| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 6, December 2023, pages 529-539
Efficacy of First-Line Immunotherapy Combined With Chemotherapy in Extensive-Stage Small Cell Lung Cancer Patients With Different Brain Metastases Status: A Systematic Review and Meta-Analysis
Wen Hua Zhaoa, d, Shou Feng Wangb, d, Cui Yun Sua, Xin Bin Panc, e
aDepartment of Respiratory Oncology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi 530021, China
bDepartment of Thoracic Surgery, Guangxi Medical University Cancer Hospital, Nanning, Guangxi 530021, China
cDepartment of Radiation Oncology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi 530021, China
dThese authors contributed equally to this work.
eCorresponding Author: Xin Bin Pan, Department of Radiation Oncology, Guangxi Medical University Cancer Hospital, Nanning, Guangxi 530021, China
Manuscript submitted September 8, 2023, accepted October 19, 2023, published online November 3, 2023
Short title: Immunotherapy and Chemotherapy in ES-SCLC With Brain Metastases
doi: https://doi.org/10.14740/wjon1726
| Abstract | ▴Top |
Background: This study aims to evaluate the efficacy of first-line immunotherapy combined with chemotherapy in extensive-stage small cell lung cancer (ES-SCLC) patients with differing brain metastasis statuses.
Methods: We conducted a comprehensive search in public databases, such as PubMed, EMBASE, and the Cochrane Library, to identify randomized controlled trials involving ES-SCLC patients, with or without brain metastases, who underwent first-line immunotherapy combined with chemotherapy. The primary outcome measure was progression-free survival (PFS), and the secondary outcome measure was overall survival (OS).
Results: Our analysis incorporated seven high-quality randomized controlled trials, encompassing 398 patients with brain metastases and 3,533 without. Among patients without brain metastases, the combination of immunotherapy and chemotherapy led to significantly improved PFS (hazard ratio (HR) = 0.72, 95% confidence interval (CI): 0.62 - 0.84, P < 0.001) and OS (HR = 0.77, 95% CI: 0.67 - 0.88, P < 0.001) in comparison to chemotherapy alone. Conversely, for patients with brain metastases, the addition of immunotherapy to chemotherapy did not result in a significant improvement in PFS (HR = 1.03, 95% CI: 0.66 - 1.61, P = 0.887) or OS (HR = 1.03, 95% CI: 0.82 - 1.31, P = 0.776) when compared to chemotherapy alone.
Conclusions: In ES-SCLC patients without brain metastases, first-line immunotherapy combined with chemotherapy demonstrated improved PFS and OS in contrast to chemotherapy alone. However, patients with brain metastases did not experience similar benefits.
Keywords: Small cell lung cancer; Extensive stage; Immunotherapy; Chemotherapy; Brain metastases
| Introduction | ▴Top |
Small cell lung cancer (SCLC) accounts for approximately 15% of all lung cancer cases [1]. It is a highly aggressive neuroendocrine malignancy, ranking among the leading causes of cancer-related mortality [2]. Within the spectrum of SCLC, extensive-stage disease (ES-SCLC) constitutes a significant portion, accounting for approximately 70% of cases [3]. Notably, 10-18% of ES-SCLC cases present with brain metastases at the time of diagnosis [4].
Extensive evidence supports the notion that the integration of first-line immunotherapy with chemotherapy leads to enhanced treatment outcomes for ES-SCLC patients [5-12]. However, it is important to recognize that several studies have suggested that various clinical factors and combined agents might influence prognosis in this context [13, 14]. Moreover, clinical trials, when subjected to subgroup analysis, have demonstrated variable efficacy in the combination of immunotherapy with chemotherapy for ES-SCLC patients who already have brain metastases [5-11].
Given the potential incidence of adverse events associated with immunotherapy [15], it becomes imperative to determine whether ES-SCLC patients with brain metastases can derive maximal benefit from this treatment approach. The existing inconsistencies in the literature underscore the necessity for a systematic review aimed at evaluating the treatment outcomes of first-line immunotherapy combined with chemotherapy in ES-SCLC patients with varying brain metastasis statuses.
| Materials and Methods | ▴Top |
Data sources
Two authors (Wen Hua Zhao and Shou Feng Wang) independently conducted data retrieval. The search spanned multiple databases, including PubMed, EMBASE, and the Cochrane Library, from their inception up to December 2022. Additionally, abstracts from key oncology conferences such as the European Society of Medical Oncology, World Conference on Lung Cancer, and American Society of Clinical Oncology were screened by the same authors. The data search strictly adhered to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [16, 17].
Search strategy
Our search strategy involved a range of terms, including “small cell lung cancer”, “SCLC”, “stage IV”, “extensive stage”, “chemotherapy”, “immunotherapy”, and “immune checkpoint inhibitor”. Furthermore, relevant randomized controlled trials were identified by reviewing references in significant literature.
Inclusion and exclusion criteria
The included studies were required to meet specific inclusion criteria, which are as follows: 1) patients with histopathologically confirmed SCLC; 2) clinical stage classified as extensive stage based on the Veterans Administration Lung Study Group (VALG) definition or stage IV according to the International Association for the Study of Lung Cancer (IASLC) staging system; 3) patients received first-line immunotherapy combined with chemotherapy; 4) study design was a randomized controlled trial; and 5) randomized controlled trials reported treatment outcomes regarding progression-free survival (PFS) and/or overall survival (OS). Case reports, reviews, comments, editorials, letters, and animal studies were excluded from this systematic review and meta-analysis.
Study selection
The selection of studies was carried out independently by two authors (Wen Hua Zhao and Shou Feng Wang). Initially, titles and abstracts of studies were assessed, and for potentially eligible studies, full manuscripts were reviewed. Duplicate studies were eliminated by combining the results of the two authors’ selections. In cases of disagreement during the selection process, a third author (Xin Bin Pan) was consulted for resolution.
Data extraction
Following the selection process, data extraction was performed by two independent authors (Cui Yun Su and Xin Bin Pan), adhering to the PRISMA guidelines. The following information was extracted from each included randomized controlled trial: 1) authors and trial names; 2) publication year; 3) patient geographical area; 4) trial design; 5) total number of participants; 6) immunotherapy and chemotherapy regimens; 7) number of patients with brain metastases; 8) number of patients without brain metastases; and 9) hazard ratios (HRs) with 95% confidence intervals (CIs) for PFS and/or OS in patients treated with immunotherapy combined with chemotherapy and those receiving chemotherapy alone.
