| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 5, October 2023, pages 382-391
Familial Risk and Interaction With Smoking and Alcohol Consumption in Bladder Cancer: A Population-Based Cohort Study
Hyun Jung Kima , Kyoung-Hoon Kimb, Sung Won Leec, Heather Swana
, Sayada Zartasha Kazmid
, Young Shin Kima
, Kyeong Uoon Kime, Minjung Kimf, Jaewoo Chaf, Taeuk Kangg, Hoo Jae Hannh
, Hyeong Sik Ahna, i
aDepartment of Preventive Medicine, College of Medicine, Korea University, Seoul 02841, Korea
bEvidence-Based Research Division, Health Insurance Review and Assessment Service, Gangwon-do (Bangok-dong) 26465, Korea
cDepartment of Urology, Samsung Medical Center, Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Seoul 06351, Korea
dDepartment of Preventive Medicine, Seoul National University College of Medicine, Seoul 03080, Korea
eDepartment of Nursing, Seojeong University, Gyeonggi-do, Korea
fDepartment of Public Health, Graduate School, Korea University, Seoul, Korea
gHealth and Wellness College, Sungshin Women’s University, Seoul, Korea
hMedical Research Institute, College of Medicine, Ewha Womans University, Seoul, Korea
iCorresponding Author: Hyeong Sik Ahn, Department of Preventive Medicine, College of Medicine, Korea University, Seoul 02841, Korea
Manuscript submitted June 21, 2023, accepted August 1, 2023, published online September 20, 2023
Short title: Familial Risk and Interaction in Bladder Cancer
doi: https://doi.org/10.14740/wjon1639
| Abstract | ▴Top |
Background: Although genetic factors are known to play a role in the pathogenesis of bladder cancer, population-level familial risk estimates are scarce. We aimed to quantify the familial risk of bladder cancer and analyze interactions between family history and smoking or alcohol consumption.
Methods: Using the National Health Insurance database, we constructed a cohort of 5,524,403 study subjects with first-degree relatives (FDRs) and their lifestyle risk factors from 2002 to 2019. Familial risk was calculated using hazard ratios (HRs) with 95% confidence intervals (CIs) that compare the risk of individuals with and without affected FDRs. Interactions between family history and smoking or alcohol intake were assessed on an additive scale using the relative excess risk due to interaction (RERI).
Results: Offspring with an affected parent had a 2.09-fold (95% CI: 1.41 - 3.08) increased risk of disease compared to those with unaffected parents. Familial risks of those with affected father and mother were 2.26 (95% CI: 1.51 - 3.39) and 1.10 (95% CI: 0.27 - 4.41), respectively. When adjusted for lifestyle factors, HR reduced slightly to 2.04 (95% CI: 1.38 - 3.01), suggesting that a genetic predisposition is the main driver in the familial aggregation. Smokers with a positive family history had a markedly increased risk of disease (HR: 3.60, 95% CI: 2.27 - 5.71), which exceeded the sum of their individual risks, with statistically significant interaction (RERI: 0.72, 95% CI: 0.31 - 1.13). For alcohol consumption, drinkers with a positive family history also had an increased risk of disease, although the interaction was not statistically significant (RERI: 0.05, 95% CI: -3.39 - 3.48).
Conclusion: Smokers and alcohol consumers with a positive family history of bladder cancer should be considered a high-risk group and be advised to undergo genetic counseling.
Keywords: Bladder cancer; Familial risk; Additive interaction; Smoking; Alcohol consumption
| Introduction | ▴Top |
Bladder cancer is one of the most commonly occurring malignancies worldwide, accounting for 7% of all cancers in men and 2% of those in women [1]. The 5-year survival rate of localized bladder cancer is estimated to be approximately 30% [2]. Previous studies have suggested that genetic predispositions may play a role in the pathogenesis of bladder cancer [3].
Familial aggregation studies have also shown 1.2- to 2-fold elevated risks among family members of bladder cancer patients [4]. However, evidence on the familial aggregation of bladder cancer remains limited due to the small number of studies that mainly used case-control study designs [5-9] and comprised a few hundred study subjects. Large-scale registry-based studies have estimated familial risks of bladder cancer in the Swedish population [10-12], but since they compared bladder cancer incidence in the database with that of the general population outside the registry, time-based incidence pattern among individuals with and without affected family members could not be provided.
Several environmental exposures including lifestyle factors are known to influence the development of bladder cancer, such as tobacco smoking, red meat or processed meat consumption, cardiovascular disease, obesity, alcohol intake, poor glycemic control and exposure to chemical carcinogens [13-17]. Given the complex pathogenesis of bladder cancer, it is possible that genetic and lifestyle factors have an interactive relationship, where the presence of lifestyle factors in genetically predisposed individuals yields a greater (or lesser) impact compared to non-predisposed persons. In this study, we quantified the familial risk of bladder cancer and examined interactions between family history and smoking or alcohol intake to assess gene-environment interactions in bladder cancer.
| Materials and Methods | ▴Top |
Data source
This study used the National Health Insurance (NHI), National Health Screening Program (NHSP) and Support for Specific Illness (SSI) databases to identify blood-related first-degree relatives (FDRs), their lifestyle factors and bladder cancer cases with confirmed diagnoses.
The NHI database is maintained by the mandatory health insurance program run by the Korean government which provides universal insurance to approximately 98% of the Korean population. The database contains demographics and healthcare utilization data of insured persons and their dependents, including primary diagnoses based on International Classification of Disease 10th revision (ICD-10) codes, prescriptions and surgical procedures. Since the NHI database contains information on family relatedness of all insured people and their dependents, it allowed us to identify FDRs.
