| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 6, December 2023, pages 505-517
Development and Validation of Nomograms Based on Nutritional Risk Index for Predicting Extracapsular Extension and Seminal Vesicle Invasion in Patients Undergoing Radical Prostatectomy
Ze Nan Liua, d, Zi Ang Lia, d, Ji De Hea, Jia Long Wua, Lei Qiua, Zhen Kun Zhaoa, Min Lub, Hai Bic, e, Jian Lua, e
aDepartment of Urology, Peking University Third Hospital, Beijing, China
bDepartment of Pathology, Peking University Third Hospital, Beijing, China
cDepartment of Urology, Shanghai General Hospital, Shanghai, China
dThese authors contributed equally to this work.
eCorresponding Author: Jian Lu, Department of Urology, Peking University Third Hospital, Beijing, China; Hai Bi, Department of Urology, Shanghai General Hospital, Shanghai, China
Manuscript submitted August 27, 2023, accepted November 6, 2023, published online November 18, 2023
Short title: Predictive Value of NRI in Prostate Cancer
doi: https://doi.org/10.14740/wjon1718
| Abstract | ▴Top |
Background: The aim of the study was to investigate the predictive value of the nutritional risk index (NRI) for extracapsular extension (ECE) and seminal vesicle invasion (SVI) in prostate cancer (PCa) patients undergoing radical prostatectomy (RP), and further develop and validate predictive nomograms for ECE and SVI based on the NRI.
Methods: We retrospectively analyzed 734 PCa patients who underwent RP between 2010 and 2020 in the Department of Urology at Peking University Third Hospital. The enrolled patients were randomly divided into a primary cohort (n = 489) and a validation cohort (n = 245) in a 2:1 manner. The baseline NRI of patients was calculated using serum albumin level and body mass index, and a malnutrition status was defined as NRI ≤ 98. Univariate and multivariate logistic regression analyses were conducted to identify predictors for ECE and SVI. Nomograms for predicting ECE and SVI were established based on the results of the multivariate logistic regression analysis. The performance of the nomograms was estimated using Harrell’s concordance index (C-index), the area under curve (AUC) of receiver operating characteristic (ROC) curves and the calibration curves.
Results: In the primary cohort, 70 (14.3%) patients with NRI ≤ 98 were classified as malnutrition, while the remaining 419 (85.7%) patients with NRI > 98 were considered to have normal nutrition. The nomograms for predicting ECE and SVI shared common factors including NRI, percentage of positive biopsy cores (PPC) and biopsy Gleason score, while prostate-specific antigen (PSA) levels and PSA density (PSAD) were only incorporated in ECE nomogram. The C-indexes of the nomograms for predicting ECE and SVI were 0.785 (95% confidence interval (CI): 0.745 - 0.826) and 0.852 (95% CI: 0.806 - 0.898), respectively. The calibration curves demonstrated excellent agreement between the predictions by the nomograms and the actual observations. The results remained reproducible when the nomograms were applied to the validation cohort.
Conclusions: The NRI is significantly associated with ECE and SVI in PCa patients. The nomogram established based on the NRI in our study can provide individualized risk estimation for ECE and SVI in PCa patients, and may be valuable for clinicians in making well-informed decisions regarding treatment strategies and patient management.
Keywords: Prostate cancer; Radical prostatectomy; Nutritional risk index; Extracapsular extension; Seminal vesicle invasion; Nomogram
| Introduction | ▴Top |
Prostate cancer (PCa) is one of the most common malignant tumors of the urogenital system and ranks as the second leading cause of male cancer-related deaths worldwide. It is projected that there will be approximately 288,300 new cases of PCa and 34,700 PCa-related deaths in the United States by 2023 [1]. Radical prostatectomy (RP) has emerged as the gold standard treatment for localized PCa due to its superior efficacy in cancer control and survival benefits [2]. However, the presences of extracapsular extension (ECE) and seminal vesicle invasion (SVI) by PCa are recognized as significant risk factors for unfavorable oncologic outcome following RP [3]. Consequently, it is crucial to identify PCa patients at a high risk for ECE and SVI before RP in order to develop appropriate surgical treatment strategies, such as avoiding positive surgical margin (PSM), as well as maximizing the preservation of erectile and urinary control function in cases where the neurovascular bundle (NVB) can be preserved. Additionally, identifying high risk patients can aid in determining the need for adjuvant treatment [4, 5]. Various clinical parameters, including prostate-specific antigen (PSA) levels, digital rectal examination (DRE) findings, biopsy Gleason score and multiparametric magnetic resonance imaging (mp-MRI), have been identified as useful tools in predicting adverse pathological features such as ECE and SVI in PCa patients [6, 7]. Several multivariable prediction tools based on these clinical parameters have been developed to enhance the accuracy of predicting ECE and SVI [8, 9]. However, the predictive models solely based on traditional clinical variables have limited accuracy. Therefore, there is need for additional reliable novel markers to improve the accuracy of predicting ECE and SVI before surgery.
Numerous studies have consistently indicated that the progression and metastasis of cancer are influenced not only by tumor type, disease stage and treatment approach [10], but also by the nutritional status of patients [11, 12]. The relationship between nutrition and the development of tumor is an intricate mechanism that plays a significant role in tumorigenesis [13]. Traditionally, the nutritional status of cancer patients was often assessed using body mass index (BMI) and serum albumin (ALB) levels [14]. However, relying solely on these parameters may not provide an accurate assessment of nutritional status [15, 16]. To address this limitation, the nutritional risk index (NRI) has emerged as a more reliable and objective tool for assessing nutritional status in various types of cancer [17-19]. The NRI takes into account the patient’s height, weight, and serum ALB level, providing a more comprehensive evaluation [20]. However, there are currently limited available data on the use of NRI in PCa [21-23]. Furthermore, no clinical studies have investigated the potential association between NRI and the aggressiveness and adverse pathological features of PCa. Therefore, the objective of this study was to bridge this research gap by exploring the predictive value of NRI for ECE and SVI in PCa patients, and further develop novel predictive nomograms for ECE and SVI based on NRI, which can provide valuable guidance for the clinical formulation of PCa treatment strategies. By exploring the relationship between NRI and adverse pathological features of PCa, this study sought to enhance our understanding of the impact of nutritional status on PCa progression and inform personalized treatment approaches.