Quality assessment
Two independent authors (Wen Hua Zhao and Shou Feng Wang) assessed the methodological quality of included randomized controlled trials. In cases of disagreement, a third author (Xin Bin Pan) was involved for discussion and consensus. Methodological quality assessment of included randomized clinical trials was performed using the Cochrane Risk of Bias tool [18], which evaluated seven domains: 1) random sequence generation; 2) blinding of participants and personnel; 3) allocation concealment; 4) incomplete outcome data; 5) blinding of outcome assessment; 6) selective reporting; and 7) other bias.
Statistical analysis
Heterogeneity between the included randomized controlled trials was evaluated using the I2 statistic and the Q Chi-squared test. P ≥ 0.10 or I2 < 50% indicated no significant heterogeneity between the included randomized controlled trials. In contrast, P < 0.10 or I2 ≥ 50% indicated significant heterogeneity between the included randomized controlled trials. The random-effect model was used in cases of significant heterogeneity between the included randomized controlled trials; otherwise, the fixed-effect model was used when no significant heterogeneity was observed.
The primary endpoint was PFS, and the secondary endpoint was OS. This systematic review and meta-analysis presented pooled analyses using forest plots. HRs with 95% CIs for PFS and OS were calculated to compare the efficacy of immunotherapy combined with chemotherapy versus chemotherapy alone in patients with varying brain metastasis statuses. Sensitivity analyses were performed to assess the influence of each study by omitting one study at a time. Publication bias was assessed using funnel plots with Begg’s and Egger’s tests.
This systematic review and meta-analysis utilized R software version 4.3.0 and SPSS Statistics Version 26.0 (IBM Co., Armonk, NY, USA) for statistical analyses. A statistically significant difference was considered at P < 0.05 (two-tailed).
Ethics approval and informed consent were waived by the Ethics Committee of Guangxi Medical University Cancer Hospital.
| Results | ▴Top |
Study selection
The study selection process is illustrated in Figure 1. Initially, 8,466 studies were identified, and after abstract screening, 8,420 were excluded. Upon a thorough examination of full-text articles, 20 studies were considered potentially eligible. Among the 20 studies, two were not focused on first-line treatment, eight were duplicative trials, and three did not provide information on brain metastases. Ultimately, seven randomized controlled trials were included in this systematic review and meta-analysis [5-11].
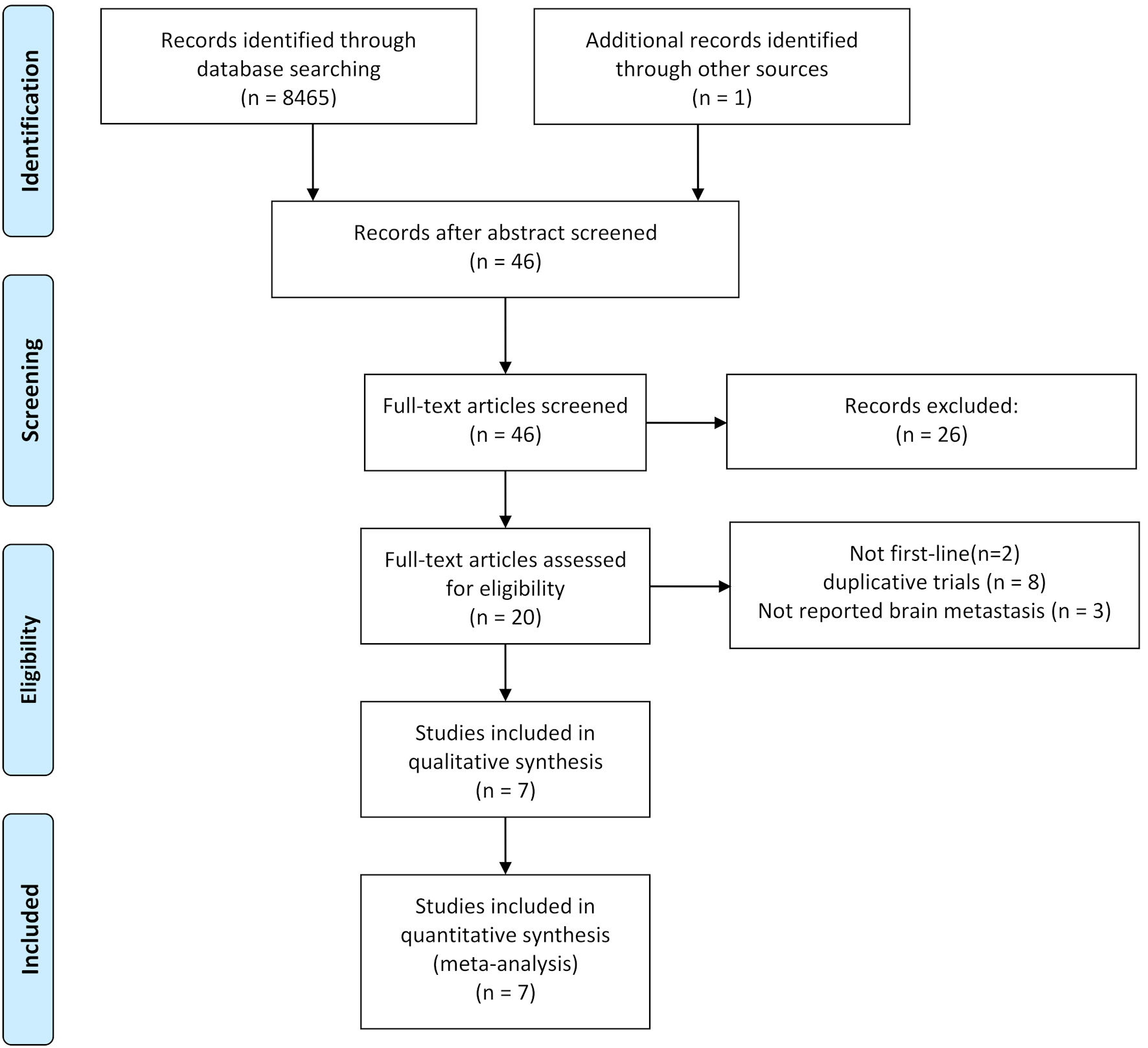 Click for large image | Figure 1. Flowchart illustrating trial selection. |
Characteristics of included trials
Table 1 provides a summary of the characteristics of the included trials. All seven randomized controlled trials were classified as phase III trials. Of these, six trials had a global scope, while one was conducted exclusively in China. In total, these trials encompassed 3,931 ES-SCLC patients, comprising 398 patients with brain metastases and 3,533 without. The methodological quality assessment consistently indicated high quality for the seven included randomized controlled trials (Fig. 2).