The NHSP is a nationwide health screening program operated by the NHI, which provides biannual standardized health screening checkups to all insured individuals over the age of 20. The health checkup includes questionnaires regarding health-related lifestyle habits, such as smoking, physical activity, as well as questions on drinking status, including the frequency of alcohol consumption and standard drink amount. It also includes information on anthropometrics and basic laboratory tests of participants.
The SSI program was launched by the NHI to provide copayment reduction for various cancers including bladder cancer. In order to be registered in this program, disease diagnosis must meet the standard criteria specified by the NHI, and be reviewed and confirmed by the corresponding institution prior to submission into the NHI. This process ensures a thorough diagnostic verification for accurate bladder cancer diagnosis prior to registration in the SSI database.
Family relationship data
Employed or self-employed individuals become insured with the NHI by paying a certain monthly amount according to their income. Spouse or children of insured individuals become dependents in the NHI following marriage or birth registration, respectively. Individuals remain insured under the NHI system even if their status (insurer/dependent) or occupation changes. This information allowed us to identify first-degree relationships, namely, individuals and their biological parents. An individual was defined as a biological offspring of a married couple if he or she was registered as a dependent at birth.
Identification of bladder cancer case diagnosis
Through follow-up, we identified individuals diagnosed with bladder cancer using the SSI registration code and ICD-10 code (C67) for bladder cancer from 2002 to 2019. Registration as a bladder cancer case in the SSI database necessitated biopsy and histological confirmation of bladder cancer, along with imaging tests (computed tomography (CT) or magnetic resonance imaging (MRI)). The verification process of the identified cases is summarized in the Supplementary Material 1 (www.wjon.org).
Assessment of smoking and alcohol consumption
We acquired information on smoking and alcohol consumption using NHSP data. The questionnaire administered at the NHSP includes information on the smoking status of participants, start and stop year of smoking, and the number of packs consumed per day. Among individuals who underwent more than one health check-up, we collected consecutive responses and constructed data for smoking status in chronological order. Participants’ latest response was cross verified with their previous responses to verify consistency.
Based on their most recent NHSP response, participants were categorized as “non-smoker”, “former smoker” or “current smoker”. Period and intensity of smoking for both former and current smokers, as well as start and stop year of smoking were obtained, and from this information, we calculated the number of pack-years smoked by multiplying the number of smoking years by the number of cigarette packs smoked per day. The total number of pack-years was calculated for each individual up to bladder cancer diagnosis, smoking cessation or the end of follow-up, whichever came first. Smoking was categorized according to pack-years of < 10, 10 to < 20 and ≥ 20.
With regards to alcohol consumption, individuals were categorized according to standardized guidelines as either non-drinker, moderate drinker (< 2 times per week or < 5 drinks on any day (male); or < 2 times per week or < 4 drinks on any day (female)), or heavy drinker (≥ 2 times per week and ≥ 5 drinks on any day (male); or ≥ 2 times per week and ≥ 4 drinks on any day (female)). We also acquired information on other lifestyle characteristics of each study subject such as age, sex, body mass index (BMI), blood pressure, fasting blood glucose, pulse pressure, proteinuria and cholesterol levels (Supplementary Material 2, www.wjon.org).
Statistical analysis
We included individuals with identifiable blood-related FDRs who underwent a NHSP screening during the study period, and excluded individuals with single parents and children who were not registered as a dependent at birth (Fig. 1). Study subjects were followed from January 1, 2002 up to whichever of the following came first: the diagnosis of bladder cancer, death or the end of follow-up on December 31, 2019. In order to maximize the number of bladder cancer cases in our study, cases diagnosed before 2002 were also included. Sensitivity analyses were performed to analyze familial risks including and excluding bladder cancer cases diagnosed prior to 2002 and the results did not show any significant difference.
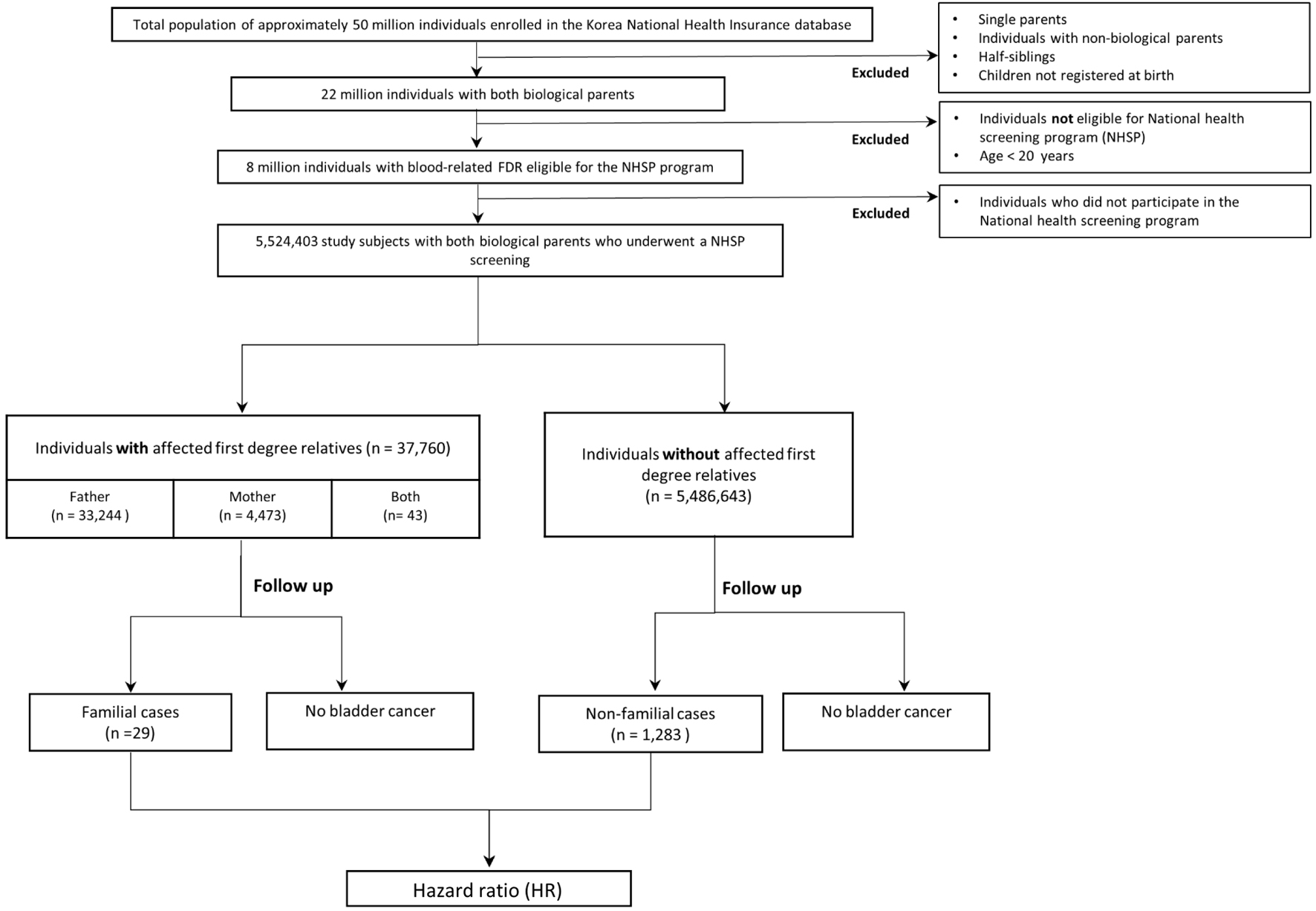 Click for large image | Figure 1. Study flow diagram. |