| Materials and Methods | ▴Top |
Study population
After obtaining approval from the Medical Science Research Ethics Committee, data of 895 PCa patients who underwent RP between 2010 and 2020 from the PCa database in the Department of Urology at Peking University Third Hospital were extracted. We reviewed and collected comprehensive clinicopathologic data for each patient carefully. To ensure the reliability and validity of our study, patients who met the following criteria were excluded: 1) those with histological types other than adenocarcinoma; 2) those who had received prior neoadjuvant therapy; 3) those with any incomplete clinicopathologic information. As a result, a total of 734 PCa patients were deemed eligible for further analysis. To ensure the generalizability of our findings, the eligible patients were randomly divided into two cohorts: a primary cohort consisting of 489 patients and a validation cohort consisting of 245 patients. The allocation was done in a 2:1 manner, respectively. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Data collection and pathological evaluation
The clinical and pathological variables of the enrolled patients were retrospectively collected from the database. These variables included age, height, weight, BMI, ALB, DRE, percentage of positive biopsy cores (PPC), biopsy Gleason score, preoperative PSA level, prostate volume (PV), PSA density (PSAD), ECE and SVI. BMI (kg/m2) was calculated by dividing weight by the square of height. The NRI was calculated using the formula: (1.489 × serum albumin concentration (g/L)) + (41.7 × present body weight (PBW)/ideal body weight (IBW)). IBW was calculated as: height2 (m) × 22. The ratio of PBW to IBW was set to 1 when the PBW exceeded the IBW [20]. Based upon the original publication describing NRI, patients were divided into a malnutrition group (NRI ≤ 98) and a normal nutrition group (NRI > 98) [20]. The PPC was calculated by dividing the total number of positive biopsy cores by the total number of biopsy cores obtained. PV was determined using transrectal ultrasonography (TURS) or mp-MRI. PV was calculated using the formula: (anteroposterior diameter) × (left and right diameter) × (upper and lower diameter) × 0.52. PSAD was calculated by dividing the total PSA (tPSA) by PV.
All surgical specimens after RP were processed according to standard pathological procedures. The pathological report was standardized based on the histological/architectural thresholds proposed by the WHO classification of tumors of the urinary system and male genital organs [24]. The Gleason scoring system, recommended by the International Society of Urological Pathology (ISUP) 2005 and 2014 consensus conferences, was used [25]. ECE was defined as the presence of tumor beyond the borders of the gland, admixed with periprostatic fat tissue, or within loose connective tissue and/or perineural spaces of the NVBs in the posterolateral area. SVI was defined as tumor invasion of the extra-prostatic seminal vesicle [26].
Statistical analysis
Continuous variables were presented as median and interquartile range (IQR), while categorical variables were expressed as the number of patients with respective percentages. Intergroup comparisons were performed using the Student’s t-test or Mann-Whitney U test for continuous variables, and the χ2 test or Fisher’s exact test for categorical variables. Univariate and multivariate logistic regression analyses were conducted to evaluate the predictors for ECE and SVI. The nomograms for predicting ECE and SVI were established based on the result of the multivariate logistic regression analysis. The performance of the nomograms was estimated using Harrell’s concordance index (C-index) and the area under curve (AUC) of receiver operating characteristic (ROC) curves, and the calibration curves of the nomograms were plotted to evaluate the consistency between the nomograms’ predication and the actual observation. In the external validation of the nomograms, the total points of each case in the validation cohort were calculated according to the established nomograms, and these points were included as factors in the logistic regression model to derive the validation C-index, AUC and calibration curves. All statistical analyses were performed using SPSS Statistics version 26.0 (IBM, Armonk, New York) and R version 3.6.1 (R Project for Statistical Computing, Vienna, Austria). A two-sided P value < 0.05 was considered statistically significant.
| Results | ▴Top |
Clinicopathologic characteristics of patients
A total of 734 PCa patients treated with RP were included in the study. The entire cohort was randomly divided into a primary cohort (n = 489) and a validation cohort (n = 245) in a 2:1 manner. Apart from BMI (P = 0.030), PPC (P = 0.034), biopsy Gleason score (P = 0.003) and ECE (P = 0.049), no statistically significant difference in baseline characteristics between the two cohorts can be observed. The baseline characteristics of the primary cohort and validation cohort are presented in Table 1.
 Click to view | Table 1. Baseline Characteristics of the Separate Patient Cohorts |
In the primary cohort, 70 (14.3%) patients with NRI ≤ 98 were classified as malnutrition, while the remaining 419 (85.7%) patients with NRI > 98 were considered to have normal nutrition. The incidences of ECE in the malnutrition group (NRI ≤ 98) and the normal nutrition group (NRI > 98) were 60.0% (n = 42) and 44.6% (n = 187), respectively. Similarly, the incidences of SVI in the two groups were 27.1% (n = 19) and 15.8% (n = 66), respectively. There were significant differences between the two groups in terms of age (P = 0.030), BMI (P = 0.01), preoperative PSA level (P = 0.020), ECE (P = 0.017) and SVI (P = 0.020). Compared to the normal nutrition group (NRI > 98), patients in the malnutrition group (NRI ≤ 98) were older, had lower BMI and higher PSA levels, and were more likely to develop ECE and SVI. The clinicopathologic characteristics of patients in the primary cohort are shown in Table 2.
 Click to view | Table 2. Clinicopathologic Characteristics of Patients in Primary Cohort |
Univariate and multivariate analyses for ECE and SVI in primary cohort
The results of univariate logistic analyses demonstrated a significant association between NRI, PPC, biopsy Gleason score, PSA and PSAD with ECE as well as SVI (P < 0.05). Incorporated the above factors into a multivariate logistic regression analysis, NRI (odds ratio (OR): 1.901, 95% confidence interval (CI): 1.050 - 3.440, P = 0.034), PPC (OR: 1.016, 95% CI: 1.008 - 1.024, P < 0.001), biopsy Gleason score (OR: 3.520, 95% CI: 2.263 - 5.475, P < 0.001), PSA (OR: 2.299, 95% CI: 1.406 - 3.758, P = 0.001) and PSAD (OR: 2.000, 95% CI: 1.077 - 3.713, P = 0.028) were identified as independent predictors for ECE (Table 3). Additionally, NRI (OR: 2.255, 95% CI: 1.088 - 4.676, P = 0.029), PPC (OR: 1.034, 95% CI: 1.023 - 1.045, P < 0.001) and biopsy Gleason score (OR: 8.626, 95% CI: 2.986 - 24.915, P < 0.001) were identified as independent predictors for SVI (Table 4).