 Click to view | Table 1. Characteristics of Included Trials |
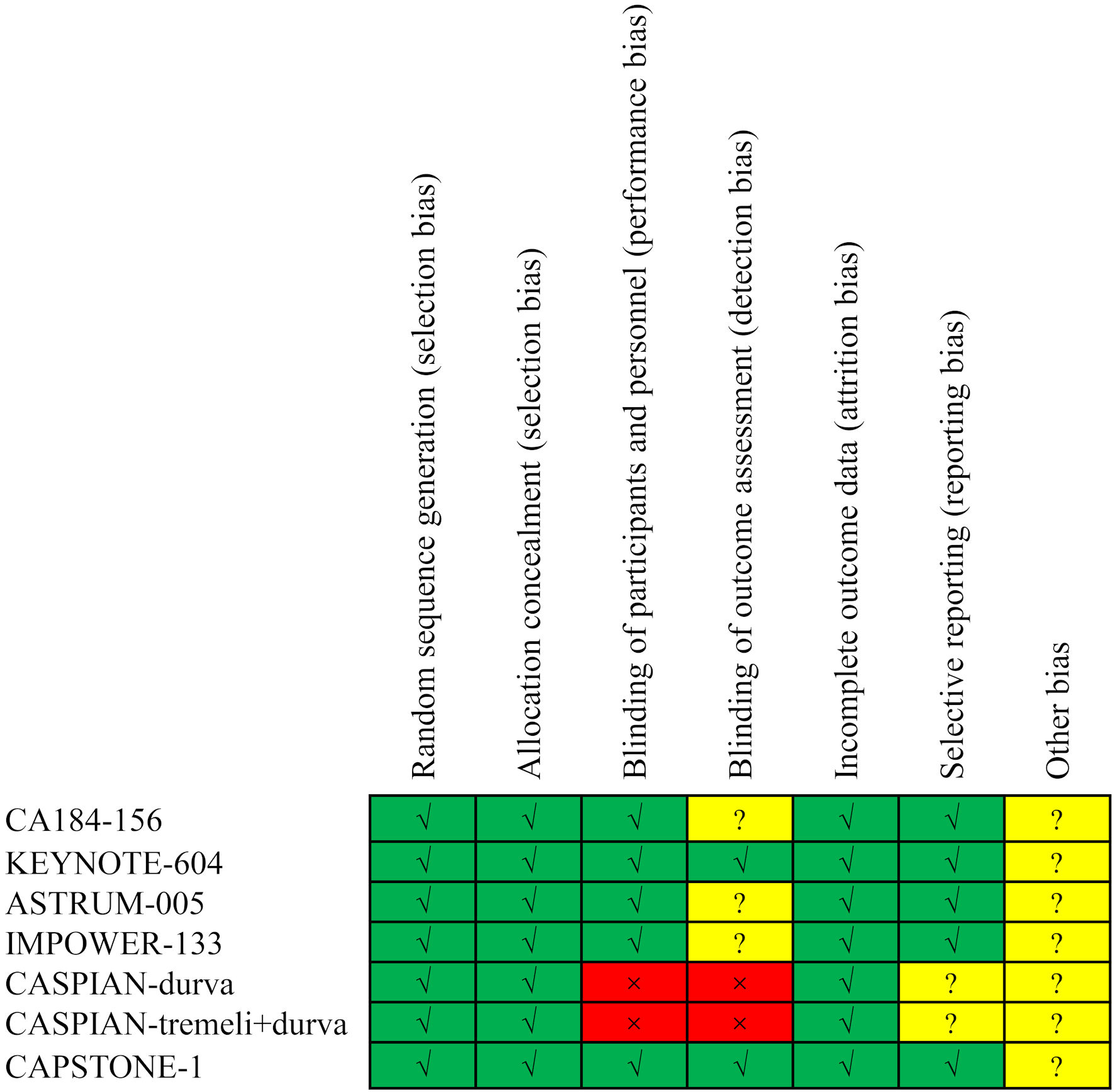 Click for large image | Figure 2. Risk of bias assessment for the included trials. |
PFS analysis
Two randomized controlled trials reported PFS data for patients with brain metastases [6, 11]. Heterogeneity was not observed between these two trials (P = 0.85, I2 = 0.00%). Therefore, the fixed-effects model was applied. The pooled analysis indicated that immunotherapy combined with chemotherapy did not significantly improve PFS compared to chemotherapy alone in patients with brain metastases (HR = 1.03, 95% CI: 0.66 - 1.61; P = 0.887) (Fig. 3a).
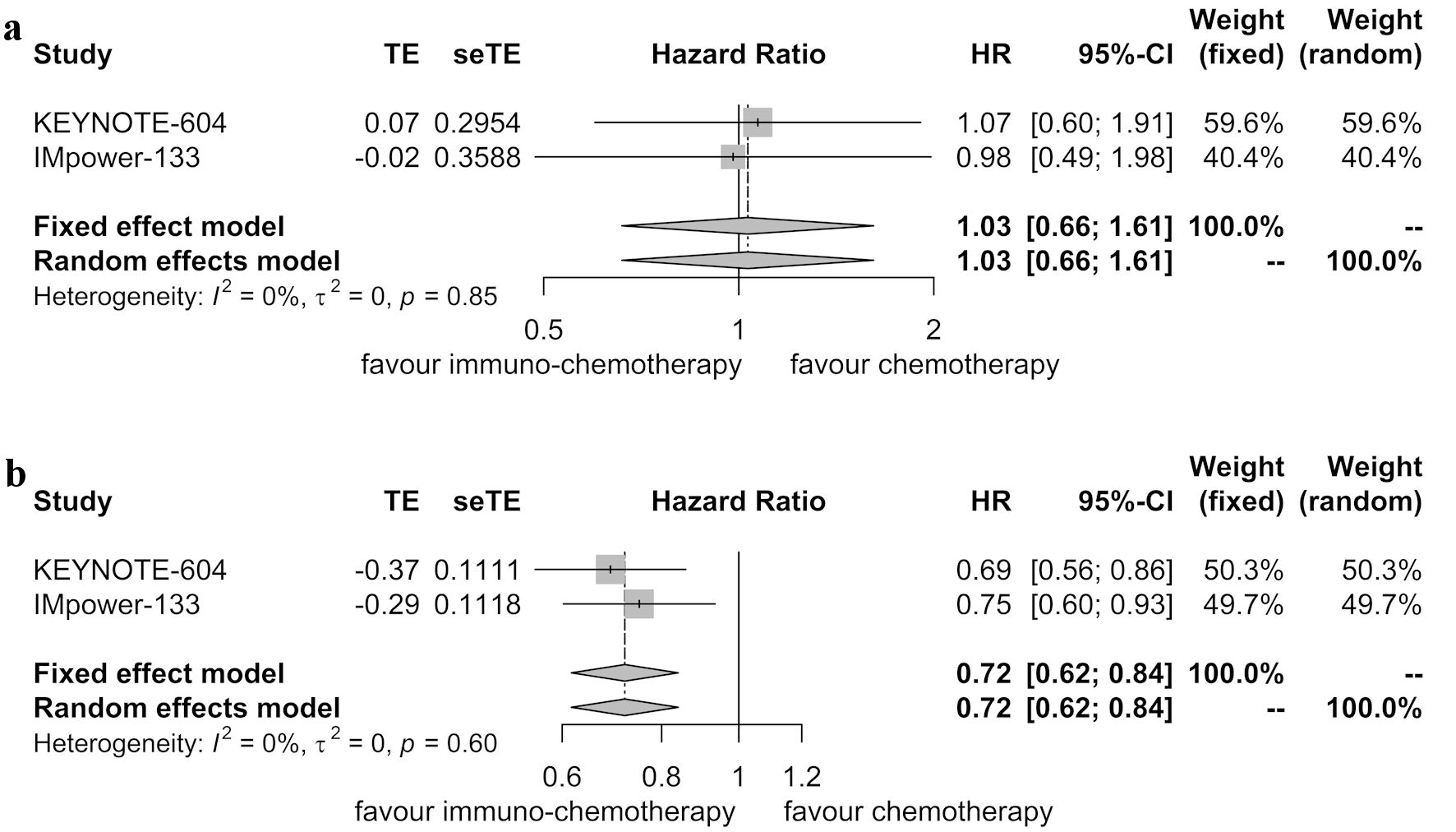 Click for large image | Figure 3. Forest plot displaying hazard ratios (HRs) for comparing progression-free survival between immunotherapy combined with chemotherapy and chemotherapy alone. (a) Patients with brain metastases. (b) Patients without brain metastases. CI: confidence interval. |
Similarly, for patients without brain metastases, the analysis of two randomized controlled trials revealed no significant heterogeneity (P = 0.60, I2 = 0.00%). Therefore, the fixed-effects model was used. The pooled analysis showed that immunotherapy combined with chemotherapy improved PFS compared to chemotherapy alone in these patients (HR = 0.72, 95% CI: 0.62 - 0.84; P < 0.001) (Fig. 3b).