During the follow-up period, once a parent was diagnosed with bladder cancer, offspring were considered exposed and identified as “with an affected parent” and also as the “familial group”. If a second parent was diagnosed, they were defined as the first “familial case” and offspring were considered exposed to two familial cases and identified as “with both affected parents”. In families with no affected patients, offspring were identified as “without affected parents” and also as the “non-familial group”, and if during follow-up either parent developed bladder cancer, they were defined as a “non-familial case”. We used person-time analysis in which individuals contributed to their corresponding age group.
The magnitude of familial aggregation was estimated as hazard ratios (HRs) with 95% confidence intervals (CIs) using Cox proportional hazard regression models by comparing the incidence of bladder cancer in offspring with versus without an affected parent. Person-years of each study subject were calculated from the date of diagnosis to the respective end of follow-up. Total person-years were defined as the sum of person-years of all study subjects. Incidence rate was measured by dividing the number of bladder cancer cases by total-person years at risk. HRs were also calculated according to each type of family relation. To assess effect of age on familial risk, separate age-stratified familial analyses were performed.
To assess the association of lifestyle factors in bladder cancer, we used Cox proportional hazards regression models to evaluate HRs with 95% CIs. Independent variables were the lifestyle factors acquired from the NHSP database and the dependent variable was development of bladder cancer. In order to account for missing data on lifestyle factors, we excluded each missing value in the univariate analysis, while for the multivariate analysis, we replaced the missing data with the most frequent values in each column. The proportional hazards assumptions were verified by using Schoenfeld and scaled Schoenfeld residuals.
To assess the contribution of lifestyle risk factors in the familial aggregation of bladder cancer, familial risk with and without adjustment for lifestyle factors was measured. Using Cox proportional hazards models, first familial risks without adjustments were estimated. Subsequently, age- and sex-adjusted familial risks were calculated. Then, in another model familial risks adjusted for lifestyle risk factors were assessed.
Interactions were examined between familial risk and smoking/alcohol consumption. Gene-environment interaction was evaluated on an additive scale using risk difference by testing the assumption that family history and lifestyle factors are independent of each other in the underlying population. Under the null hypothesis, the risk difference associated with one exposure (e.g., familial risk) is constant across levels of another exposure (e.g., smoking). We analyzed whether the presence of family history and either smoking or alcohol consumption yielded a greater or reduced risk than their separate risks. For this, four disjointed categories were constructed for the combinations of lifestyle factors and family history of bladder cancer and each category was coded as a dichotomous variable. Incidence was calculated as a multivariate analysis for individuals in each group and HRs were estimated by comparing the incidence of each group to that of a reference group, defined as individuals with neither family history nor smoking/alcohol intake. In the case of interaction, having both a family history and smoking/alcohol intake would increase the risk of bladder cancer more than expected.
The amount of interaction and its statistical significance as a departure from additivity between family history and a given lifestyle factor was represented by relative excess risk due to interaction (RERI) and its corresponding 95% CI. When RERI is zero, it indicates that there is no interaction between the two exposures, while any deviation suggests an interaction (Supplementary Material 3, www.wjon.org). This study was approved by the institutional review board of Korea University and was conducted in compliance with the ethical standards of the responsible institution on human subjects.
| Results | ▴Top |
Cohort description
Using the study database, we identified 5,524,403 individuals with biological mother and father, comprising 1.7 million families. During the study period, 24,651 individuals developed bladder cancer. From our familial relationship analysis, we determined that 21,608 of them were fathers and 3,043 were mothers, and 37,760 individuals (males: 26,608, females: 11,152) had an affected parent, while the remaining 5,486,643 individuals (males: 3,542,561, females: 1,944,082) had no affected mother or father. Table 1 summarizes the demographics and lifestyle factors of individuals with and without affected parents. No significant difference was observed between the two groups in terms of lifestyle risk factors except for smoking, where the offspring with affected parents had a higher proportion of smokers compared to those without affected parents (standardized difference 0.27).