 Click to view | Table 3. Univariate and Multivariate Analyses for ECE in Primary Cohort |
 Click to view | Table 4. Univariate and Multivariate Analyses for SVI in Primary Cohort |
Nomograms for ECE and SVI based on NRI in primary cohort
The nomograms presented in Figure 1a (ECE) and Figure 1b (SVI) integrated all significant independent risk factors identified in the primary cohort. The C-index of the nomogram for predicting ECE was 0.785 (95% CI: 0.745 - 0.826), indicating a moderate level of accuracy. Similarly, the C-index of the nomogram for predicting SVI was 0.852 (95% CI: 0.806 - 0.898), indicating a higher level of accuracy. The corresponding AUC of the ROC cures, as shown in Figure 2a, further supported the predictive performance of the nomograms. In addition, the calibration curves for ECE and SVI also showed excellent agreement between the predictions made by the nomogram and the actual observation in the primary cohort, as depicted in Figure 3a, b.
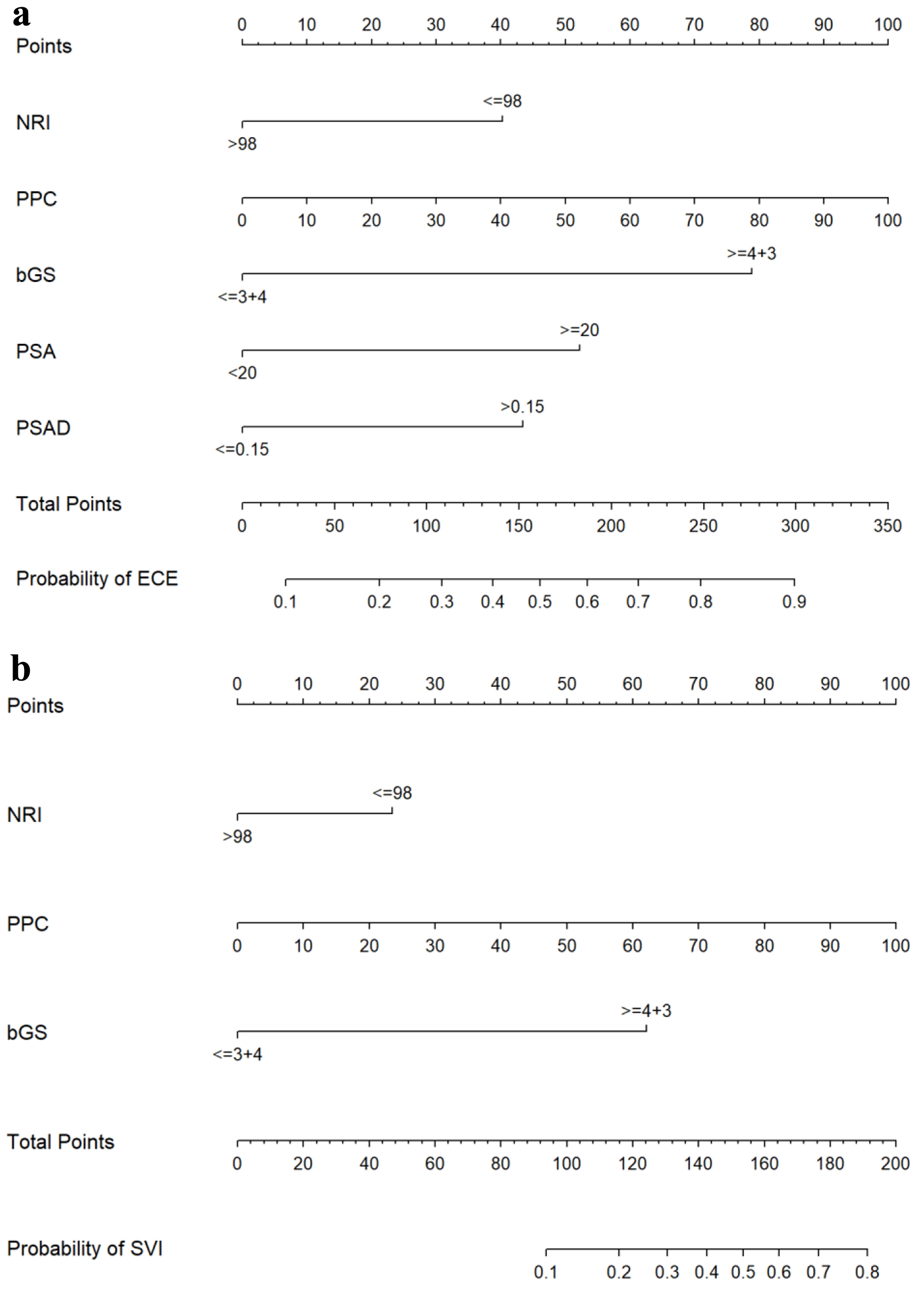 Click for large image | Figure 1. Nomograms for predicting ECE (a) and SVI (b). bGS: biopsy Gleason score; ECE: extracapsular extension; NRI: nutritional risk index; PPC: percentage of positive biopsy cores; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density; SVI: seminal vesicle invasion. |
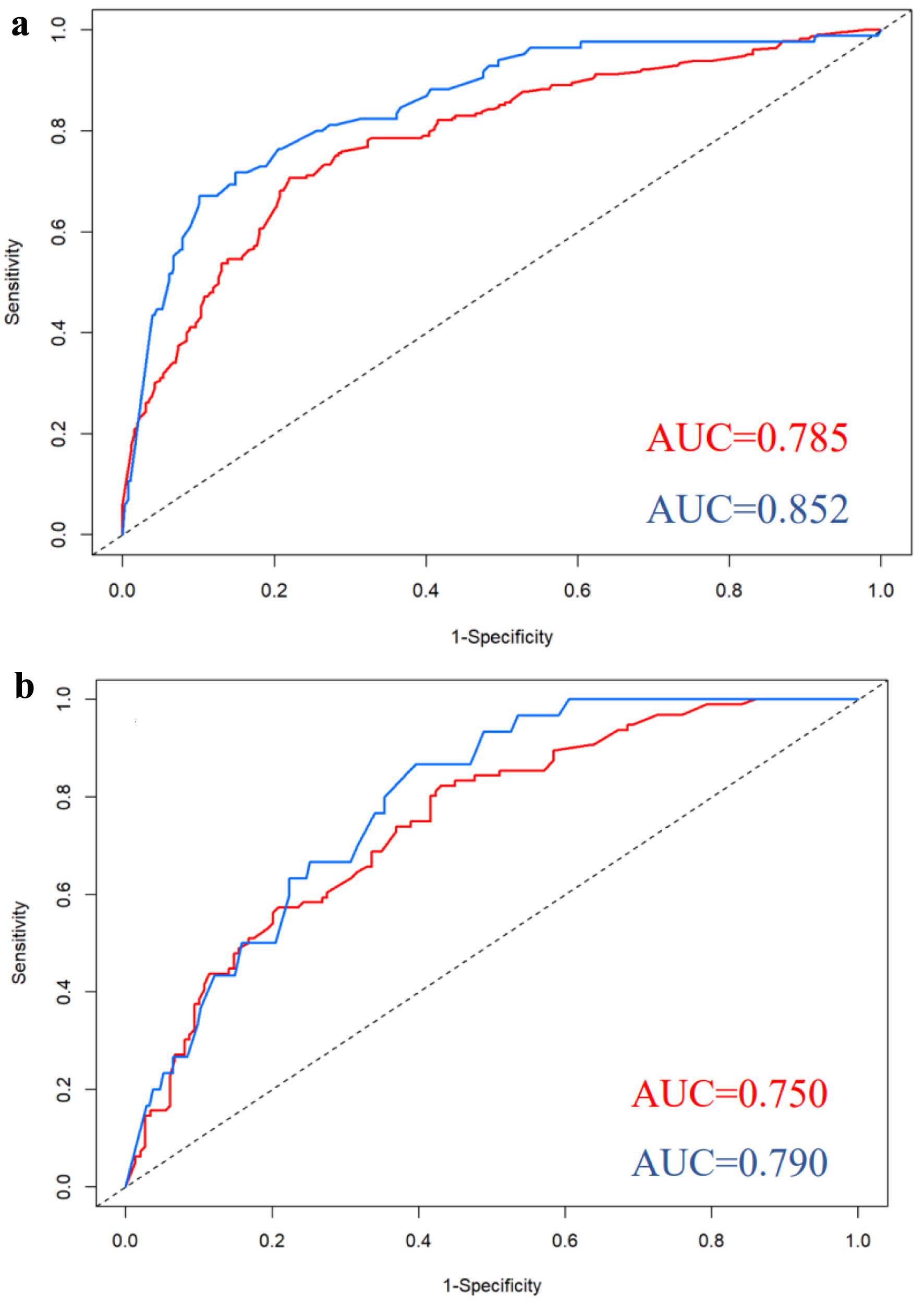 Click for large image | Figure 2. ROC curves for nomograms predicting ECE and SVI in the primary cohort (a) and validation cohort (b). Red curves for ECE; blue curves for SVI. AUC: area under curve; ECE: extracapsular extension; ROC: receiver operating characteristic; SVI: seminal vesicle invasion. |
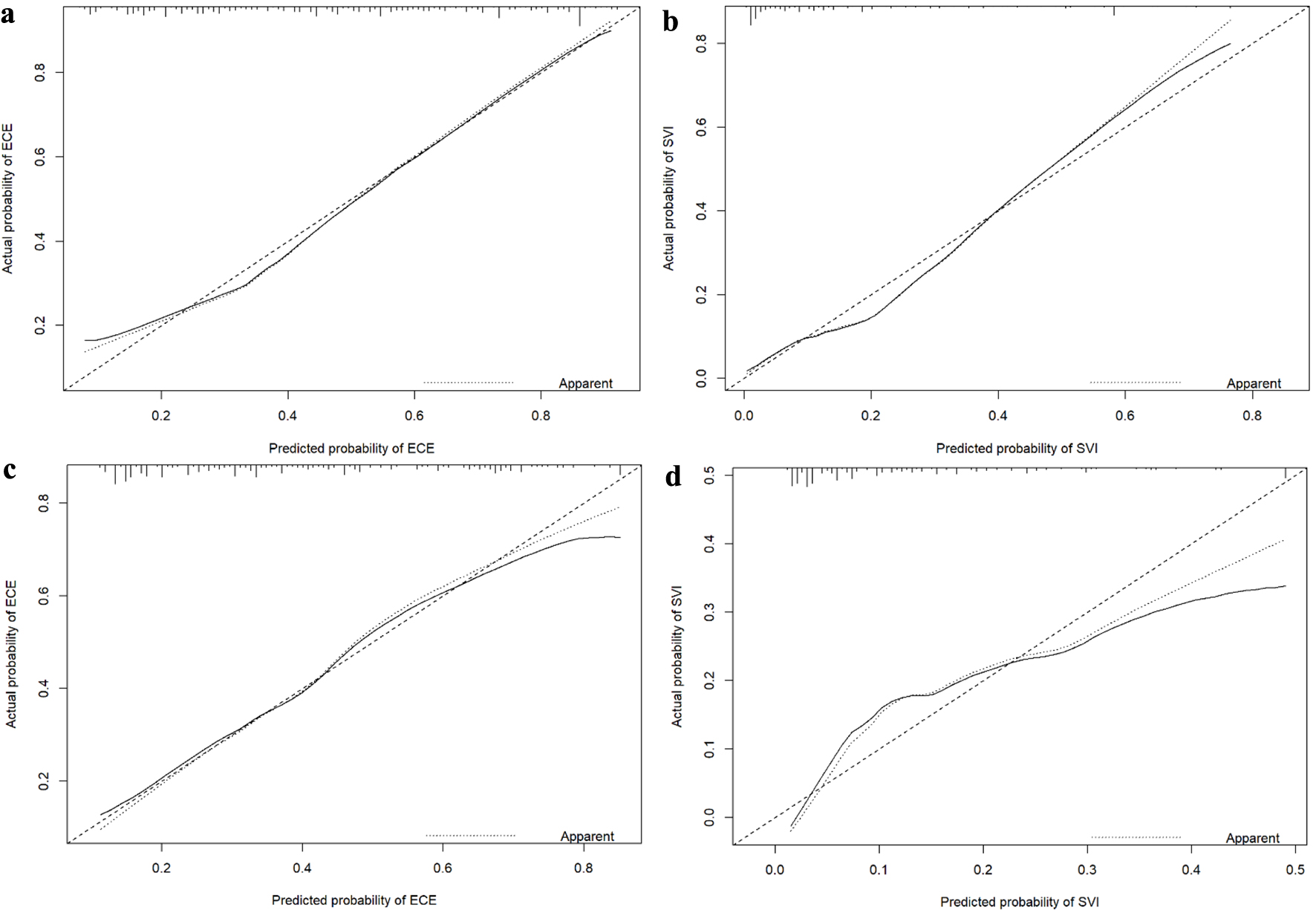 Click for large image | Figure 3. Calibration curves for nomograms predicting ECE and SVI in the primary cohort (a: ECE; b: SVI) and validation cohort (c: ECE; d: SVI). ECE: extracapsular extension; SVI: seminal vesicle invasion. |
External validation of nomograms for ECE and SVI
We conducted external validation of the created nomograms in a separate validation cohort. The nomogram for predicting ECE achieved a C-index of 0.750 (95% CI: 0.689 - 0.811), indicating a moderate level of discrimination. In comparison, the nomogram for predicting SVI achieved a slightly higher C-index of 0.790 (95% CI: 0.719 - 0.861), suggesting a slightly better discriminatory ability than the ECE prediction. The corresponding AUC of ROC curves, as shown in Figure 2b, further supported the predictive performance of the nomograms. The ROC curves demonstrated that the nomograms exhibited strong discriminatory power for both ECE and SVI. Furthermore, the calibration curves for nomograms predicting ECE and SVI also displayed great agreement between the predicted probabilities by the nomogram and the actual observations in the validation cohort, as depicted in Figure 3c, d.