Sensitivity analyses confirmed the robustness of these findings. For patients with brain metastases, the pooled HRs for PFS ranged from 0.98 to 1.07 (Fig. 4a). In patients without brain metastases, the pooled HRs for PFS ranged from 0.69 to 0.75 (Fig. 4b). No significant publication bias was detected in the two randomized controlled trials (Fig. 5).
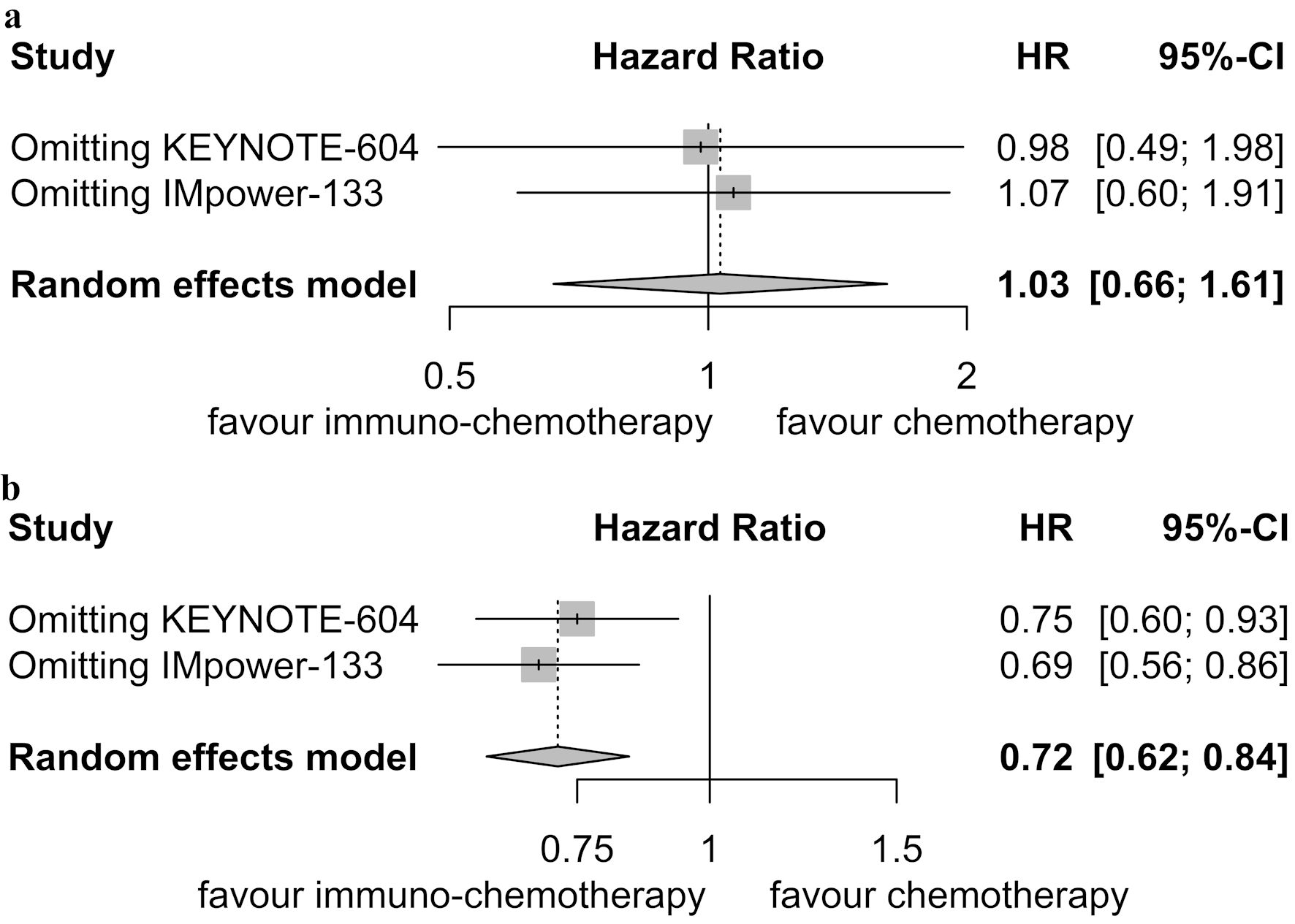 Click for large image | Figure 4. Sensitivity analysis for progression-free survival. (a) Patients with brain metastases. (b) Patients without brain metastases. HR: hazard ratio; CI: confidence interval. |
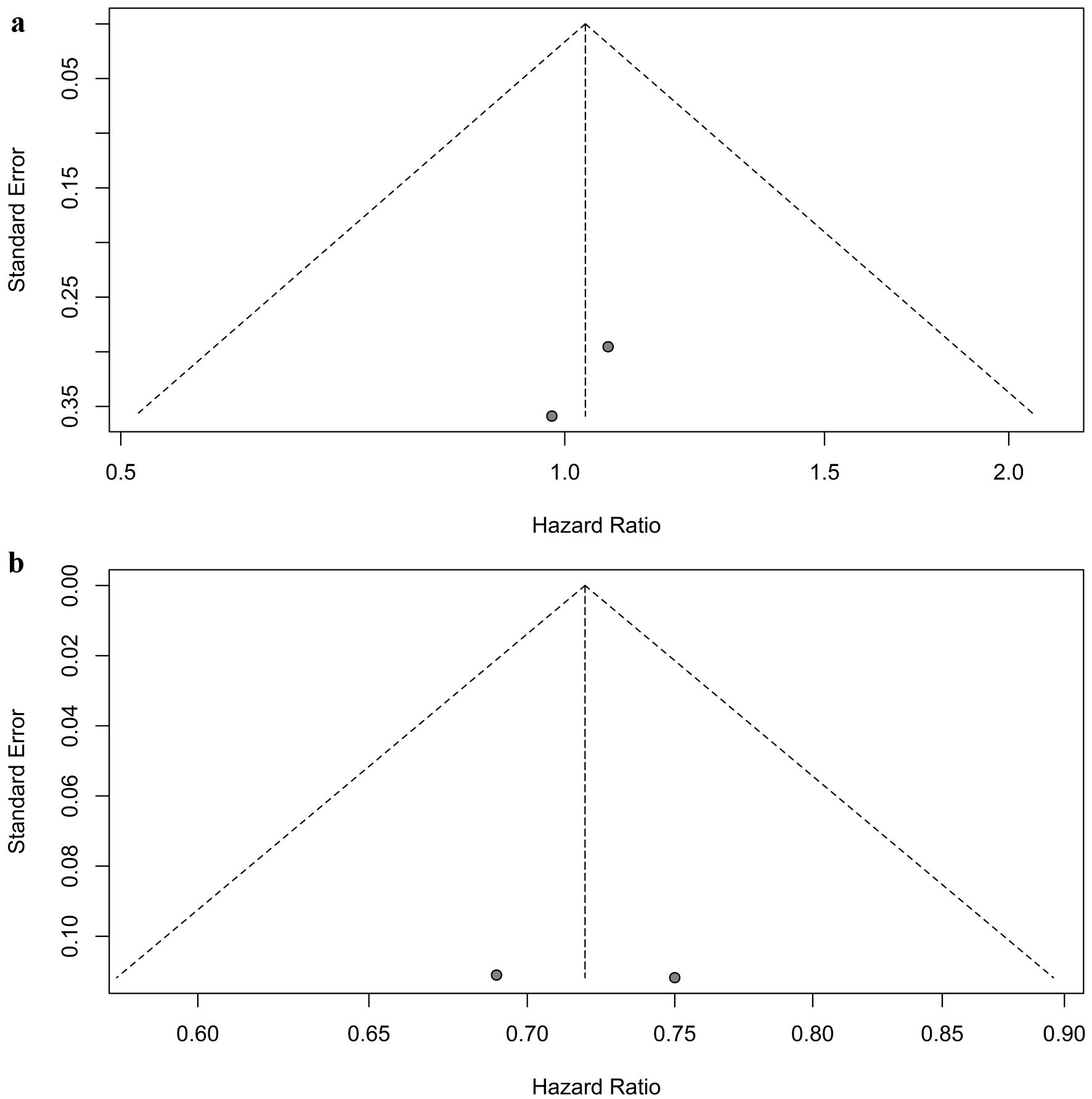 Click for large image | Figure 5. Assessment of publication bias for progression-free survival. (a) Patients with brain metastases. (b) Patients without brain metastases. |
OS analysis
Six randomized controlled trials reported OS data for patients with brain metastases [5-9, 11]. These trials did not exhibit significant heterogeneity (P = 0.12, I2 = 43.00%), leading to the application of the fixed-effects model. The pooled analysis indicated that immunotherapy combined with chemotherapy did not improve OS compared to chemotherapy alone in patients with brain metastases (HR = 1.03, 95% CI: 0.82 - 1.31; P = 0.776) (Fig. 6a).
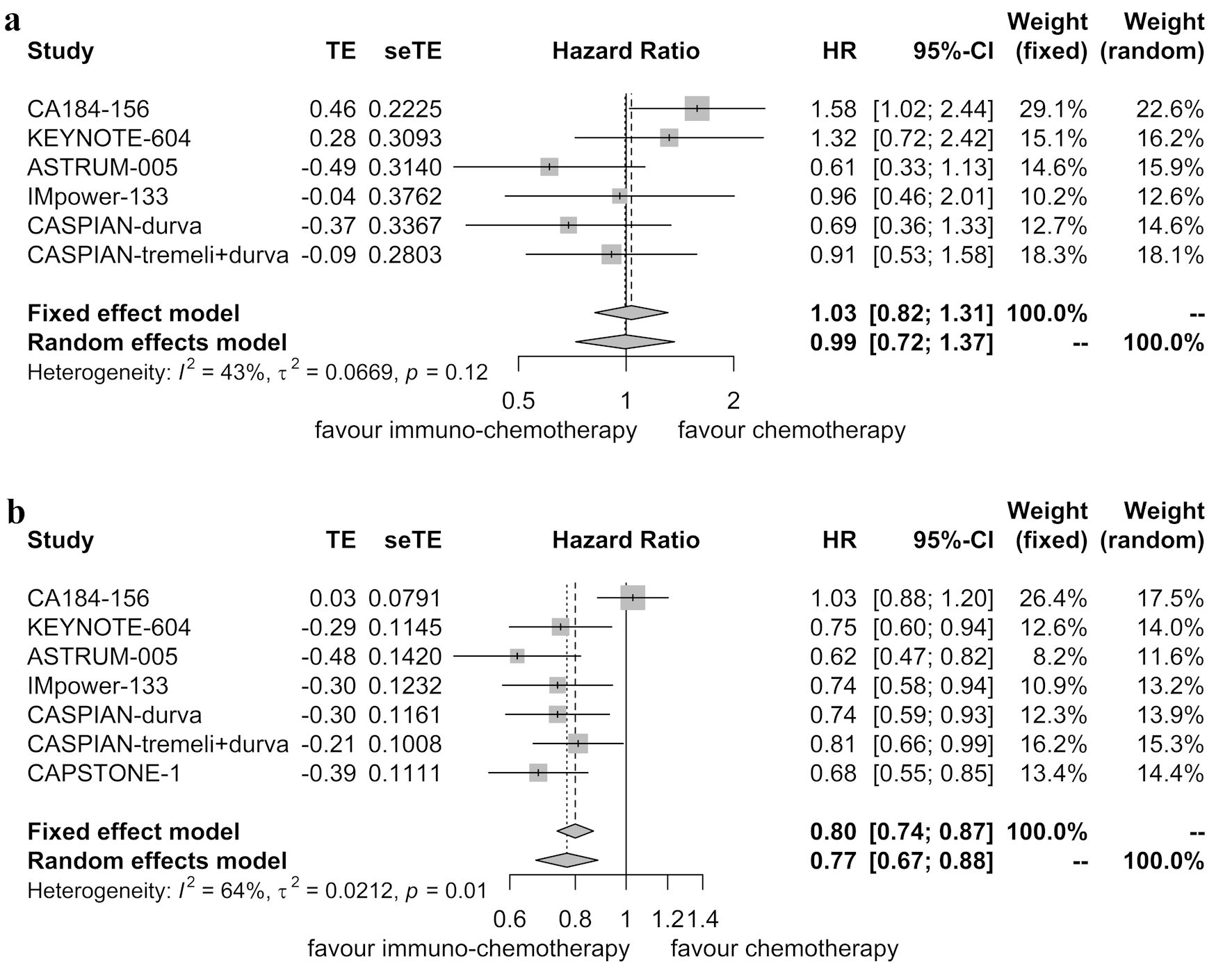 Click for large image | Figure 6. Forest plot illustrating hazard ratios for overall survival between immunotherapy combined with chemotherapy and chemotherapy alone. (a) Patients with brain metastases. (b) Patients without brain metastases. HR: hazard ratio; CI: confidence interval. |