 Click to view | Table 1. Distribution of Lifestyle Factors Among Individuals With and Without Affected Parents For Bladder Cancer |
Familial risk of bladder cancer
Table 2 shows that among individuals with affected parents, the incidence of bladder cancer was 0.44 per 10,000 person-years; and among individuals without affected parents, the incidence was 0.13 per 10,000 person-years. Accordingly, the age- and sex-adjusted HR for bladder cancer in individuals with versus without affected parents was 2.09 (95% CI: 1.41 - 3.08). According to family relationship, offspring of affected father and mother had HRs of 2.26 (95% CI: 1.51 - 3.39) and 1.10 (95% CI: 0.27 - 4.41) and incidences of 0.43 (95% CI: 0.29 - 0.63) and 0.51 (0.19 - 1.35) per 10,000 person-years, respectively.
 Click to view | Table 2. Familial Risk of Bladder Cancer Among Offspring of Affected Parents |
Age- and sex-specific familial risks for bladder cancer
Age-specific analyses for familial and non-familial groups are presented in Supplementary Material 4 (www.wjon.org). According to age group, familial risk was higher among younger age groups and decreased with advancing age. The HR for persons aged 30 - 40 years was 3.08 (95% CI: 0.99 - 7.31), which reduced to 1.06 (95% CI: 0.34 - 2.48) among those aged 40 - 50 years. According to sex, males had an increased risk of disease, as we observed a consistent 4:1 ratio between men and women across age groups.
Risk of smoking and alcohol intake on bladder cancer
Figure 2 shows the association between smoking/alcohol consumption and bladder cancer. Smoking was associated with an increased risk of disease. According to pack-years, the risk of bladder cancer was increased across all categories of smoking and the magnitude was higher for ≥ 20 pack-years (2.73, 95% CI: 2.21 - 3.36) compared to 10 to < 20 pack-years (1.85, 95% CI: 1.50 - 2.27) and < 10 pack-years (1.35, 95% CI: 1.09 - 1.66). For alcohol consumption, the HR for heavy drinking was 1.30 (95% CI: 1.04 - 1.63), compared to non-drinkers; however, moderate drinking was not associated with an increased risk of disease (HR 0.87, 95% CI: 0.74 - 1.02).
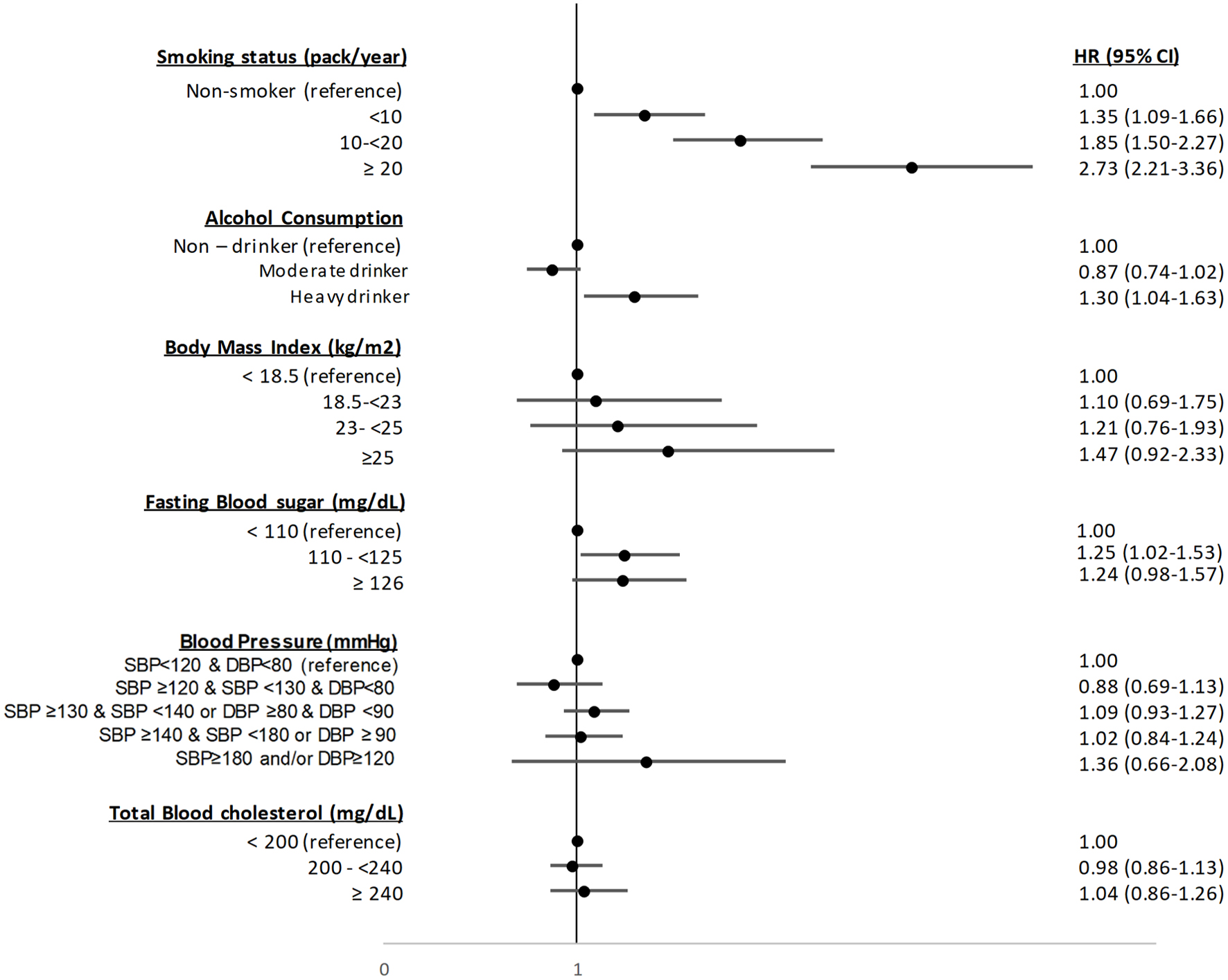 Click for large image | Figure 2. Association of lifestyle factors with the total study population. CI: confidence interval; DBP: diastolic blood pressure; HR: hazard ratio; SBP: systolic blood pressure. |
To assess the relative contribution of shared lifestyle factors in the familial aggregation of bladder cancer, familial risks were calculated before and after adjusting for lifestyle factors (Table 2). The adjusted familial risk decreased slightly from HR 2.09 (95% CI: 1.41 - 3.08) to 2.04 (95% CI: 1.38 - 3.01), suggesting that the impact of lifestyle factors may be limited.