| Discussion | ▴Top |
To the best of our knowledge, this study is the first to investigate the potential impact of NRI on the risk of ECE and SVI in PCa patients who underwent RP. Our results indicated that malnutrition as assessed by NRI was identified as an independent predictor for both ECE and SVI in the multivariate analysis. Additionally, we further incorporated other independent risk factors identified in the multivariate analysis and developed nomograms to predict the likelihood of ECE and SVI in PCa patients. The performance of these nomograms was evaluated using the C-index, the AUC of the ROC curves and the calibration curves. The results demonstrated great reliability and accuracy of the prediction models both in the primary and validation cohorts. Our findings might be beneficial for providing valuable insights for clinicians in making informed decisions regarding treatment strategies and patient management in PCa.
Research evidence suggests that malnutrition and cachexia contribute to approximately 20-30% of cancer-related deaths, rather than the cancer itself [27]. Malnutrition can weaken the body’s immune system and reduce the effectiveness of treatment, thereby accelerating disease progression, local recurrence, and distant metastasis [28, 29]. Therefore, early nutritional assessment has a crucial impact on improving the incidence or mortality of tumor associated with malnutrition [30]. The NRI, which combines BMI and ALB levels, has become a widely used nutritional evaluation indicator due to its simplicity and interpretability. NRI not only serves as a nutritional screening tool but has also been proven to be an independent prognostic factor for various types of cancer [14, 31]. In the field of PCa, several studies have pointed out that poor nutritional status, as indicated by an NRI < 92, was an independent prognostic factor for overall survival in patients with both metastatic hormone-naive prostate cancer (mHNPC) and metastatic castration-resistant prostate cancer (mCRPC) [22, 23]. In addition, Shu et al [32] found that malnutrition patients (NRI ≤ 98) had a significantly higher incidence and severity of postoperative complications compared with those of normal nutrition patients (NRI > 98). However, there is currently no research exploring the potential association between NRI and adverse pathology of PCa. Therefore, we conducted the study to fill the above research gap and determined the independent predictive role of NRI in ECE and SVI in PCa. Similar studies in other fields can support our findings. For instance, a multicenter retrospective study from Korea showed that a low NRI (92 - 98) was associated with aggressive tumor characteristics including large tumor size, advanced