In contrast, seven randomized controlled trials reported OS data for patients without brain metastases [5-11]. These trials demonstrated significant heterogeneity (P = 0.01, I2 = 64.00%), leading to the use of the random-effects model. The pooled analysis indicated that immunotherapy combined with chemotherapy improved OS compared to chemotherapy alone in patients without brain metastases (HR = 0.77, 95% CI: 0.67 - 0.88; P < 0.001) (Fig. 6b).
Sensitivity analyses supported the stability of these results, with pooled HRs for OS in patients with brain metastases ranging from 0.87 to 1.10 (Fig. 7a), and in patients without brain metastases ranging from 0.73 to 0.79 (Fig. 7b). While no publication bias was detected among the six randomized controlled trials involving patients with brain metastases (Egger’s test: P = 0.133, Begg’s test: P = 0.352) (Fig. 8a), publication bias was identified among the seven randomized controlled trials involving patients without brain metastases in the Egger’s test (P = 0.001), but not in the Begg’s test (P = 0.051) (Fig. 8b).
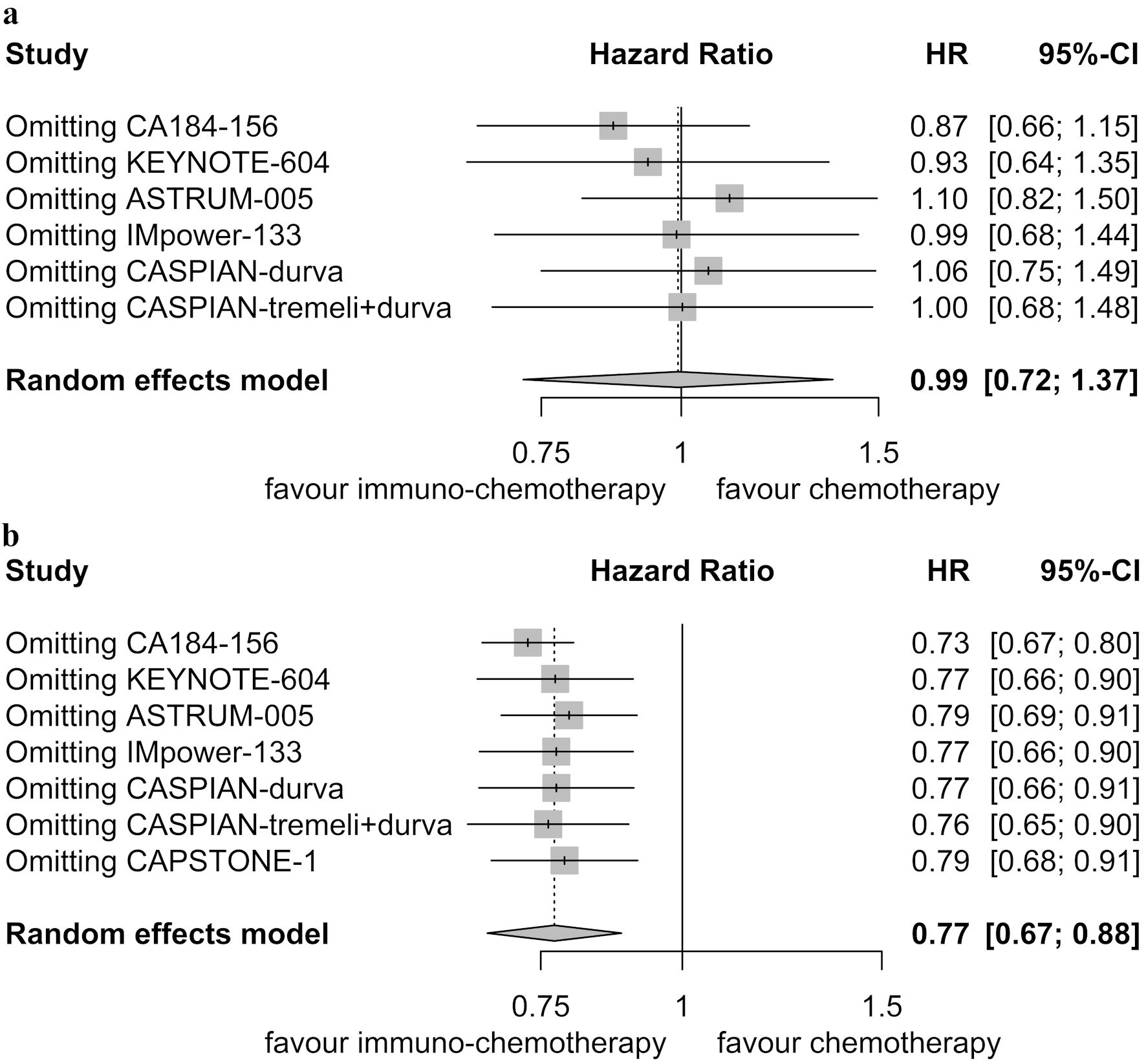 Click for large image | Figure 7. Sensitivity analysis for overall survival. (a) Patients with brain metastases. (b) Patients without brain metastases. HR: hazard ratio; CI: confidence interval. |
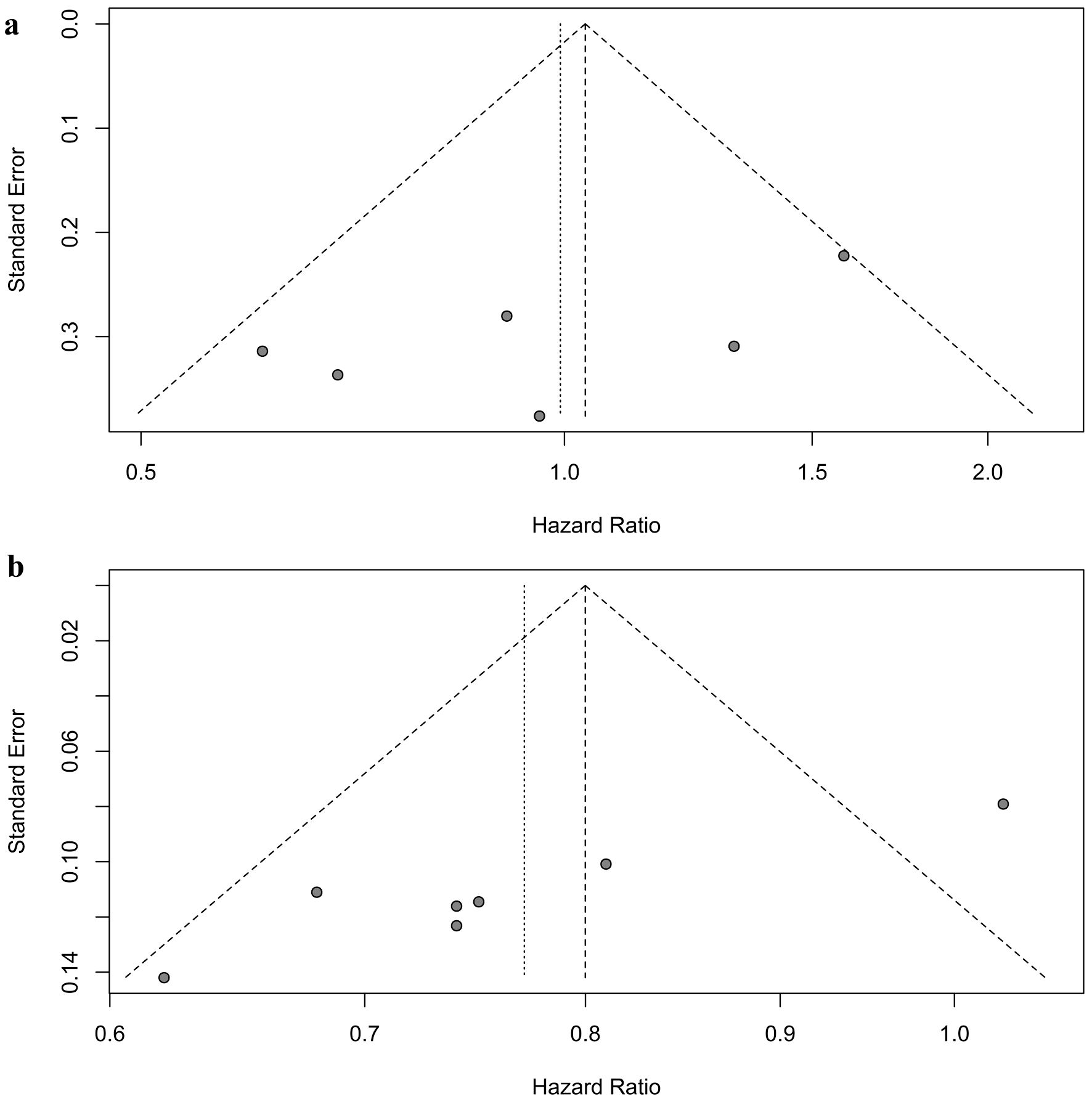 Click for large image | Figure 8. Assessment of publication bias for overall survival. (a) Patients with brain metastases. (b) Patients without brain metastases. |
| Discussion | ▴Top |