Interaction between familial risk and smoking/alcohol consumption
Figure 3 presents the interaction analyses and combined effects of family history and smoking or alcohol consumption on bladder cancer risk. Smokers with a positive family history had a significantly increased risk of bladder cancer (HR 3.60, 95% CI: 2.27 - 5.71) and the combined effect of these factors exceeded the sum of their individual risks (HRs 3.60 vs. 2.89). This indicates the presence of a statistically significant interactive relationship (RERI 0.72, 95% CI: 0.31 - 1.13).
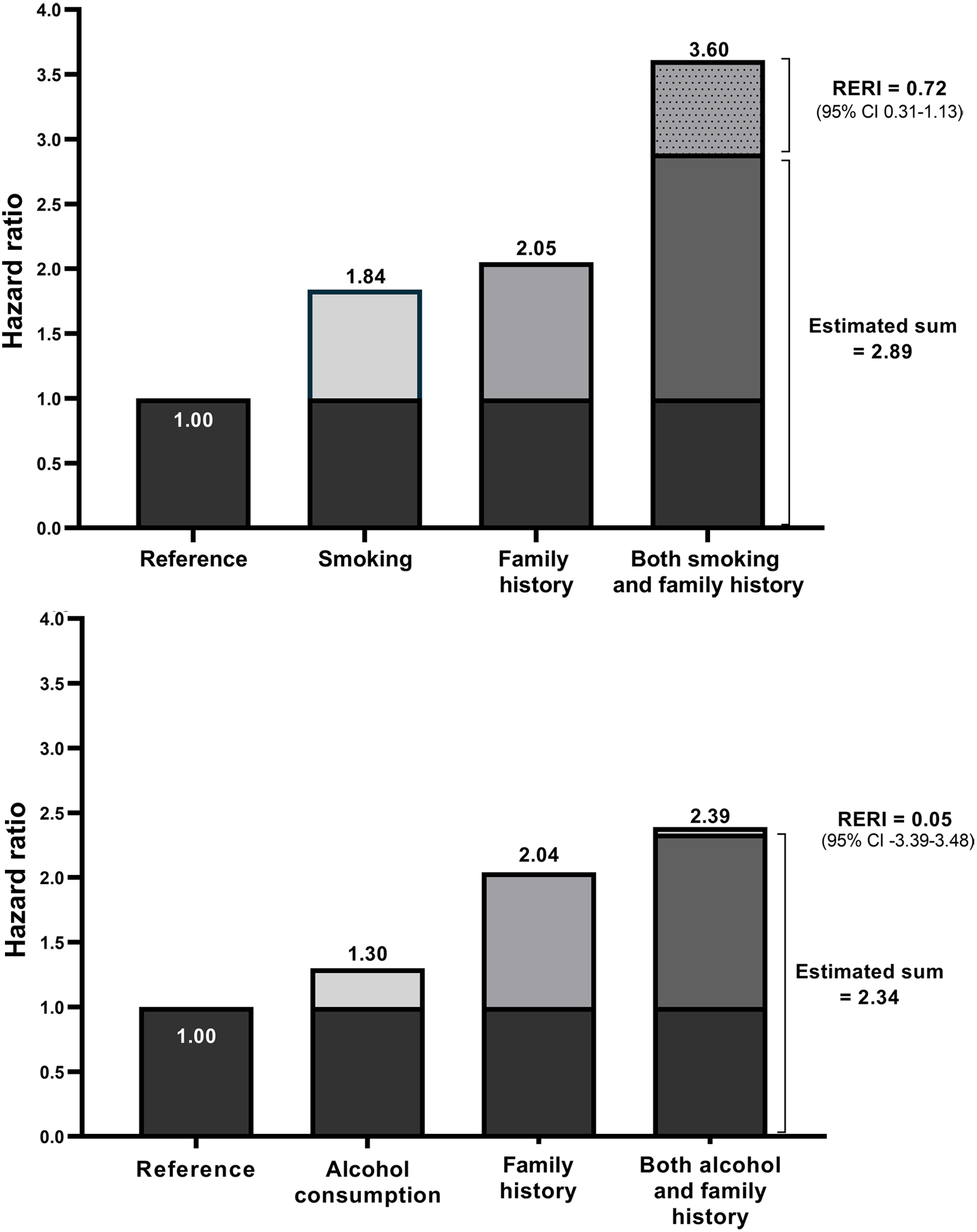 Click for large image | Figure 3. Combined effect of family history and smoking and alcohol consumption on the risk of bladder cancer. CI: confidence interval; RERI: relative excess risk due to interaction. |
Drinkers with a positive family history of bladder cancer had an increased risk of disease (HR 2.39, 95% CI: 0.59 - 9.58) and their combined effect was slightly higher than the sum of their individual effects (HR 2.39 vs. 2.34), although this was not statistically significant and the excess risk was lower than smoking (RERI 0.05, 95% CI: -3.39 - 3.48).
