, Shan H, Wang D, et al. Neoadjuvant chemotherapy plays an adverse role in the prognosis of grade 2 breast cancer. J Cancer. 2019;10(23):5661-5670.
doi pubmed pmc - Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361-387.
doi pubmed - Lemeshow S, Hosmer DW, Jr. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115(1):92-106.
doi pubmed - Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, et al. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis B virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151(4):356-363.
doi pubmed - Liu C, Jiang Y, Gu X, Xu Z, Ai L, Zhang H, Chen G, et al. Predicting level 2 axillary lymph node metastasis in a Chinese breast cancer population post-neoadjuvant chemotherapy: development and assessment of a new predictive nomogram. Oncotarget. 2017;8(45):79147-79156.
doi pubmed pmc - Jiang Y, Xu H, Zhang H, Ou X, Xu Z, Ai L, Sun L, et al. Nomogram for prediction of level 2 axillary lymph node metastasis in proven level 1 node-positive breast cancer patients. Oncotarget. 2017;8(42):72389-72399.
doi pubmed pmc - Van Maele-Fabry G, Lombaert N, Lison D. Dietary exposure to cadmium and risk of breast cancer in postmenopausal women: A systematic review and meta-analysis. Environ Int. 2016;86:1-13.
doi pubmed - Reis-Filho JS, Pusztai L. Gene expression profiling in breast cancer: classification, prognostication, and prediction. Lancet. 2011;378(9805):1812-1823.
doi pubmed - Kennecke H, Yerushalmi R, Woods R, Cheang MC, Voduc D, Speers CH, Nielsen TO, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271-3277.
doi pubmed - Kim MY. Breast cancer metastasis. Adv Exp Med Biol. 2021;1187:183-204.
doi pubmed - Erber R, Hartmann A. Histology of luminal breast cancer. Breast Care (Basel). 2020;15(4):327-336.
doi pubmed pmc - Leone JP, Cole BF, Regan MM, Thurlimann B, Coates AS, Rabaglio M, Giobbie-Hurder A, et al. Clinical behavior of recurrent hormone receptor-positive breast cancer by adjuvant endocrine therapy within the Breast International Group 1-98 clinical trial. Cancer. 2021;127(5):700-708.
doi pubmed - Alanko A, Heinonen E, Scheinin T, Tolppanen EM, Vihko R. Significance of estrogen and progesterone receptors, disease-free interval, and site of first metastasis on survival of breast cancer patients. Cancer. 1985;56(7):1696-1700.
doi pubmed - Chia S, Norris B, Speers C, Cheang M, Gilks B, Gown AM, Huntsman D, et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J Clin Oncol. 2008;26(35):5697-5704.
doi pubmed - Soerjomataram I, Louwman MW, Ribot JG, Roukema JA, Coebergh JW. An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat. 2008;107(3):309-330.
doi pubmed pmc - Su J, Miao LF, Ye XH, Cui MS, He XF. Development of prognostic signature and nomogram for patients with breast cancer. Medicine (Baltimore). 2019;98(11):e14617.
doi pubmed pmc - Xiong Y, Cao H, Zhang Y, Pan Z, Dong S, Wang G, Wang F, et al. Nomogram-predicted survival of breast cancer brain metastasis: a SEER-based population study. World Neurosurg. 2019;128:e823-e834.
doi pubmed - Min Y, Liu X, Hu D, Chen H, Chen J, Xiang K, Yin G, et al. Risk factors, prognostic factors, and nomogram for distant metastasis in breast cancer patients without lymph node metastasis. Front Endocrinol (Lausanne). 2021;12:771226.
doi pubmed pmc - Hsieh SM, Lintell NA, Hunter KW. Germline polymorphisms are potential metastasis risk and prognosis markers in breast cancer. Breast Dis. 2006;26:157-162.
doi pubmed - Tabor S, Szostakowska-Rodzos M, Fabisiewicz A, Grzybowska EA. How to predict metastasis in luminal breast cancer? Current Solutions and Future Prospects. Int J Mol Sci. 2020;21(21):8415.
doi pubmed pmc - Chiang AC, Massague J. Molecular basis of metastasis. N Engl J Med. 2008;359(26):2814-2823.
doi pubmed pmc - Cetintas S, Tezcan G, Tunca B, Egeli U, Gokgoz MS, Cecener G. Prediction of breast cancer metastasis risk using circulating tumor markers: A follow-up study. Bosn J Basic Med Sci. 2019;19(2):172-179.
doi pubmed pmc - Anwar SL, Avanti WS, Nugroho AC, Choridah L, Dwianingsih EK, Harahap WA, Aryandono T, et al. Risk factors of distant metastasis after surgery among different breast cancer subtypes: a hospital-based study in Indonesia. World J Surg Oncol. 2020;18(1):117.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