This systematic review and meta-analysis have provided valuable insights into the treatment outcomes of ES-SCLC patients with and without brain metastases when undergoing first-line immunotherapy combined with chemotherapy. The findings highlight clear distinctions in the efficacy of this combined treatment based on brain metastasis status.
In ES-SCLC patients without brain metastases, the addition of immunotherapy to chemotherapy as a first-line treatment led to substantial benefits. The significant improvements in both PFS and OS in this subgroup underscore the clinical efficacy of this combination therapy. These results align with existing evidence supporting the role of immunotherapy in improving outcomes for ES-SCLC patients.
Conversely, for ES-SCLC patients with brain metastases, the incorporation of immunotherapy into chemotherapy did not result in a significant enhancement of PFS or OS. This observation raises critical questions about the management of this particular subgroup, indicating that more aggressive therapeutic strategies, such as radiotherapy and surgery, might be needed in the era of immunotherapy for patients with brain metastases.
Historically, the brain has been considered an immune-privileged organ [19]. However, recent insights into the tumor microenvironment within brain metastases challenge this notion. Emerging evidence suggests that the brain can be a viable target for immunotherapy, with promising results seen in controlling brain metastases from melanoma [20]. This has prompted discussions about the potential effectiveness of immunotherapy in lung cancer brain metastases, with some studies indicating improved treatment outcomes for lung cancer patients with brain metastases when immunotherapy is combined with chemotherapy [21-26].
However, this meta-analysis suggests that immunotherapy combined with chemotherapy as a first-line treatment did not improve PFS in ES-SCLC patients with brain metastases. One possible explanation is that many of these patients may have been asymptomatic, and some might have received radiotherapy before being included in the randomized controlled trials. Additionally, the use of prophylactic cranial irradiation following immunotherapy and chemotherapy remains unclear in all of the included trials. As such, it is essential to exercise caution when translating the results of this meta-analysis into clinical practice.
Moreover, the study indicates that immunotherapy combined with chemotherapy did not result in improved OS compared to chemotherapy alone in patients with brain metastases. This finding may be attributed to the fact that all the included trials recommended second-line systemic treatments for patients experiencing recurrences, and the primary patterns of first progression were thoracic and brain metastases [8]. None of the trials allowed for consolidation or salvage local treatments such as surgery or radiotherapy on brain metastases, thoracic disease, or other metastases. Notably, thoracic radiotherapy and brain irradiation have demonstrated the potential to improve OS in ES-SCLC patients when used as consolidation or salvage local treatments [27, 28].