| Discussion | ▴Top |
Study summary
This population-based cohort study followed 5.5 million individuals with blood-related FDR and demonstrated a 2.09-fold increased familial risk for bladder cancer, being 2.26- and 1.10-fold among those with an affected father and mother, respectively. While lifestyle factors including smoking and alcohol consumption were significantly associated with cancer risk, familial risk adjusted for lifestyle factors reduced only slightly to 2.04-fold, suggesting that genetic factors were the predominant driver in the familial aggregation of bladder cancer. Our findings indicate the possibility of a gene-environment interaction, as the combination of both a family history of bladder cancer and smoking was associated with a markedly increased risk of disease that exceeded the sum of their individual effects.
Previous studies
Since the 1960s, several studies have been performed on the familial aggregation of bladder cancer. We identified 10 studies that provided familial risk estimates, six of which were performed using case-control study designs. Three were hospital-based case-control studies from USA [5], Italy [6] and Spain [7] that included 319, 690 and 1,158 participants, respectively, and reported 2- to 2.3-fold increased bladder cancer risks among FDRs. The remaining three were population-based case-control studies conducted in Iceland [18], Netherlands [8] and Utah [19] that yielded 1.2- to 1.73-fold increased familial risks. Most of these previous studies used a case-control study design and collected information on family relationships and cancer diagnoses of family members from interviews or questionnaires.
Three reports from Sweden used registry-based data and provided familial risk estimates of up to 1.5-fold, which are lower than our study [10-12]. However, these studies did not specifically estimate familial risk for bladder cancer, but rather examined familial occurrence of other cancers among family members of bladder cancer cases. In our study, we concomitantly followed persons with affected FDR as well as those without affected FDR and provided temporal incidence pattern specifically for bladder cancer.
While both genetic and shared lifestyle risk factors may affect familial aggregation [20-22], the findings of our study suggest that genetic factors are the primary determinants of the familial aggregation in bladder cancer. After adjusting for lifestyle risk factors, the magnitude of familial risk only slightly decreased from 2.09- to 2.04-fold and a similar pattern was observed in the familial risks according to relationships.
This study demonstrated that smokers with a positive family history had a 3.60-fold increased risk for bladder cancer and the combined effect of smoking with family history on the risk of bladder cancer exceeded the sum of their individual risks, showing an interactive relationship between these factors. Our findings suggest that genetic factors and smoking may potentiate each other rather than operating independently. It is also possible that the impact of smoking is more sensitive towards those with a genetic predisposition. Although the combined risk of a family history and alcohol intake was high, our statistical analysis showed that the interaction was statistically insignificant, which may be due to the low number of bladder cancer cases included in our study. Smokers or drinkers with a positive family history may be considered a high-risk group and should be advised to undergo genetic counseling, as well as be informed of the increased risk of disease associated with these factors.
Gene-environment interactions between bladder cancer-related genes and environmental risk factors such as smoking have been reported by a few previous studies. For smoking, eight SNPs (NAT2, GSTM1, UGT1A6, PSCA, MYC, CBX5 APOBEC3A [23, 24] FOXF2 and RSPH3-TAGAP-EZR) [25] have been identified that showed additive gene-smoking interactions [23, 26-29]. While for alcohol consumption, one Japanese study demonstrated that the genetic variants ALDH2 Glu/Lys and ADH1B Arg+ increased the risk of bladder cancer among subjects who consumed alcohol and not in subjects who were non-alcoholics [30]. However, gene-environment interactions in bladder cancer remain unclear due to the limited number of studies and non-replicability of findings.
To date, GWAS and candidate gene research suggests that 15 genomic regions play a significant role in bladder carcinogenesis and have identified a dozen susceptibility variants for bladder cancer in genes GSTM1, NAT2, UGT1A, SLC14A1, TP63, TERT/CLPTM1L, PSCA, FGFR3/TACC3. In particular, FGFR3 has recently emerged as a therapeutic target in bladder cancer, with studies showing that approximately 50% of bladder cancers have somatic mutations in the FGFR3 coding sequence [31]. While therapies targeting the FGFR3 protein have demonstrated clinical benefit, further studies are needed to improve the management of FGFR3 bladder cancer patients [32]. Furthermore, our interaction analyses represent the “average” effects of these bladder cancer-related genes, and therefore further studies are needed to assess gene-environment interactions between genes and smoking, especially at the genome-wide level.
Suggested mechanisms for smoking in relation to bladder cancer include DNA damage caused by carcinogenic compounds present in tobacco smoke via impaired detoxification [33], along with smoking-associated defective inflammatory and cytokine responses. While for alcohol, its metabolite acetaldehyde has been implicated to induce accumulation of DNA mutations in bladder mucosa. Several genes have been reported to be involved in these mechanisms [29], such as those regulating DNA repair (ERCC2, FANCD2), proinflammatory cytokine-related responses (ILF4), cell proliferation (TP53, PTEN) and oxidation (ALDH2). It is plausible that these genes related to the mechanisms of smoking and alcohol intake may also be involved in the oncogenic pathways of bladder cancer, and possibly facilitate the interaction with bladder cancer carcinogenesis. Further research is required to better elucidate these associations which might offer probable explanations on the interactions found in our study.