stage, and high nuclear grade in patients with renal cell carcinoma after nephrectomy [33]. Prijovic et al [34] also observed statistically significant inverse correlations between NRI and risk of muscle layer invasion, lymphovascular invasion (LVI), and lymph nodes metastases in bladder cancer patients treated with radical cystectomy. These findings highlight the importance of considering the nutritional status of patients when assessing tumor characteristics and prognosis. Further research in different populations and tumor types is needed to validate these results and explore potential mechanisms underlying the association between NRI and aggressive tumor behavior.
ECE and SVI are two crucial pathologic features of PCa, which have a significant impact on patient prognosis and surgical strategy [35]. The incidence of ECE and SVI appeared to be heterogeneous, with recent studies reporting rates ranging from 17% to 54% for ECE [6, 7, 36] and 8% to 17.6% for SVI [37-39]. In our study cohort, the incidence of ECE and SVI was found to be 46.8% and 17.4%, respectively, which is consistent with these reported in recent research. The presence of ECE directly affects the surgical approach taken towards the pericapsular structures of the prostate, particularly the posterior lateral NVBs [40]. In case of patients with localized PCa, the current guidelines from American Urological Association (AUA) recommend performing nerve-sparing RP [41]. This technique has been shown to significantly improve postoperative urinary control and erectile function [42, 43]. However, it is important to note that it also carries an increased risk of PSMs in patients with ECE. Thus, urologists face the great challenge of striking a balance between the potential surgical risks and benefits associated with ECE. On the other hand, ECE has also been identified as an independent predictor for biochemical recurrence (BCR), and a study has reported that the 5-year BCR rate in patients with organ-confined PCa was relatively low at 13%, whereas in PCa patients with ECE, the rate can be as high as 27% [44]. Similarly, in PCa patients with SVI, the 5- and 10-year biochemical failure rates were reported to be 60% and 72%, respectively, which were significantly higher than those observed in patients with pT2 disease [45]. Patients with SVI are considered as a very high-risk group, and their treatment should be approached with caution. Asymptomatic patients with a life expectancy of less than 5 years are typically only considered for androgen deprivation therapy (ADT), radiotherapy or active surveillance. Therefore, it is crucial to accurately predict the risk of ECE and SVI before surgery in order to make informed decisions regarding patient selection, treatment planning, and surgical strategy for PCa.