Several limitations of this systematic review should be acknowledged. First, four of the randomized controlled trials did not stratify patients randomly based on brain metastasis status [5, 6, 8, 9], potentially introducing imprecision in subgroup analyses. Second, the small sample size of ES-SCLC patients with brain metastases may have limited the statistical power for analysis.
In conclusion, this study highlights the significant benefit of first-line immunotherapy combined with chemotherapy in ES-SCLC patients without brain metastases, leading to improvements in both PFS and OS. However, this benefit is not observed in patients with brain metastases. The intricate nature of ES-SCLC with brain metastases, combined with the challenges of balancing systemic therapy and localized treatments, underscores the imperative need for further research to optimize the management of this specific subgroup.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Conceptualization: Xin Bin Pan. Methodology: Wen Hua Zhao and Shou Feng Wang. Formal analysis: Cui Yun Su. Validation: Wen Hua Zhao and Shou Feng Wang. Writing - original draft preparation: Wen Hua Zhao. Writing - review and editing: Xin Bin Pan.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
SCLC: small cell lung cancer; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-analyses; RCT: randomized controlled trial; HR: hazard ratio; CI: confidence interval; PFS: progression-free survival; OS: overall survival
| References | ▴Top |
- van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378(9804):1741-1755.
doi pubmed - Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021;7(1):3.
doi pubmed pmc - Rudin CM, Ismaila N, Hann CL, Malhotra N, Movsas B, Norris K, Pietanza MC, et al. Treatment of small-cell lung cancer: American Society of Clinical Oncology Endorsement of the American College of Chest Physicians Guideline. J Clin Oncol. 2015;33(34):4106-4111.
doi pubmed - Reck M, Luft A, Szczesna A, Havel L, Kim SW, Akerley W, Pietanza MC, et al. Phase III randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J Clin Oncol. 2016;34(31):3740-3748.
doi pubmed - Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csoszi T, Cheema PK, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369-2379.
doi pubmed pmc - Cheng Y, Han L, Wu L, Chen J, Sun H, Wen G, Ji Y, et al. Effect of first-line serplulimab vs placebo added to chemotherapy on survival in patients with extensive-stage small cell lung cancer: the ASTRUM-005 randomized clinical trial. JAMA. 2022;328(12):1223-1232.
doi pubmed pmc - Paz-Ares L, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939.
doi pubmed - Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51-65.
doi pubmed - Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, Xu X, et al. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23(6):739-747.
doi pubmed - Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220-2229.
doi pubmed - Santoni M, Rizzo A, Kucharz J, Mollica V, Rosellini M, Marchetti A, Tassinari E, et al. Complete remissions following immunotherapy or immuno-oncology combinations in cancer patients: the MOUSEION-03 meta-analysis. Cancer Immunol Immunother. 2023;72(6):1365-1379.
doi pubmed - Rizzo A, Cusmai A, Giovannelli F, Acquafredda S, Rinaldi L, Misino A, Montagna ES, et al. Impact of proton pump inhibitors and histamine-2-receptor antagonists on non-small cell lung cancer immunotherapy: a systematic review and meta-analysis. Cancers (Basel). 2022;14(6):1404.
doi pubmed pmc - Santoni M, Rizzo A, Mollica V, Matrana MR, Rosellini M, Faloppi L, Marchetti A, et al. The impact of gender on The efficacy of immune checkpoint inhibitors in cancer patients: The MOUSEION-01 study. Crit Rev Oncol Hematol. 2022;170:103596.
doi pubmed - Longo V, Rizzo A, Catino A, Montrone M, Galetta D. Safety evaluation of immune checkpoint inhibitors combined with chemotherapy for the treatment of small cell lung cancer: A meta-analysis of randomized controlled trials. Thorac Cancer. 2023;14(11):1029-1035.
doi pubmed pmc - Phan K, Tian DH, Cao C, Black D, Yan TD. Systematic review and meta-analysis: techniques and a guide for the academic surgeon. Ann Cardiothorac Surg. 2015;4(2):112-122.
doi pubmed pmc - Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
doi pubmed pmc - Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
doi pubmed pmc - Lauko A, Thapa B, Venur VA, Ahluwalia MS. Management of brain metastases in the new era of checkpoint inhibition. Curr Neurol Neurosci Rep. 2018;18(10):70.
doi pubmed - Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, Khushalani NI, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722-730.
doi pubmed pmc - Teixeira Loiola de Alencar V, Guedes Camandaroba MP, Pirolli R, Fogassa CAZ, Cordeiro de Lima VC. Immunotherapy as single treatment for patients with NSCLC with brain metastases: a systematic review and meta-analysis-the META-L-BRAIN study. J Thorac Oncol. 2021;16(8):1379-1391.
doi pubmed - Gadgeel SM, Lukas RV, Goldschmidt J, Conkling P, Park K, Cortinovis D, de Marinis F, et al. Atezolizumab in patients with advanced non-small cell lung cancer and history of asymptomatic, treated brain metastases: Exploratory analyses of the phase III OAK study. Lung Cancer. 2019;128:105-112.
doi pubmed - Powell SF, Rodriguez-Abreu D, Langer CJ, Tafreshi A, Paz-Ares L, Kopp HG, Rodriguez-Cid J, et al. Outcomes with pembrolizumab plus platinum-based chemotherapy for patients with NSCLC and stable brain metastases: pooled analysis of KEYNOTE-021, -189, and -407. J Thorac Oncol. 2021;16(11):1883-1892.
doi pubmed - Novello S, Kowalski DM, Luft A, Gumus M, Vicente D, Mazieres J, Rodriguez-Cid J, et al. Pembrolizumab plus chemotherapy in squamous non-small-cell lung cancer: 5-year update of the phase III KEYNOTE-407 study. J Clin Oncol. 2023;41(11):1999-2006.
doi pubmed pmc - Awad MM, Gadgeel SM, Borghaei H, Patnaik A, Yang JC, Powell SF, Gentzler RD, et al. Long-term overall survival from KEYNOTE-021 cohort G: pemetrexed and carboplatin with or without pembrolizumab as first-line therapy for advanced nonsquamous NSCLC. J Thorac Oncol. 2021;16(1):162-168.
doi pubmed - Gadgeel S, Rodriguez-Abreu D, Speranza G, Esteban E, Felip E, Domine M, Hui R, et al. Updated analysis from KEYNOTE-189: pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol. 2020;38(14):1505-1517.
doi pubmed - Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, Keijser A, et al. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385(9962):36-42.
doi pubmed - Ni M, Jiang A, Liu W, Sheng Y, Zeng H, Liu N, Gao Q, et al. Whole brain radiation therapy plus focal boost may be a suitable strategy for brain metastases in SCLC patients: a multi-center study. Radiat Oncol. 2020;15(1):70.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