Limitations
While we assessed gene-environment interactions assuming that familial risk is a surrogate for genetic factors, the concern may be raised that familial risk is influenced not only by genetic factors, but also by shared lifestyle factors. However, our study was unable to precisely separate lifestyle factors from genetic factors. Although we controlled for several bladder cancer risk factors, such as blood pressure and hyperglycemia, some relevant risk factors, including dietary factors, could not be included in our study. Moreover, the length of our study period might not have been long enough to cover all familial occurrences and may have led to an under-identification of older relatives. Another issue is our retrospective study design and reliance on pre-existing databases. Possible concerns may be raised regarding the validity of bladder cancer diagnosis and misclassification bias. However, as the SSI database requires histological diagnosis for diagnosis confirmation, the bladder cancer diagnoses may be considered valid. Additionally, our study did not include information on genes and therefore future genetic studies are needed to further assess and confirm our findings. Finally, information on smoking and alcohol consumption were acquired from self-reported questionnaires administered at the NHSP check-up, which may be subject to bias. However, given that the questionnaire inquires on participants’ current lifestyle habits, the risk of recall bias may be considered low.
This nationwide population-based study demonstrated a 2.09-fold increased familial risk of bladder cancer and suggested that substantial interactions exist between family history of bladder cancer and smoking. This finding implies that smokers with a genetic predisposition are more susceptible to developing bladder cancer and therefore should be warned of this high-risk behavior for preventing the risk of developing the cancer.
| Supplementary Material | ▴Top |
Suppl 1. Assessing Diagnostic Accuracy.
Suppl 2. Lifestyle Factors Subgroups.
Suppl 3. Statistical Interaction Analyses.
Suppl 4. Age-Specific Familial Risk of Bladder Cancer Among Offspring of Affected Parents.
Acknowledgments
None to declare.
Financial Disclosure
The authors have no relevant financial or non-financial interests to disclose.
Conflict of Interest
None to declare.
Informed Consent
We did not collect informed consent from each study subject. We used secondary data obtained from the National Health Insurance database which includes the healthcare utilization of the entire Korean population. This dataset is encrypted and strictly anonymized, and does not contain any personal information.
Author Contributions
HJ Kim and KH Kim: protocol/project development, data collection or management, and data analysis. SW Lee and HJ Hann: protocol/project development, data collection or management. H Swan, SZ Kazmi, and MJ Kim: manuscript writing/editing. YS Kim: data analysis, manuscript writing/editing. KU Kim and JW Cha: data collection or management. TU Kang: data analysis. HS Ahn: protocol/project development, data collection or management, data analysis, and manuscript writing/editing.
Data Availability
The data analyzed in our study can be acquired from the Korean National Health Insurance Service, but restrictions apply to the availability of the data, which was used with permission for the current study and therefore not publicly available. Data are however available upon reasonable request and with permission from the NHI.
Abbreviations
BMI: body mass index; CI: confidence interval; FDRs: first-degree relatives; HRs: hazard ratios; ICD-10: International Classification of Disease 10th revision; NHI: National Health Insurance; NHSP: National Health Screening Program; RERI: relative excess risk due to interaction; SSI: Support for Specific Illness
| References | ▴Top |
- Malats N, Real FX. Epidemiology of bladder cancer. Hematol Oncol Clin North Am. 2015;29(2):177-189.
doi pubmed - Crocetto F, Buonerba C, Caputo V, Ferro M, Persico F, Trama F, Iliano E, et al. Urologic malignancies: advances in the analysis and interpretation of clinical findings. Future Sci OA. 2021;7(4):FSO674.
doi pubmed pmc - Gu J, Wu X. Genetic susceptibility to bladder cancer risk and outcome. Per Med. 2011;8(3):365-374.
doi pubmed pmc - Kiemeney LA. Hereditary bladder cancer. Scand J Urol Nephrol Suppl. 2008;218:110-115.
doi pubmed - Kramer AA, Graham S, Burnett WS, Nasca P. Familial aggregation of bladder cancer stratified by smoking status. Epidemiology. 1991;2(2):145-148.
doi pubmed - Turati F, Bosetti C, Polesel J, Serraino D, Montella M, Libra M, Facchini G, et al. Family history of cancer and the risk of bladder cancer: A case-control study from Italy. Cancer Epidemiol. 2017;48:29-35.
doi pubmed - Murta-Nascimento C, Silverman DT, Kogevinas M, Garcia-Closas M, Rothman N, Tardon A, Garcia-Closas R, et al. Risk of bladder cancer associated with family history of cancer: do low-penetrance polymorphisms account for the increase in risk? Cancer Epidemiol Biomarkers Prev. 2007;16(8):1595-1600.
doi pubmed - Aben KK, Witjes JA, Schoenberg MP, Hulsbergen-van de Kaa C, Verbeek AL, Kiemeney LA. Familial aggregation of urothelial cell carcinoma. Int J Cancer. 2002;98(2):274-278.
doi pubmed - Martin C, Leiser CL, O'Neil B, Gupta S, Lowrance WT, Kohlmann W, Greenberg S, et al. Familial cancer clustering in urothelial cancer: a population-based case-control study. J Natl Cancer Inst. 2018;110(5):527-533.
doi pubmed pmc - Plna K, Hemminki K. Familial bladder cancer in the National Swedish Family Cancer Database. J Urol. 2001;166(6):2129-2133.
pubmed - Dong C, Hemminki K. Modification of cancer risks in offspring by sibling and parental cancers from 2,112,616 nuclear families. Int J Cancer. 2001;92(1):144-150.