Several models have been constructed to accurately predict ECE and SVI in PCa prior to operation. One such model is the probability table developed by Partin for ECE prediction considering clinical stage, PSA levels, and biopsy Gleason score, which achieved high predictive accuracy (AUC = 0.84) and gained widely recognized internationality [46]. However, the model could not provide side-specific ECE prediction, a limitation that was addressed by Martini et al [7] and Soeterik et al [8]. Tosoian et al [9] recently updated the Partin tables to predict the pathological stage in patients who underwent RP in the contemporary setting, and the AUCs of their binary logistic models for predicting ECE and SVI were 0.724 and 0.856, respectively. Despite these models’ complexity, there is still a requirement for further refinement to enhance their generalizability in light of the advancements in imaging technology and the excavation of new predictive markers [47]. Additionally, Nyarangi-Dix et al have proposed a novel risk model that integrated clinical and mp-MRI parameters to predict ECE in RP specimens. The model exhibits remarkable discrimination (AUC = 0.86) and calibration, indicating its potential clinical utility [48]. However, it should be noted that the model incorporates certain intricate features that may not be easily accessible in real-world clinical setting. Furthermore, the model has not yet undergone external validation, which raises uncertainties regarding its performance when applied to diverse patient populations. Similarly, Martini et al [38] also established a nomogram for the prediction of SVI based on a combination of clinical variables such as preoperative PSA, biopsy Gleason grade and maximal percentage of core involvement along with documented SVI on mp-MRI. The nomogram demonstrated excellent discriminatory ability, as evidenced by an AUC value of 0.847. However, these results may be limited due to the relatively small number of cases of SVI included in the model. Moreover, it is worth mentioning that these models containing mp-MRI findings are heavily dependent on the presence or absence of ECE and SVI on mp-MRI. In our study, we determined the significant predictive value of nutritional status, particularly the NRI, in relation to adverse pathological features of PCa based on a sufficient number of cases with ECE and SVI. Focused on NRI combined with variables such as PSA, biopsy Gleason score and PPC that are easily applicable in clinical practice, we established a predictive nomogram, and conducted external validation of the model. The results indicate that the prediction model we established exhibits great prediction performance in both the primary and validation cohorts.
There are several limitations of the study that should be acknowledged. Firstly, both the primary cohort and validation cohort of the study were derived from the same retrospective dataset, which introduced an inevitable potential for selection bias despite of randomizing the grouping. Secondly, due to the relatively small sample size, the accuracy of the models still needed to be validated internally and externally in large multicenter studies to estimate its wider applicability. Thirdly, our study mainly focused on the patients with PCa who underwent RP. However, it is important to note that there has been a growing trend in recent years towards the utilization of RP in patients with more aggressive disease characteristics [49, 50]. Consequently, the results of our study may not be directly applicable to patients receiving alternative therapeutic approaches, such as active surveillance or radiotherapy. Additionally, the optimal cut-off value of NRI for malnutrition among patients with PCa may differ across various populations, which may potentially impact the generalizability of our results to different populations. Finally, although the prediction nomograms established in our study showed great predictive performance, the accuracy of prediction models based solely on clinicopathological factors could be limited. Model refinement may be necessary to refine the nomograms by adjusting coefficients or adding/removing variables to improve their predictive accuracy. Recent research has highlighted the value of preoperative MRI in evaluating ECE and SVI in PCa patients [6, 7], suggesting that the integration of radiological information into predictive models may enhance the accuracy of prediction. Therefore, it is imperative that future prospective studies are supposed to be conducted to explore the combination of the model with imaging tools, with the aim of improving the prediction of ECE and SVI in PCa.
Conclusion
In conclusion, the NRI can be considered a reliable tool for assessing nutritional status, which is also significantly associated with adverse pathological features, particularly ECE and SVI, in patients with PCa. Moreover, the nomogram established based on the NRI in our study can provide an individualized risk estimation for ECE and SVI in PCa patients, and may be valuable for clinicians in making well-informed decisions regarding treatment strategies and patient management.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by grants from Beijing Natural Science Foundation (No. Z200027 and No. L212051) and National Natural Science Foundation of China (No. 62331001).
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Jian Lu and Hai Bi designed the study and controlled the structure and quality of the manuscript. Ze Nan Liu analyzed the data and wrote this manuscript. Zi Ang Li, Ji De He, Jia Long Wu, Lei Qiu and Zhen Kun Zhao collected and arranged the data. Min Lu collected and improved the pathological data. All authors contributed to the article and approved the final version.
Data Availability
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
ADT: androgen deprivation therapy; ALB: albumin; AUA: American Urological Association; AUC: area under curve; BCR: biochemical recurrence; BMI: body mass index; CI: confidence interval; C-index: concordance index; DRE: digital rectal examination; ECE: extracapsular extension; IBW: ideal body weight; IQR: interquartile range; ISUP: International Society of Urological Pathology; LVI: lymphovascular invasion; mCRPC: metastatic castration-resistant prostate cancer; mHNPC: metastatic hormone-naive prostate cancer; mp-MRI: multiparametric magnetic resonance imaging; NRI: nutritional risk index; NVB: neurovascular bundle; OR: odds ratio; PBW: present body weight; PCa: prostate cancer; PPC: percentage of positive biopsy cores; PSA: prostate-specific antigen; PSAD: prostate-specific antigen density; PSM: positive surgical margin; PV: prostate volume; Ref: reference; ROC: receiver operating characteristic; RP: radical prostatectomy; SVI: seminal vesicle invasion; TURS: transrectal ultrasonography
| References | ▴Top |
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48.
doi pubmed - Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on prostate cancer-2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2021;79(2):243-262.
doi pubmed - Tollefson MK, Karnes RJ, Rangel LJ, Bergstralh EJ, Boorjian SA. The impact of clinical stage on prostate cancer survival following radical prostatectomy. J Urol. 2013;189(5):1707-1712.
doi pubmed - Wiegel T, Bartkowiak D, Bottke D, Bronner C, Steiner U, Siegmann A, Golz R, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96-02/AUO AP 09/95 trial. Eur Urol. 2014;66(2):243-250.