pubmed - Yu H, Hemminki O, Forsti A, Sundquist K, Hemminki K. Familial urinary bladder cancer with other cancers. Eur Urol Oncol. 2018;1(6):461-466.
doi pubmed - Cumberbatch MGK, Jubber I, Black PC, Esperto F, Figueroa JD, Kamat AM, Kiemeney L, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74(6):784-795.
doi pubmed - Lin J, Spitz MR, Dinney CP, Etzel CJ, Grossman HB, Wu X. Bladder cancer risk as modified by family history and smoking. Cancer. 2006;107(4):705-711.
doi pubmed - Cantiello F, Cicione A, Salonia A, Autorino R, De Nunzio C, Briganti A, Gandaglia G, et al. Association between metabolic syndrome, obesity, diabetes mellitus and oncological outcomes of bladder cancer: a systematic review. Int J Urol. 2015;22(1):22-32.
doi pubmed - Aveta A, Cacciapuoti C, Barone B, Di Zazzo E, Del Giudice F, Maggi M, Ferro M, et al. The impact of meat intake on bladder cancer incidence: is it really a relevant risk? Cancers (Basel). 2022;14(19):4775.
doi pubmed pmc - Barone B, Finati M, Cinelli F, Fanelli A, Del Giudice F, De Berardinis E, Sciarra A, et al. Bladder cancer and risk factors: data from a multi-institutional long-term analysis on cardiovascular disease and cancer incidence. J Pers Med. 2023;13(3):512.
doi pubmed pmc - Kiemeney LA, Moret NC, Witjes JA, Schoenberg MP, Tulinius H. Familial transitional cell carcinoma among the population of Iceland. J Urol. 1997;157(5):1649-1651.
pubmed - Goldgar DE, Easton DF, Cannon-Albright LA, Skolnick MH. Systematic population-based assessment of cancer risk in first-degree relatives of cancer probands. J Natl Cancer Inst. 1994;86(21):1600-1608.
doi pubmed - Volanis D, Kadiyska T, Galanis A, Delakas D, Logotheti S, Zoumpourlis V. Environmental factors and genetic susceptibility promote urinary bladder cancer. Toxicol Lett. 2010;193(2):131-137.
doi pubmed - Letasiova S, Medve'ova A, Sovcikova A, Dusinska M, Volkovova K, Mosoiu C, Bartonova A. Bladder cancer, a review of the environmental risk factors. Environ Health. 2012;11(Suppl 1):S11.
doi pubmed pmc - Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, Kassouf W, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63(2):234-241.
doi pubmed - Garcia-Closas M, Rothman N, Figueroa JD, Prokunina-Olsson L, Han SS, Baris D, Jacobs EJ, et al. Common genetic polymorphisms modify the effect of smoking on absolute risk of bladder cancer. Cancer Res. 2013;73(7):2211-2220.
doi pubmed pmc - Selinski S, Blaszkewicz M, Ickstadt K, Gerullis H, Otto T, Roth E, Volkert F, et al. Identification and replication of the interplay of four genetic high-risk variants for urinary bladder cancer. Carcinogenesis. 2017;38(12):1167-1179.
doi pubmed pmc - Figueroa JD, Han SS, Garcia-Closas M, Baris D, Jacobs EJ, Kogevinas M, Schwenn M, et al. Genome-wide interaction study of smoking and bladder cancer risk. Carcinogenesis. 2014;35(8):1737-1744.
doi pubmed pmc - Lipunova N, Wesselius A, Cheng KK, van Schooten FJ, Bryan RT, Cazier JB, Zeegers MP. Gene-environment interaction with smoking for increased non-muscle-invasive bladder cancer tumor size. Transl Androl Urol. 2020;9(3):1329-1337.
doi pubmed pmc - Hung RJ, Boffetta P, Brennan P, Malaveille C, Hautefeuille A, Donato F, Gelatti U, et al. GST, NAT, SULT1A1, CYP1B1 genetic polymorphisms, interactions with environmental exposures and bladder cancer risk in a high-risk population. Int J Cancer. 2004;110(4):598-604.
doi pubmed - Hemminki K, Bermejo JL, Ji J, Kumar R. Familial bladder cancer and the related genes. Curr Opin Urol. 2011;21(5):386-392.
doi pubmed - Rouissi K, Ouerhani S, Hamrita B, Bougatef K, Marrakchi R, Cherif M, Ben Slama MR, et al. Smoking and polymorphisms in xenobiotic metabolism and DNA repair genes are additive risk factors affecting bladder cancer in Northern Tunisia. Pathol Oncol Res. 2011;17(4):879-886.
doi pubmed - Masaoka H, Ito H, Soga N, Hosono S, Oze I, Watanabe M, Tanaka H, et al. Aldehyde dehydrogenase 2 (ALDH2) and alcohol dehydrogenase 1B (ADH1B) polymorphisms exacerbate bladder cancer risk associated with alcohol drinking: gene-environment interaction. Carcinogenesis. 2016;37(6):583-588.
doi pubmed - Ascione CM, Napolitano F, Esposito D, Servetto A, Belli S, Santaniello A, Scagliarini S, et al. Role of FGFR3 in bladder cancer: Treatment landscape and future challenges. Cancer Treat Rev. 2023;115:102530.
doi pubmed - Kacew A, Sweis RF. FGFR3 Alterations in the era of immunotherapy for urothelial bladder cancer. Front Immunol. 2020;11:575258.
doi pubmed pmc - Pelucchi C, Bosetti C, Negri E, Malvezzi M, La Vecchia C. Mechanisms of disease: The epidemiology of bladder cancer. Nat Clin Pract Urol. 2006;3(6):327-340.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