doi pubmed - Diamand R, Ploussard G, Roumiguie M, Oderda M, Benamran D, Fiard G, Quackels T, et al. External validation of a multiparametric magnetic resonance imaging-based nomogram for the prediction of extracapsular extension and seminal vesicle invasion in prostate cancer patients undergoing radical prostatectomy. Eur Urol. 2021;79(2):180-185.
doi pubmed - Gandaglia G, Ploussard G, Valerio M, Mattei A, Fiori C, Roumiguie M, Fossati N, et al. The key combined value of multiparametric magnetic resonance imaging, and magnetic resonance imaging-targeted and concomitant systematic biopsies for the prediction of adverse pathological features in prostate cancer patients undergoing radical prostatectomy. Eur Urol. 2020;77(6):733-741.
doi pubmed - Martini A, Gupta A, Lewis SC, Cumarasamy S, Haines KG, 3rd, Briganti A, Montorsi F, et al. Development and internal validation of a side-specific, multiparametric magnetic resonance imaging-based nomogram for the prediction of extracapsular extension of prostate cancer. BJU Int. 2018;122(6):1025-1033.
doi pubmed - Soeterik TFW, van Melick HHE, Dijksman LM, Kusters-Vandevelde H, Stomps S, Schoots IG, Biesma DH, et al. Development and external validation of a novel nomogram to predict side-specific extraprostatic extension in patients with prostate cancer undergoing radical prostatectomy. Eur Urol Oncol. 2022;5(3):328-337.
doi pubmed - Tosoian JJ, Chappidi M, Feng Z, Humphreys EB, Han M, Pavlovich CP, Epstein JI, et al. Prediction of pathological stage based on clinical stage, serum prostate-specific antigen, and biopsy Gleason score: Partin Tables in the contemporary era. BJU Int. 2017;119(5):676-683.
doi pubmed - McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223-226.
doi pubmed - Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin. 2012;62(4):243-274.
doi pubmed - Liu X, Meng QH, Ye Y, Hildebrandt MA, Gu J, Wu X. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancer. Carcinogenesis. 2015;36(2):243-248.
doi pubmed - Mayne ST, Playdon MC, Rock CL. Diet, nutrition, and cancer: past, present and future. Nat Rev Clin Oncol. 2016;13(8):504-515.
doi pubmed - Lin F, Xia W, Chen M, Jiang T, Guo J, Ouyang Y, Sun H, et al. A prognostic model based on nutritional risk index in operative breast cancer. Nutrients. 2022;14(18):3783.
doi pubmed pmc - Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3(3):141-146.
doi pubmed - Spinella R, Sawhney R, Jalan R. Albumin in chronic liver disease: structure, functions and therapeutic implications. Hepatol Int. 2016;10(1):124-132.
doi pubmed - Haas M, Lein A, Fuereder T, Brkic FF, Schnoell J, Liu DT, Kadletz-Wanke L, et al. The Geriatric Nutritional Risk Index (GNRI) as a prognostic biomarker for immune checkpoint inhibitor response in recurrent and/or metastatic head and neck cancer. Nutrients. 2023;15(4):880.
doi pubmed pmc - Chen XY, Lin Y, Yin SY, Shen YT, Zhang XC, Chen KK, Zhou CJ, et al. The geriatric nutritional risk index is an effective tool to detect GLIM-defined malnutrition in rectal cancer patients. Front Nutr. 2022;9:1061944.
doi pubmed pmc - Grinstead C, George T, Han B, Yoon SL. Associations of overall survival with geriatric nutritional risk index in patients with advanced pancreatic cancer. Nutrients. 2022;14(18):3800.
doi pubmed pmc - Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777-783.
doi pubmed - Guo Y, Wang R, Wu P, Zhang W, Mao S, Wu Y, Liu J, et al. Preoperative nutritional risk index predicts recurrence of oligometastatic prostate cancer in patients undergoing cytoreductive radical prostatectomy. Nutr Cancer. 2021;73(8):1440-1447.
doi pubmed - Chang LW, Hung SC, Li JR, Chiu KY, Yang CK, Chen CS, Lu K, et al. Geriatric nutritional risk index as a prognostic marker for patients with metastatic castration-resistant prostate cancer receiving docetaxel. Front Pharmacol. 2020;11:601513.
doi pubmed pmc - Okamoto T, Hatakeyama S, Narita S, Takahashi M, Sakurai T, Kawamura S, Hoshi S, et al. Impact of nutritional status on the prognosis of patients with metastatic hormone-naive prostate cancer: a multicenter retrospective cohort study in Japan. World J Urol. 2019;37(9):1827-1835.
doi pubmed - Eble JN, Sauter G, Epstein JI. Pathology and genetics of tumours of the urinary system and male genital organs. IARC Press. 2004; p. 1-353.
- Epstein JI, Zelefsky MJ, Sjoberg DD, Nelson JB, Egevad L, Magi-Galluzzi C, Vickers AJ, et al. A contemporary prostate cancer grading system: a validated alternative to the gleason score. Eur Urol. 2016;69(3):428-435.
doi pubmed pmc - Fine SW, Amin MB, Berney DM, Bjartell A, Egevad L, Epstein JI, Humphrey PA, et al. A contemporary update on pathology reporting for prostate cancer: biopsy and radical prostatectomy specimens. Eur Urol. 2012;62(1):20-39.
doi pubmed - Caccialanza R, Goldwasser F, Marschal O, Ottery F, Schiefke I, Tilleul P, Zalcman G, et al. Unmet needs in clinical nutrition in oncology: a multinational analysis of real-world evidence. Ther Adv Med Oncol. 2020;12:1758835919899852.
doi pubmed pmc - Barao K, Abe Vicente Cavagnari M, Silva Fucuta P, Manoukian Forones N. Association between nutrition status and survival in elderly patients with colorectal cancer. Nutr Clin Pract. 2017;32(5):658-663.
doi pubmed - van der Wal HH, Grote Beverborg N, Dickstein K, Anker SD, Lang CC, Ng LL, van Veldhuisen DJ, et al. Iron deficiency in worsening heart failure is associated with reduced estimated protein intake, fluid retention, inflammation, and antiplatelet use. Eur Heart J. 2019;40(44):3616-3625.
doi pubmed pmc - Xie H, Ruan G, Zhang Q, Ge Y, Song M, Zhang X, Liu X, et al. Combination of nutritional risk index and handgrip strength on the survival of patients with cancer cachexia: a multi- center cohort study. J Inflamm Res. 2022;15:1005-1015.
doi pubmed pmc - Xie H, Wei L, Yuan G, Liu M, Liang Y, Gao S, Wang Q, et al. Combination of geriatric nutritional risk index and carcinoembryonic antigen to predict the survival of patients with colorectal cancer. Front Nutr. 2022;9:902080.
doi pubmed pmc - Shu W, Tao W, Chunyan H, Jie F, Yuan L, Yan X, Huan Z, et al. Preoperative nutritional evaluation of prostate cancer patients undergoing laparoscopic radical prostatectomy. PLoS One. 2022;17(2):e0262630.
doi pubmed pmc - Kang HW, Seo SP, Kim WT, Yun SJ, Lee SC, Kim WJ, Hwang EC, et al. A low geriatric nutritional risk index is associated with aggressive pathologic characteristics and poor survival after nephrectomy in clear renal cell carcinoma: a multicenter retrospective study. Nutr Cancer. 2020;72(1):88-97.
doi pubmed - Prijovic N, Acimovic M, Santric V, Stankovic B, Nikic P, Vukovic I, Soldatovic I, et al. Predictive value of inflammatory and nutritional indexes in the pathology of bladder cancer patients treated with radical cystectomy. Curr Oncol. 2023;30(3):2582-2597.
doi pubmed pmc - Mikel Hubanks J, Boorjian SA, Frank I, Gettman MT, Houston Thompson R, Rangel LJ, Bergstralh EJ, et al. The presence of extracapsular extension is associated with an increased risk of death from prostate cancer after radical prostatectomy for patients with seminal vesicle invasion and negative lymph nodes. Urol Oncol. 2014;32(1):26.e1-e7.
- Pedraza AM, Parekh S, Joshi H, Grauer R, Wagaskar V, Zuluaga L, Gupta R, et al. Side-specific, Microultrasound-based Nomogram for the Prediction of Extracapsular Extension in Prostate Cancer. Eur Urol Open Sci. 2023;48:72-81.
doi pubmed pmc - Jambor I, Falagario U, Ratnani P, Perez IM, Demir K, Merisaari H, Sobotka S, et al. Prediction of biochemical recurrence in prostate cancer patients who underwent prostatectomy using routine clinical prostate multiparametric MRI and decipher genomic score. J Magn Reson Imaging. 2020;51(4):1075-1085.
doi pubmed - Martini A, Gupta A, Cumarasamy S, Lewis SC, Haines KG, 3rd, Briganti A, Montorsi F, et al. Novel nomogram for the prediction of seminal vesicle invasion including multiparametric magnetic resonance imaging. Int J Urol. 2019;26(4):458-464.
doi pubmed - Wang H, Ruan M, Wang H, Li X, Hu X, Liu H, Zhou B, et al. Predictive model containing PI-RADS v2 score for postoperative seminal vesicle invasion among prostate cancer patients. Transl Androl Urol. 2021;10(2):584-593.
doi pubmed pmc - Walz J, Epstein JI, Ganzer R, Graefen M, Guazzoni G, Kaouk J, Menon M, et al. A critical analysis of the current knowledge of surgical anatomy of the prostate related to optimisation of cancer control and preservation of continence and erection in candidates for radical prostatectomy: an update. Eur Urol. 2016;70(2):301-311.
doi pubmed - Eastham JA, Auffenberg GB, Barocas DA, Chou R, Crispino T, Davis JW, Eggener S, et al. Clinically localized prostate cancer: AUA/ASTRO guideline, Part II: principles of active surveillance, principles of surgery, and follow-up. J Urol. 2022;208(1):19-25.
doi pubmed - Michl U, Tennstedt P, Feldmeier L, Mandel P, Oh SJ, Ahyai S, Budaus L, et al. Nerve-sparing surgery technique, not the preservation of the neurovascular bundles, leads to improved long-term continence rates after radical prostatectomy. Eur Urol. 2016;69(4):584-589.
doi pubmed - Nguyen LN, Head L, Witiuk K, Punjani N, Mallick R, Cnossen S, Fergusson DA, et al. The risks and benefits of cavernous neurovascular bundle sparing during radical prostatectomy: a systematic review and meta-analysis. J Urol. 2017;198(4):760-769.
doi pubmed - de Rooij M, Hamoen EH, Witjes JA, Barentsz JO, Rovers MM. Accuracy of magnetic resonance imaging for local staging of prostate cancer: a diagnostic meta-analysis. Eur Urol. 2016;70(2):233-245.
doi pubmed - Algarra R, Barba J, Merino I, Tienza A, Tolosa E, Robles JE, Zudaire J. Prognostic value of seminal vesicle involvement due to prostate cancer in radical prostatectomy specimens. Actas Urol Esp. 2015;39(3):144-153.
doi pubmed - Eifler JB, Feng Z, Lin BM, Partin MT, Humphreys EB, Han M, Epstein JI, et al. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int. 2013;111(1):22-29.
doi pubmed pmc - Rouviere O, Puech P, Renard-Penna R, Claudon M, Roy C, Mege-Lechevallier F, Decaussin-Petrucci M, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 2019;20(1):100-109.
doi pubmed - Nyarangi-Dix J, Wiesenfarth M, Bonekamp D, Hitthaler B, Schutz V, Dieffenbacher S, Mueller-Wolf M, et al. Combined clinical parameters and multiparametric magnetic resonance imaging for the prediction of extraprostatic disease - a risk model for patient-tailored risk stratification when planning radical prostatectomy. Eur Urol Focus. 2020;6(6):1205-1212.
doi pubmed - Lu J, Wirth GJ, Wu S, Chen J, Dahl DM, Olumi AF, Young RH, et al. A close surgical margin after radical prostatectomy is an independent predictor of recurrence. J Urol. 2012;188(1):91-97.
doi pubmed - van den Bergh R, Gandaglia G, Tilki D, Borgmann H, Ost P, Surcel C, Valerio M, et al. Trends in radical prostatectomy risk group distribution in a european multicenter analysis of 28 572 patients: towards tailored treatment. Eur Urol Focus. 2019;5(2):171-178.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


